Abstract
The incidence of menstrual irregularities, both primary and secondary amenorrhea, has been reported to be as high as 60%, with the highest incidence in younger athletes, suggesting possible adverse effects on bone development. It was hypothesized that in a rat model, suppressed hypothalamic activity via a gonadotropin-releasing hormone antagonist (GnRH-a) before onset of puberty would result in a relatively larger bone strength deficit compared with suppression after puberty. Hypothalamic suppression was achieved by providing GnRH injections. Animals received injections for 25 days either before puberty (pre group) (age 23–46 days) or after puberty (post group) (age 65–90 days). Body weights and uterine weights were measured. Serum estradiol was assayed. Mechanical strength of the right femora and histomorphometry of the left femur were measured. Suppression of the hypothalamic– pituitary–gonadal axis was confirmed by significant atrophy of uterine tissue and suppressed estradiol levels. The peak moment was significantly lower in the pre and post GnRH-a groups compared with control. The percentage difference of the average peak moment and stiffness values from the respective age-matched control groups yielded a greater percentage difference in the pre group. The cortical area was less in the GnRH-a-treated groups, but no significant difference between the relative deficits between pre and post groups were found. Hypothalimic–pituitary–gonadal axis suppression before puberty resulted in a significantly larger deficit in mechanical strength compared with postpubertal animals. The time before puberty may represent a time when skeletal strength is more compromised. Women experience both primary and secondary amenorrhea; however, the treatment may need to be different for each condition.
Keywords: Puberty, Estradiol, Bone strength, GnRH antagonist, Bone histomorphometry
The incidence of menstrual irregularities, both primary and secondary amenorrhea, has been reported as high as 60%, with the highest incidence in younger athletes, suggesting possible adverse effects on bone development [1, 2]. Studies have reported bone densities in young athletic women to be similar to those of 51-year-old women [3]. In a recent study, 72% of amenorrheic athletes had bone densities that met the diagnostic criterion for osteopenia or osteoporosis [4]. In 1992, a syndrome, the female athlete triad, was identified and includes the connection between energy availability, menstrual function, and bone strength [5]. The delay of menarche and infrequent menstrual cycles decrease estrogen levels during adolescence and decrease peak bone mass [3, 6, 7]. Suboptimal skeletal development may affect long-term bone strength and increase the incidence of fracture during growth and at maturity. The failure to accrue peak bone mass during the adolescent years represents a missed opportunity to optimize bone mass during one’s life [2, 4].
Osteoporosis has been called ‘‘a pediatric disease with geriatric consequences’’ [8]. Peak bone mineral accrual rate occurs at puberty [9], with an accrual of 26% of total adult bone mineral within 2 years [10]. However, a delay in the onset of puberty (primary amenorrhea) correlates with both low bone mass and an increased incidence of stress fracture in young women [11]. In a comparison of elite female athletes who experienced fracture vs. those who did not, bone mineral density (BMD) was not different between groups; however, there was a significantly later age at menarche (puberty) in the group that experienced fracture [12]. Young girls with childhood fracture had lower bone mineral content compared with a nonfracture group, and they also had lower bone mass at pubertal maturity [13]. Several studies have reported that women with secondary amenorrhea had lower bone mass when compared with control groups or women with normal menstruation [3, 6, 7]. Reproductive abnormalities such as primary or secondary amenorrhea are highly prevalent in athletes, dancers, and patients with anorexia nervosa [14, 15]. It is estimated that between 3% and 66% of the female athletic population exhibit menstrual irregularities [1, 2].
Although amenorrhea is associated with compromised bone mass [2, 11, 16], a suppression of estrogen should be beneficial to the cortical bone structure and strength. Increased estrogen levels in females at puberty inhibit periosteal modeling resulting in smaller bones compared with males. As a result, a delay in puberty should result in an increased periosteal diameter, potentially producing stronger bones because the resistance to bending or torsional forces is exponentially related to bone diameter. Suppression of estradiol by ovariectomy in older rats resulted in increased periosteal bone formation rates in females and suppressed rates in males [17, 18]. Furthermore, increased femoral periosteal circumference and moments of inertia have been reported after ovariectomy [19, 20]. As a result, mechanical strength after ovariectomy may not decrease and has actually been reported to increase in some studies [19–21]. These data support the hypothesis that suppressed estradiol should result in stronger bone; however, an animal model of delayed puberty (suppressed estrogen levels) has reported short-term decreases in peak moment and stiffness without changes in total area or bone area [22].
Therefore, the purpose of this analysis was to determine whether hypothalamic suppression affects bone strength to a greater extent before vs. after the onset of puberty (primary vs. secondary amenorrhea). These two time points both occur during increased growth rates in rats [23, 24], but one time point is before the onset of puberty and the other is after puberty. It was hypothesized that suppressed hypothalamic activity via a gonadotropin-releasing hormone antagonist (GnRH-a) before the onset of puberty would result in a relatively larger bone strength deficit compared with suppression after puberty.
Materials and Methods
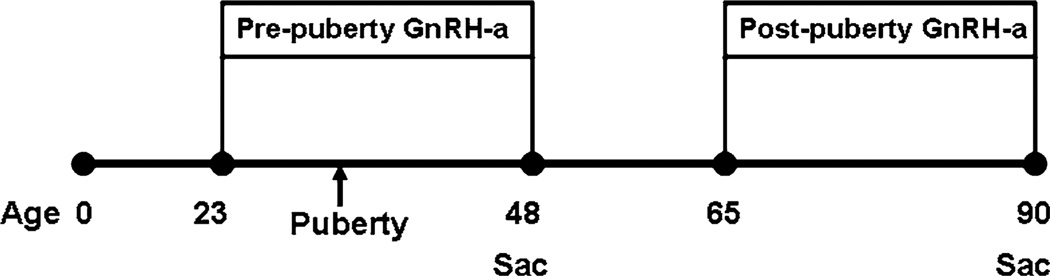
An analysis was conducted to determine the effect of hypothalamic suppression on mechanical parameters, bone structure parameters, estradiol, and body weight at two ages: before and after puberty (Fig. 1). Hypothalamic suppression was achieved by providing GnRH-a injections [25, 26].
