Abstract
Anhedonia is a core feature of major depressive disorder (MDD), but the precise nature of anhedonic symptoms is unknown. While anhedonia has traditionally been viewed as a deficit in the experience of pleasure, more recent evidence suggests that reduced anticipation and motivation may also be a core feature of this symptom. Here, we provide data from a study in MDD patients and healthy controls using a translational measure of reward motivation, the Effort Expenditure for Rewards Task (EEfRT or “effort”). This task offers subjects a series of trials where they may choose to expend more or less effort for the opportunity to win varying amounts of monetary rewards. We found that MDD patients were less willing to expend effort for rewards than controls. Additionally, we observed that patients were less able to effectively use reward information about magnitude and probability of rewards to guide their choice behavior. Finally, within the MDD patient group, duration of the current episode was a significant negative predictor of EEfRT task performance. These findings offer novel support for theoretical models proposing that anhedonia in MDD may reflect specific impairments in motivation and reward-based decision-making.
Anhedonia, a symptom characterized by reduced motivation and reported enjoyment of positive life experiences, is a core symptom of major depressive disorder (MDD). While early theoretical formulations defined anhedonia as primarily—if not exclusively—an inability to experience pleasure (Meehl, 2001; Ribot, 1896), more recent work highlights the importance of considering other aspects of reward processing in anhedonic symptomatology (Treadway & Zald, 2011). This broadening of focus is partly due to past findings that affective ratings of positively-valenced stimuli —a common laboratory probe of hedonic responsiveness in depression—do not necessarily differ in MDD patients as compared to controls; indeed, a recent meta-analysis of affective responses in depressed patients and controls found that depression was associated with blunted reactivity to both positively and negatively valenced stimuli, raising the possibility that affective responses to these stimuli may reflect an affective flattening, rather than a true deficit in reward experience (Bylsma, Morris, & Rottenberg, 2008). Indeed, studies of affective responses to basic rewards, such as sucrose samples in the sweet-taste test, have failed to identify differences between patients and controls (Amsterdam, Settle, Doty, Abelman, & Winokur, 1987; Berlin, 1998; Dichter, Smoski, Kampov-Polevoy, Gallop, & Garbutt; Kazes, et al., 1994).
Previously, we have suggested that anergic and anhedonic behavioral patterns commonly observed in the course of a major depressive episode (MDE) do not necessarily reflect the incapacity to enjoy rewards, nor the inability to mobilize effort to obtain them. Rather, we proposed that these symptoms may result from a core deficit in cost/benefit decision-making, such that individuals fail to engage in rewarding behaviors because they either over-estimate the costs of obtaining rewards, under-estimate the anticipated benefits, or simply fail to integrate cost/benefit information in an optimal manner (Treadway & Zald, 2011). Consistent with this idea, several studies suggest that MDD symptoms are associated with reduced reward anticipation (Chentsova-Dutton & Hanley, 2010; McFarland & Klein, 2009; Sherdell, Waugh, & Gotlib, 2011), with diminishment in anticipation predicting reduced motivation for rewards (Sherdell et al., 2011).
Supporting the idea that reward-related deficits in depression may involve multiple sub-components, animal models of reward processing strongly suggest the presence of critical neurochemical and systems-level distinctions between motivation and reward (Berridge & Robinson, 2003). Substantial evidence suggests that dopamine (DA) signaling is neither necessary nor sufficient for the experience of pleasure, but is crucial for reward motivation (Salamone, Correa, Farrar, & Mingote, 2007). This has been empirically demonstrated by studies of effort-based decision-making, in which an animal must choose between a freely available, but smaller or less palatable food reward (Low-Cost/Low-Reward [LC/LR]), as compared to a larger or more preferred food reward that the animal must expend effort to receive (High-Cost/High-Reward [HC/HR]) (Salamone et al., 2007). Interference with DA function through lesions or receptor blockade produces a shift towards more LC/LR choices (Denk et al., 2005; Salamone et al., 2007; Salamone, Cousins, McCullough, Carriero, & Berkowitz, 1994), while pharmacological enhancement of DA signaling can increase HC/HR choices (Bardgett, Depenbrock, Downs, Points, & Green, 2009). Similarly in humans, transient attenuation and potentiation of DA can respectively decrease and increase willingness to work for rewards (Venugopalan et al., 2011; Wardle, Treadway, Mayo, Zald, & de Wit, 2011). In contrast to the effects of DA blockade on motivation, DA lesions and antagonists do not reduce overall consumption of freely available food reward (Salamone et al., 2007).