Fig. 1.
Pre-experimental group was injected with GnRH-a beginning on day 23 (before the onset of puberty) until day 48, when they were killed. The post experimental group was injected from days 65–90, when they were killed
Injections (0.2 mL) of either saline or GnRH-a (Zentaris GmbH) were provided intraperitoneally. The postpuberty group consisted of 24 female Sprague Dawley rats randomly assigned to an age-matched control group (n = 15) and to an experimental group (post group) (n = 9). The post group received injections for 25 days, from the age of 65 days to the age of 90 days, at a dose of 2.5 mg/kg. This represents a time point after puberty but during a period of increased growth rate.
The outcome measures of the postpubertal animals were compared with those of a group of animals with prepubertal hypothalamic suppression (reported elsewhere [27]). Thirty animals (23 days of age) were randomly assigned to a control group (n = 15) and an experimental group (n = 15) (pre group) that received injections of GnRH-a. The experimental group was injected with a dose of 1.25 mg/kg every day for a 25-day period. Animals were killed at 48 days.
All animals were housed three per cage with a 12 h light–dark cycle. They received standard rat chow and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Brooklyn College, City University of New York.
When the animals were killed (at 48 days of age for the pre group and 90 days of age for the post group), animals were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (16 mg/kg). Blood was taken via cardiac puncture, after which the animals were killed by an overdose of pentobarbital. After death, body weight was measured, and uterine tissue was collected and weighed. The femurs were removed and cleaned of soft tissue. The right femurs were tested for mechanical strength, the left femurs were used for histomorphometry analysis, and tibiae were ashed for mineral content.
Blood Chemistry
Serum estradiol was measured with a radioimmunoassay (3rd Generation Estradiol RIA, DSL-39100, Diagnostic Systems Laboratories, Inc., Webster, TX). Interassay coefficient of variation was less than 6% and sensitivity was 0.6 pg/mL.
Bone Histomorphometry
Left femurs were fixed in 10% buffered formalin for 24–48 h and thereafter kept in 70% ethanol until processing. The bones were bulk stained with a Villanueva mineralized bone stain (Arizona Histology and Histomorphometry Services, Phoenix, AZ) for 7 days. After staining, the femurs were dehydrated with ethylene glycol monoethyl (Fisher, Fair Lawn, NJ), cleared in methyl salicylate (J. T. Baker, Phillipsburg, NJ), and embedded in methyl methacrylate with 15% dibutyl phthalate (Fisher Scientific). Undecalcified cross sections (200 µm thick) were cut at the middiaphysis with an Isomet 1000 precision saw with a diamond wafering blade (Buehler, Lake Bluff, IL), polished to a final thickness of 50–100 µm, and coverslipped for analysis.
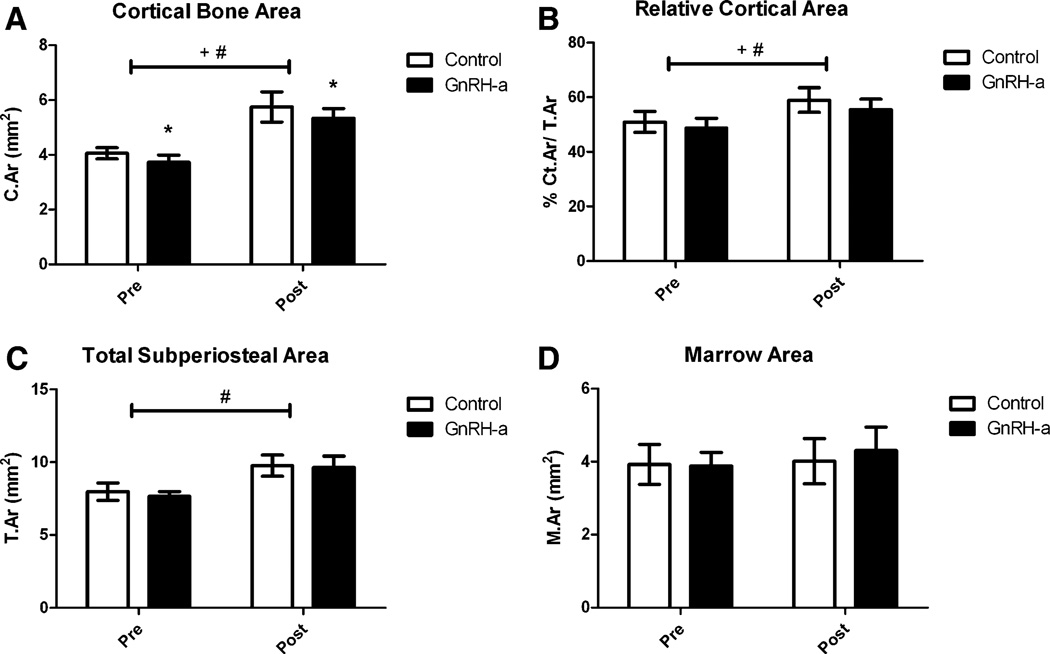
Cortical bone changes were assessed with bright-field microscopy. Histomorphometry was performed by a bio-quantification system (Osteometrics, Atlanta, GA, and Bioquant, Nashville, TN) following the standard measures described by Parfitt et al. [28]. Static histomorphometric indices included total subperiosteal area (T.Ar; mm2), cortical area [Ct.Ar = (T.Ar– Ma.Ar); mm2] and marrow area (Ma.Ar; mm2). The relative cortical area was calculated as a percentage of Ct.Ar per T.Ar (% Ct.Ar/T.Ar).
Cortical Bone Mechanical Properties
Breaking strength of each femur was measured under three-point bending using a materials testing machine (Instron, Canton, MA) fitted with a 1000-N load cell. Femurs were placed on the loading fixture anterior-side down and loaded in the anteroposterior plane. Because of the difference in femoral length between the pre and post animals, and in order to minimize the effect of shear loading, the distance between the lower support points was maximized for each group: 16 mm for the pre animals and 19 mm for the post animals. Before testing, the right femurs were thawed in saline at room temperature to ensure hydration. The femurs were loaded to failure at a rate of 0.05 mm/s, during which displacement and force were collected (100 Hz). The force and displacement values were normalized by using terms derived from engineering analysis of three-point bending [29]. Bending moments were calculated from the force (F) data (M = FL/4) (Nmm). Displacement data were divided by (L2/12) (mm/mm2), where L is the distance between the lower supports. Whole bone mechanical properties were then determined from the moment vs. normalized displacement curves including; peak moment (Nmm) (ultimate load the specimen sustained), stiffness (Nmm) (the slope of the initial linear portion of the moment–displacement curve), postyield displacement (mm/mm2) (displacement at failure minus the displacement at the yield point), and work to failure (Nmm-mm/mm2) (the area under the moment-displace-ment curve before failure).
Bone Composition
The right tibiae were removed and the marrow flushed with phosphate-buffered saline. Dry weight of the tibia was determined after drying in an oven at 100°C for 12 h. Ash weight was determined after ashing the bone in a muffle furnace (Fisher Scientific) at 800°C for 24 h. Ash fraction was calculated as ash weight/dry weight [30]. Only the diaphyses were ashed for the pre groups.
Data Analysis
Before statistical evaluation, the mechanical outcome measures that were found to scale with body weight were normalized with a linear regression-based correction [31]. All variables with an R2 level greater than 0 were normalized to avoid choosing an arbitrary R2 value as a cutoff for normalization. The correction decreased the variability in the data. Results are presented as mean (standard deviation) values. A two-way analysis of variance and a Bonferroni post hoc test assessed differences between the control and experimental groups for the pre and post groups at a significance level of P < 0.05 (GraphPadPrism v5.01, GraphPad Software, Inc.). To compare the relative deficits in bone strength and structure between the pre and post groups, the difference between the average control value and the GnRH-a groups were calculated and expressed as a percentage difference. An unpaired t-test assessed differences between the pre and post groups at a significance level of P < 0.05 (GraphPadPrism v5.01, GraphPad Software, Inc.). Differences in any outcome measure greater than twice the standard error of the mean were considered to be a trend.
Results
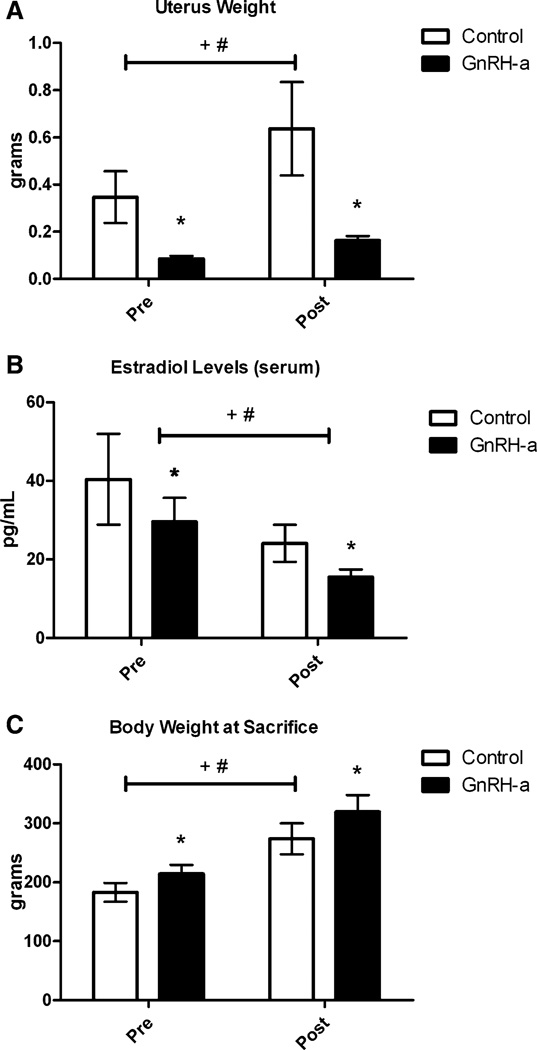
Suppression of the hypothalamic–pituitary–gonadal axis was confirmed by a significant atrophy of uterine tissue. Uterine weight decreased by 62.5–75.5% in both the pre and post groups (Fig. 2a). Fifty percent of the pre group had complete suppression of puberty during the 25-day protocol [27]. Estradiol levels were suppressed by 27% in the pre group and 36% in the post group (Fig. 2b). Body weight increased significantly (17–18%) in both the pre and post groups (Fig. 2c).
Fig. 2.