Given that DA impairment does not appear to impact hedonic capacity, it might be predicted that possible DA-mediated impairments in reward motivation would not be detected by experimental paradigms that emphasize hedonic responsiveness. Consequently, determining the role of DA and related motivational circuitry in MDD requires experimental paradigms that explicitly evaluate motivation to obtain rewards, rather than subjective responses to reward receipt. To date however, such paradigms are lacking. In the present study, we explored performance on a laboratory-based measure of effort-based decision-making in a sample of 20 currently depressed patients and 15 healthy control subjects. All subjects performed the Effort-Expenditure for Rewards Task (EEfRT or “effort”), a behavioral measure of motivation for rewards in humans (Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009) that was adapted from effort-based decision-making paradigms used in preclinical studies (Salamone, et al., 2007). As with animal studies, the EEfRT requires subjects to make a series of choices between HC/HR and LC/LR options. We hypothesized that MDD patients would make fewer HC/HR options as compared to controls. Additionally, we examined whether individuals with MDD differ in their use of reinforcement parameters (e.g., monetary value, probability of reward receipt) when making effort-based decisions.
Methods
All protocols were approved by Vanderbilt University Institutional Review Board. Written informed consent was obtained from all participants prior to any study procedure.
Participants
20 individuals diagnosed with MDD (14/20 female) and 15 healthy controls (9/15 female) participated. All participants were community volunteers who either responded to online recruitment advertisements or were referred from the Vanderbilt University Department of Psychiatry Mood Disorders Program outpatient clinic. Following initial screening, subjects were given an interview of their medical history and a structured psychiatric interview (SCID-P) (First, Spitzer, Gibbon, & Williams, 2005) and completed the Beck Depression Inventory II (BDI-II) (Beck, Steer, Ball, & Ranieri, 1996) and the Chapman Anhedonia Scales (Chapman, Chapman, & Raulin, 1976). For individuals in the MDD group, subjects were required to meet criteria for a current MDE. Subjects were excluded if they met criteria for bipolar disorder, psychotic and schizoaffective disorders, current substance abuse or dependence, past stimulant abuse, or past substance dependence. MDD subjects were also excluded for any current or past use of prescription drugs that act on DA (e.g., amphetamines, methylphenidate, l-dopa). Of the 20 participants in the MDD group, 17 subjects were on an antidepressant medication at the time of the study (15 SSRI alone, 2 SNRI alone). Additionally, 8 of the 20 subjects in the MDD group met criteria for a co-morbid anxiety disorder as assessed by the SCID.1
Subjects in the control group were excluded if they met criteria for any current or past Axis I disorder other than specific phobia, past adjustment disorder, or past substance abuse of non-stimulants. Control participants were also excluded if they exhibited significant trait-anhedonia despite not meeting clinical criteria for an Axis I disorder as determined by a score on the Chapman Anhedonia Scales that was two-standard deviations higher than published normative data for this instrument (Chapman et al., 1976). This exclusion was based on prior work showing that elevated trait anhedonia in a non-patient sample may reduce willingness to expend effort for rewards (Treadway et al., 2009), and resulted in the exclusion of one potential control subject. No control subjects were on any form of psychotropic medication at the time of the study.
Effort-Expenditure for Rewards Task (“EEfRT”)
The EEfRT is a multi-trial task where participants are given an opportunity on each trial to choose between two different task difficulty levels associated with varying levels of monetary reward (Figure 1). A detailed description of the task has been published previously (Treadway et al., 2009). Briefly, each trial presents the subject with a choice between, a ‘hard task’ (HC/HR) and an ‘easy task’ (LC/LR) option, which require different amounts of speeded manual button pressing. For easy-task choices, subjects were eligible to win the same amount, $1.00, on each trial if they successfully completed the task. For hard-task choices, subjects were eligible to win higher amounts that varied per trial within a range of $1.24 – $4.30 (“reward magnitude”). Subjects were not guaranteed to win the reward if they completed the task; some trials were “win” trials, in which the subject received the stated reward amount, while others were “no win” trials, in which the subject received no money for that trial. To help subjects determine which trials were more likely to be win trials, subjects were provided with accurate probability cues at the beginning of each trial. Trials had three levels of probability: “high” 88% probability of being a win trial, “medium” 50% and “low” 12%. Probability levels always applied to both the hard task and easy task, and there were equal proportions of each probability level across the experiment. Probability is manipulated in the EEfRT, because like mobilization of effort, probability discounting appears to be highly sensitive to DA function (Floresco, Tse, & Ghods-Sharifi, 2008; St Onge & Floresco, 2009). Additionally, the inclusion of a probability manipulation improves the overall ecological validity of the task, as most real-world choices that require motivation are usually associated with some level of uncertainty in the outcome.