Control and GnRH-a-treated groups for both the pre and post groups for uterus weight (a), serum estradiol levels (b), and body weight at the time they were killed (c). *P< 0.05 compared with control, +P< 0.05 significant effect of experimental protocol (control vs. GnRH-a), #P < 0.05 significant effect of age (pre vs. post)
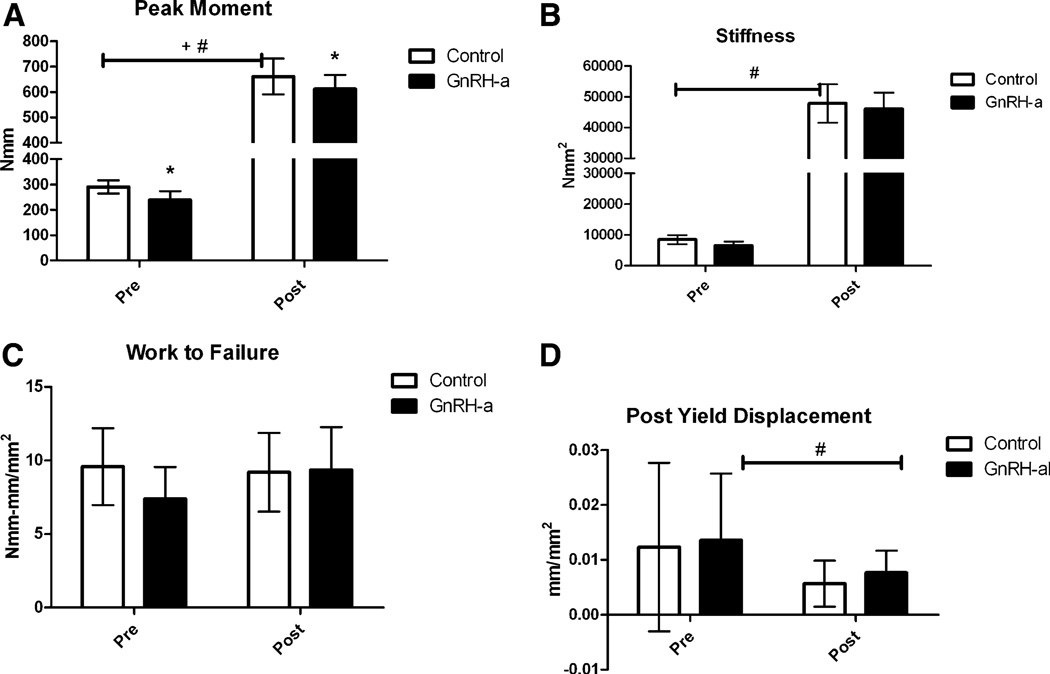
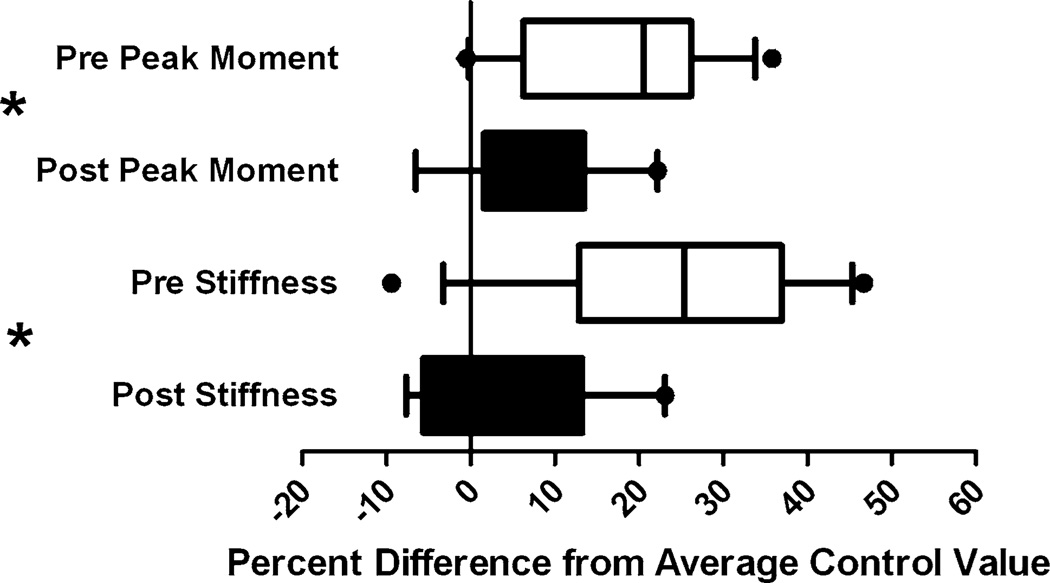
GnRH-a injections before and after onset of puberty result in lower mechanical strength after 25 days of injection (Fig. 3). The peak moment was significantly lower in the GnRH-a-treated groups compared with control. The differences were 17.6% lower in the pre group and 7.5% lower in the post group. There was also a significant increase in peak moment from the pre to post groups as a result of the difference in age between the groups (48 days vs. 90 days) (Fig. 3a). The stiffness values were significantly lower in the GnRH-a groups compared with control, with a significant increase between the pre and post groups. But the 23.3% decrease in stiffness in the pre group and the 3.6% decrease in the post group were not significantly different in the post hoc analysis (Fig. 3b), although there was a trend toward a lower stiffness in the pre GnRH-a-treated group. The work to failure or the toughness of the bone was not different between experimental conditions or between the pre and post groups (Fig. 3c). The postyield displacement was significantly lower in the post groups compared with the pre groups (Fig. 3d), indicating a more brittle bone in the older group. The trend toward an increase in postyield displacement in the GnRH-a post group combined with the decrease in peak moment maintain a similar work to failure compared with the postpubertal control group (Fig. 3). The percentage difference between peak moment and stiffness values of the GnRH-a-treated groups and the average value of the age-matched control group was larger in the pre group (Fig. 4). The percentage difference in the peak moment in the post group (7.5%) was significantly lower that the pre group (17.6%). The percentage difference between the stiffness values was also significantly lower in the post group—a difference of 3.6% compared with 23.3% in the post and pre groups, respectively (Fig. 4).
Fig. 3.
Control and GnRH-a-treated groups for both the pre and post groups for a peak moment, b stiffness, c work to failure, and d postyield deformation. *P < 0.05 compared with control, +P < 0.05 significant effect of experimental protocol (control vs. GnRH-a), #P<0.05 significant effect of age (pre vs. post)
Fig. 4.
Percentage difference between the GnRH-a groups both before and after puberty, and the average control value of peak moment and stiffness. Sold bars, post group; open bars, pre group. *P< 0.05 pre compared with post group
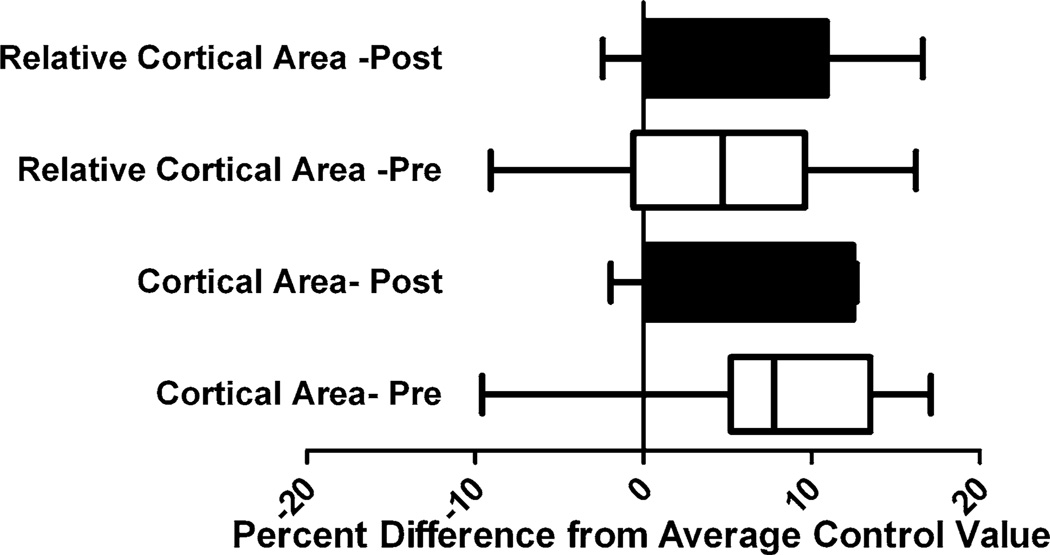
GnRH-a injections before and after the onset of puberty resulted in a lower cortical bone area (Ct.Ar) after 25 days of injection (Fig. 5). The cortical bone area was significantly lower in the GnRH-a groups compared with control. The areas were 11% lower in the pre group and 7% lower in the post group. There was a significant increase in cortical bone area from the pre to the post groups as a result of the difference in age between the groups (48 days vs. 90 days) (Fig. 5a). The relative cortical area (% Ct.Ar/T.Ar) values were significantly lower in the GnRH-a groups compared with control, with a significant increase between the pre and post groups. Post hoc analysis did not yield significant changes in relative cortical area after GnRH-a injections compared with control in both the pre and post groups (Fig. 5b). However, the data were trending toward lower relative cortical area in both groups. The total subperiosteal area was significantly lower in the pre groups compared with the post groups (Fig. 5c), but there was no difference between the GnRH-a and control groups in either the pre or post groups. The marrow area was not different between experimental conditions or between the pre and post groups (Fig. 5d). The decreased cortical bone area and relative cortical area (an indicator of cortical width) were not decreased as a result of significant changes in either the total area or marrow area, but possibly as a result of subtle shifts in bone surfaces. A comparison of the percentage difference of the relative cortical area and cortical area of the GnRH-a groups and the average value from the respective age-matched control groups resulted in no significant difference between the pre and post groups (Fig. 6); indicating that the structural changes were not more dramatic, depending on the age of hypothalamic suppression. There were no significant differences between ash fraction in the GnRH-a and control groups either before or after puberty. Ash fraction was 69.7% and 70.0% for the control and GnRH-a groups, respectively, in the pre groups and 60.7% and 60.7% in the post groups.
Fig. 5.
Control and GnRH-a-treated groups for pre and post groups for a cortical bone area, b relative cortical area, c total subperiosteal area, and d marrow area. *P<0.05 compared with control, +P < 0.05 significant effect of experimental protocol (control vs. GnRH-a), #P<0.05 significant effect of age (pre vs. post)
Fig. 6.
Percentage difference between the GnRH-a-treated pre and post groups, and the average control value of relative cortical area and cortical area. Solid bars, post group; open bars, pre group. *P < 0.05 pre compared with post group
Discussion
GnRH-a injections administered to rats before and after puberty suppressed the hypothalamic–pituitary–gonadal axis, as evidenced by a significant atrophy of uterine tissue and suppressed serum estradiol levels (Fig. 2). The decrease in estradiol levels after hypothalamic suppression may have resulted from decreased pulsatile secretion of gonadotropin-releasing hormone from the hypothalamus, which then resulted in decreased luteinizing hormone and follicle-stimulating hormone secretion from the anterior pituitary [26]. The pre group’s percentage differences between GnRH-a peak moment and stiffness values compared with the average value of the control group were larger compared with the post group. The current data suggest that both a decreased exposure to estrogen and the timing of that decreased exposure may affect bone strength. The animals had the same relative decrease in estradiol levels (Fig. 2b) for the same duration (25 days); only the timing of the suppression differed (before vs. after the onset of puberty). Less cortical area was measured in the GnRH-a groups in both the pre and post groups; however, a similar relative percentage difference between control and GnRH-a-treated groups was found in the structural parameters in the pre and post groups. Similar bone mineralization was found between the pre and post groups.
The association of primary amenorrhea (delayed puberty) and decreased areal BMD and thus a higher risk of hip, forearm, and vertebral fractures is hypothesized to result from the decreased exposure to estrogen between puberty and the time of peak bone mass [32]. Decreased estrogen may lower endocortical deposition, which decreases the cortical thickness of bone and therefore bone strength. However, an adaptation of increased periosteal expansion after endocortical resorption has been reported specifically during aging and at menopause [33–35]. This increase in periosteal bone has a greater positive effect on bone strength compared with endocortical apposition. Both pre and post groups had decreased cortical area and relative cortical area after hypothalamic suppression with GnRH-a injections. Yet there was no shift in bone structure with the decreased bone area toward the periosteal surface, as would be indicated by an increase in total area. Furthermore, the strength values were significantly lower in the GnRH-a-treated groups compared with control and to a greater degree in the pre groups, yet changes in bone area were similar between the pre and post groups. Thus, it does not completely explain the larger strength deficit in prepubertal GnRH-a-treated animals.
In the current data, the similar structural deficits between pre and post groups but larger strength deficits could result from composition or microarchitectural changes. The percentage of ash fraction, an indicator of bone mineralization, was not different between groups, suggesting that mineral differences do not explain the strength deficit. Furthermore, the decrease in cortical bone area in both groups was not due to age-related effects of GnRH-a on total area or marrow area. Both factors were not significantly affected by the primary or secondary suppression of estradiol. Interestingly, estrogen is hypothesized to be a negative regulator of body size, bone mass, and bone size. Increased bone formation specifically on the periosteal surface has been reported in both young and old rats after ovariectomy and GnRH-a injections [17–19, 27, 36, 37]. The lack of a significant change in total area and marrow area is not indicative of a lack of periosteal accrual. Modeling is the mechanism of large bone size and shape changes during growth in rats and humans [38, 39]. This growth is also accompanied by a drift, or a posterolateral shift of the bone. The relative rates of resorption and formation on the periosteal and endocortical surfaces would affect the cortical width, total area, and moment of inertia, and thus bone strength [40]. An increase in periosteal bone formation rate has resulted in increased bone strength due to an increased moment of inertia [19–21, 41]. However, in prepubescent animals that receive GnRH-a injections, the increased periosteal formation did not rescue bone strength [27]. This difference may be due in part to the type of bone formed on the periosteal surface (lamellar or woven). Therefore, young animals, although accruing bone on the periosteal surface, may accrue woven bone, which is less mechanically competent than lamellar bone, resulting in decreased strength. Younger animals (before puberty) may be more vulnerable to this accrual of woven bone.
Body weight has been considered to be positively correlated to bone density and has been considered to have a protective effect on bone mass and thus bone strength. Hypothalamic suppression through GnRH-a injections resulted in a significantly lower bone strength both before and after the onset of puberty, but also resulted in a significant increase in body weight (Figs. 2, 3). Therefore, bone mass and strength are not wholly dependent on body weight, and other factors affect bone strength development. Therefore, clinically, it should not be assumed that heavier women have stronger bones.
These data support the hypothesis that the age at which estradiol is suppressed affects bone strength and therefore may affect long-term bone health. Women experience both primary and secondary amenorrhea, which may result from hypothalamic suppression; however, the treatment may need to be different for each condition. The mechanism of lower estradiol levels at different ages remains elusive, as does the extent to which bone strength may catch up after estradiol suppression. Animal models of delayed puberty have suggested the existence of catch-up growth [42] or a recovery of bone strength. One study reported a transient decrease in bone strength (day 50) but a full recovery of bone strength at 6 months of age after a significant delay of the onset of puberty when provided with GnRH-a injections. However, the mechanism of this recovery remains elusive [43], and data from human studies suggest a sustained bone deficit after amenorrhea. Warren et al. [44] treated amenorrheic dancers for 2 years with hormone replacement therapy and found no difference in BMD between treated and placebo groups. To sum up, animal studies suggest that one negative perturbation during bone development may not have a long-term effect on bone strength, but clinically, women with a history of amenorrhea have experienced bone loss [11, 44]. Other factors, such as severity of exposure and duration of amenorrhea, must be investigated.
Young, growing rats are useful models for studying factors that modulate bone strength development, specifically hypothalamic suppression resulting in lower estradiol levels. Animal models that use ovariectomy represent the most extreme condition of delayed reproductive development with the complete cessation of estrogen. However, GnRH-a injections have successfully delayed the onset of puberty in female rats as determined by delayed vaginal opening, lower ovarian and uterine weights, and lower serum estradiol levels [22, 25, 45, 46]. Furthermore, the withdrawal of GnRH-a injections restores normal hypothalamic–pituitary function, allowing control of estrogen levels for a finite (transient) period of time. This model offers an opportunity to reproduce the environment of delayed pubertal development to investigate adaptation mechanisms, both positive and negative, on bone strength.
Limitations do exist in this analysis. First, there is an increase in body weight from the GnRH-a injection protocol. This does not mimic some clinical manifestations of delayed puberty or secondary amenorrhea, in which case body weights are lower than normally menstruating women. There may be an interaction of hypothalamic suppression and energy restriction. Second, this particular analysis is a cross-sectional approach that uses two groups, one before and one after onset of puberty. However, the advantage of using rats is that their development and environmental exposure is similar, and we can directly measure bone strength through a three-point bending test. Clinically, BMD measurements provide a surrogate for bone strength measures. However, studies on animals suggest that changes in BMD underestimate effects on bone strength. Although Fyhrie et al. [47] reported a correlation between trabecular bone volume and bone strength and stiffness, bone volume accounted for 65–80% of the variance in bone strength. Bone mass is not the only factor contributing to bone strength; bone architecture and microarchitecture are also bone strength determinants [48].
In conclusion, environmental modifications may greatly affect peak bone mass. Many young women experience primary and secondary hypothalamic amenorrhea and may have long-term bone strength deficits as a result. The timing of the suppressed estradiol to puberty (before vs. after) may affect the relative strength deficit. There is a greater deficit with hypothalamic suppression before the onset of puberty, suggesting that this time point may have more severe consequences. The long-term effect of the timing of amenorrhea and the effect of repeated bouts of amenorrhea on bone strength need to be investigated prospectively.
Contributor Information
Vanessa Yingling, Email: yingling@temple.edu, Department of Anatomy and Cell Biology, Temple University, 1800 N Broad Street, Philadelphia, PA 19122, USA; Department of Kinesiology, Temple University, Philadelphia, PA, USA.
McKayla Elle Saine, Department of Anthropology, Temple University, Philadelphia, PA, USA.
Rupali Joshi, Department of Kinesiology, Temple University, Philadelphia, PA, USA.
References
- 1.Redman LM, Loucks AB. Menstrual disorders in athletes. Sports Med. 2005;35:747–755. doi: 10.2165/00007256-200535090-00002. [DOI] [PubMed] [Google Scholar]
- 2.Warren MP, Stiehl AL. Exercise and female adolescents: effects on the reproductive and skeletal systems. J Am Med Womens Assoc. 1999;54:115–120. 138. [PubMed] [Google Scholar]
- 3.Drinkwater BL, Nilson K, Chesnut CHIII, Bremner WJ, Shainholtz S, Southworth MB. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984;311:277–281. doi: 10.1056/NEJM198408023110501. [DOI] [PubMed] [Google Scholar]
- 4.Rencken ML, Chesnut CHIII, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276:238–240. [PubMed] [Google Scholar]
- 5.Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. The female athlete triad. Med Sci Sports Exerc. 2007;39:1867–1882. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 6.Pettersson U, Stalnacke B, Ahlenius G, Henriksson-Larsen K, Lorentzon R. Low bone mass density at multiple skeletal sites, including the appendicular skeleton in amenorrheic runners. Calcif Tissue Int. 1999;64:117–125. doi: 10.1007/s002239900589. [DOI] [PubMed] [Google Scholar]
- 7.Warren MP, Brooks-Gunn J, Fox RP, Lancelot C, Newman D, Hamilton WG. Lack of bone accretion and amenorrhea: evidence for a relative osteopenia in weight-bearing bones. J Clin Endocrinol Metab. 1991;72:847–853. doi: 10.1210/jcem-72-4-847. [DOI] [PubMed] [Google Scholar]
- 8.Golden NH. Osteoporosis prevention: a pediatric challenge. Arch Pediatr Adolesc Med. 2000;154:542–543. doi: 10.1001/archpedi.154.6.542. [DOI] [PubMed] [Google Scholar]
- 9.Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med. 1997;18(suppl 3):S191–S194. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 10.Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15:2245–2250. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 11.Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162–3168. doi: 10.1210/jcem.87.7.8637. [DOI] [PubMed] [Google Scholar]
- 12.Carbon R, Sambrook PN, Deakin V, Fricker P, Eisman JA, Kelly P, Maguire K, Yeates MG. Bone density of elite female athletes with stress fractures. Med J Aust. 1990;153:373–376. doi: 10.5694/j.1326-5377.1990.tb125491.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res. 2006;21:501–507. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- 14.Rauch F, Klein K, Allolio B, Schonau E. Age at menarche and cortical bone geometry in premenopausal women. Bone. 1999;25:69–73. doi: 10.1016/s8756-3282(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 15.Kalu DN, Hardin RH, Cockerham R, Yu BP. Aging and dietary modulation of rat skeleton and parathyroid hormone. Endocrinology. 1984;115:1239–1247. doi: 10.1210/endo-115-4-1239. [DOI] [PubMed] [Google Scholar]
- 16.Myerson M, Gutin B, Warren MP, Wang J, Lichtman S, Pierson RN., Jr Total body bone density in amenorrheic runners. Obstet Gynecol. 1992;79:973–978. [PubMed] [Google Scholar]
- 17.Turner RT, Wakley GK, Hannon KS. Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J Orthop Res. 1990;8:612–617. doi: 10.1002/jor.1100080418. [DOI] [PubMed] [Google Scholar]
- 18.Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987;2:115–122. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Zhao J, Genant HK, Dequeker J, Geusens P. Long-term changes in bone mineral and biomechanical properties of vertebrae and femur in aging, dietary calcium restricted, and/or estrogen-deprived/-replaced rats. J Bone Miner Res. 1997;12:820–831. doi: 10.1359/jbmr.1997.12.5.820. [DOI] [PubMed] [Google Scholar]
- 20.Peng Z, Tuukkanen J, Vaananen HK. Exercise can provide protection against bone loss and prevent the decrease in mechanical strength of femoral neck in ovariectomized rats. J Bone Miner Res. 1994;9:1559–1564. doi: 10.1002/jbmr.5650091008. [DOI] [PubMed] [Google Scholar]
- 21.Katsumata T, Nakamura T, Ohnishi H, Sakurama T. Intermittent cyclical etidronate treatment maintains the mass, structure and the mechanical property of bone in ovariectomized rats. J Bone Miner Res. 1995;10:921–931. doi: 10.1002/jbmr.5650100613. [DOI] [PubMed] [Google Scholar]
- 22.Yingling VR, Khaneja A. Short-term delay of puberty causes a transient reduction in bone strength in growing female rats. Bone. 2006;38:67–73. doi: 10.1016/j.bone.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner RT, Maran A, Lotinun S, Hefferan T, Evans GL, Zhang M, Sibonga JD. Animal models for osteoporosis. Rev Endocr Metab Disord. 2001;2:117–127. doi: 10.1023/a:1010067326811. [DOI] [PubMed] [Google Scholar]
- 24.Li XQ, Klein L. Age-related inequality between rates of formation and resorption in various whole bones of rats. Proc Soc Exp Biol Med. 1990;195:350–355. doi: 10.3181/00379727-195-43154. [DOI] [PubMed] [Google Scholar]
- 25.Roth CL, Neu C, Jarry H, Schoenau E. Different effects of agonistic vs. antagonistic gnrh-analogues (triptorelin vs. cetrorelix) on bone modeling and remodeling in peripubertal female rats. Exp Clin Endocrinol Diabetes. 2005;113:451–456. doi: 10.1055/s-2005-865710. [DOI] [PubMed] [Google Scholar]
- 26.Roth C, Leonhardt S, Seidel C, Luft H, Wuttke W, Jarry H. Comparative analysis of different puberty inhibiting mechanisms of two GnRH agonists and the GnRH antagonist cetrorelix using a female rat model. Pediatr Res. 2000;48:468–474. doi: 10.1203/00006450-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Yingling VR, Taylor G. Delayed pubertal development by hypothalamic suppression causes an increase in periosteal modeling but a reduction in bone strength in growing female rats. Bone. 2008;42:1137–11343. doi: 10.1016/j.bone.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 29.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 30.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14:2159–2166. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 31.Di Masso RJ, Font MT, Capozza RF, Detarsio G, Sosa F, Ferretti JL. Long-bone biomechanics in mice selected for body conformation. Bone. 1997;20:539–545. doi: 10.1016/s8756-3282(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 32.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Influence of age at menarche on forearm bone microstructure in healthy young women. J Clin Endocrinol Metab. 2008;3:2594–2601. doi: 10.1210/jc.2007-2644. [DOI] [PubMed] [Google Scholar]
- 33.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 34.Riggs BL, O’Fallon WM, Muhs J, O’Connor MK, Kumar R, Melton LJ3rd. Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res. 1998;13:168–174. doi: 10.1359/jbmr.1998.13.2.168. [DOI] [PubMed] [Google Scholar]
- 35.Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21:1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- 36.Kim BT, Mosekilde L, Duan Y, Zhang XZ, Tornvig L, Thomsen JS, Seeman E. The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J Bone Miner Res. 2003;18:150–155. doi: 10.1359/jbmr.2003.18.1.150. [DOI] [PubMed] [Google Scholar]
- 37.Bagi CM, Ammann P, Rizzoli R, Miller SC. Effect of estrogen deficiency on cancellous and cortical bone structure and strength of the femoral neck in rats. Calcif Tissue Int. 1997;61:336–344. doi: 10.1007/s002239900344. [DOI] [PubMed] [Google Scholar]
- 38.Rauch F, Travers R, Glorieux FH. Cellular activity on the seven surfaces of iliac bone: a histomorphometric study in children and adolescents. J Bone Miner Res. 2006;21:513–519. doi: 10.1359/jbmr.060108. [DOI] [PubMed] [Google Scholar]
- 39.Rauch F, Travers R, Glorieux FH. Intracortical remodeling during human bone development—a histomorphometric study. Bone. 2007;40:274–280. doi: 10.1016/j.bone.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22:251–259. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 41.Bagi C, van der Meulen M, Brommage R, Rosen D, Sommer A. The effect of systemically administered rhIGF-I/IGFBP-3 complex on cortical bone strength and structure in ovariectomized rats. Bone. 1995;16:559–565. doi: 10.1016/8756-3282(95)00078-r. [DOI] [PubMed] [Google Scholar]
- 42.Gafni RI, McCarthy EF, Hatcher T, Meyers JL, Inoue N, Reddy C, Weise M, Barnes KM, Abad V, Baron J. Recovery from osteoporosis through skeletal growth: early bone mass acquisition has little effect on adult bone density. FASEB J. 2002;16:736–738. doi: 10.1096/fj.01-0640fje. [DOI] [PubMed] [Google Scholar]
- 43.Gafni RI, Baron J. Catch-up growth: possible mechanisms. Pediatr Nephrol. 2000;14:616–619. doi: 10.1007/s004670000338. [DOI] [PubMed] [Google Scholar]
- 44.Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG, Hamilton L. Persistent osteopenia in ballet dancers with amenorrhea and delayed menarche despite hormone therapy: a longitudinal study. Fertil Steril. 2003;80:398–404. doi: 10.1016/s0015-0282(03)00660-5. [DOI] [PubMed] [Google Scholar]
- 45.Yingling VR, Xiang Y, Raphan T, Schaffler MB, Koser K, Malique R. The effect of a short-term delay of puberty on trabecular bone mass and structure in female rats: a texture-based and histomorphometric analysis. Bone. 2007;40:419–424. doi: 10.1016/j.bone.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth C, Leonhardt S, Seidel C, Lakomek M, Wuttke W, Jarry H. GnRH antagonist cetrorelix prevents sexual maturation of peripubertal male rats. Exp Clin Endocrinol Diabetes. 2000;108:358–363. doi: 10.1055/s-2000-8129. [DOI] [PubMed] [Google Scholar]
- 47.Fyhrie DP, Fazzalari NL, Goulet R, Goldstein SA. Direct calculation of the surface-to-volume ratio for human cancellous bone. J Biomech. 1993;26:955–967. doi: 10.1016/0021-9290(93)90057-l. [DOI] [PubMed] [Google Scholar]
- 48.Schaffler MB, Reimann DA, Parfitt AM, Fyhrie DP. Which stereological methods offer the greatest help in quantifying trabecular structure from biological and mechanical perspectives? Forma. 1997;12:197–207. [Google Scholar]