Figure 1.
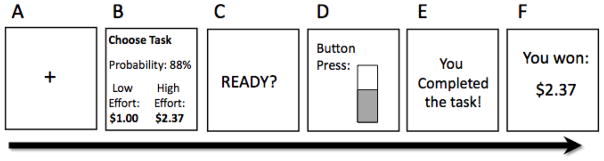
Schematic diagram of a single trial of the Effort Expenditure for Rewards Task (‘EEfRT’). A) Subjects begin by seeing a 1s fixation cue. B) 5s choice period in which subjects are presented with information regarding the reward magnitude of the hard task for that trial, and the probability of receiving any reward for that trial. C) 1s “ready” screen. D) Subjects make rapid button presses to complete the chosen task for 7s (easy task) or 21s (hard task). E) Subjects receive feedback on whether they have completed the task. F) Subjects receive reward feedback as to whether they received any money for that trial.
Statistical Analyses
Statistical analyses of choice behavior during the EEfRT was performed using Generalized Estimating Equation (GEE) models (Liang, Beaty, & Cohen, 1986; Zeger & Liang, 1986). The use of GEE is advantageous for the EEfRT, in that it can simultaneously model time-varying parameters (e.g., trial-wise changes in reward magnitude of the HC/HR option) as well as time-invariant parameters (e.g., estimates of stimulated DA release or MDD status). GEE models were implemented in SPSS 19 (IBM, Armok, NY) using an unstructured working correlation matrix. The dependent measure was the dichotomous outcome of HC/HR or LC/LR choice, and we used a binary logistic distribution to model the probability of choosing the HC/HR option. Consistent with our prior analytical approach using the EEfRT, all GEE models included reward magnitude of the HC/HR option, probability and expected value (reward magnitude x probability), which represents an ability to integrate probability and reward information simultaneously. Separate models were computed to test the effects of group on HC/HR choices, as well as interactions between group and reinforcement variables (reward magnitude, probability and expected value). All models included trial number as a nuisance covariate to control for possible effects of fatigue over the course of the task. For between-group analyses, models also included any demographic variables where groups showed significant differences. For within-group individual differences analyses (e.g., using the BDI-II scales), sex was included as a covariate, as sex has been shown previously to be a significant predictor of EEfRT task performance in individual differences analysis. This approach replicates prior analytical methods using the EEfRT (Treadway et al., 2009; Wardle et al., 2011).
Results
Sample Characteristics
Subject demographics and clinical variables are included in Table 1. The depressed and control groups did not differ in terms of sex (X2 = 0.38, p = 0.537), or age (t33 = −0.839, p = 0.41), but did differ in years of education (t33 = 3.00, p = 0.005), with the control subjects having approximately 2 more years of education on average. Subjects in the MDD group reported significantly higher depressive symptoms on the BDI-II (Mean = 24.6, SD = 9.25) than controls (Mean = 2.83, SD = 3.65) (t26.2 = −9.57, p < 0.001). MDD patients also reported significantly higher scores on the Chapman Anhedonia Scales (Mean = 37.05, SD = 15.86) as compared to controls (Mean = 11.87, SD = 7.50) (t28.6 = 6.23, p < 0.001).
Table 1.
Clinical and Demographic Characteristics
| Measure | MDD (n=20) | Controls (n=15) | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | |
|
|
|
|
|
||
| Sex | 14/20 female | 9/15 female | 0.64 | ||
| Age | 42.4 | 10.1 | 39.7 | 8.2 | 0.41 |
| Years of Education | 14.4 | 1.9 | 16.3 | 1.83 | 0.005 |
| BDI-II | 24.6 | 9.2 | 2.8 | 3.7 | < 0.001 |
| Chapman Anhedonia Scales | 37.1 | 15.9 | 11.9 | 7.5 | <0.001 |
| Average Duration of Current MDE (months) | 12.5 | 14.2 | - | - | - |
| Average Number of Prior MDEs | 2.3 | 1.3 | - | - | - |
| Average Age of MDE Onset | 39.5 | 10.6 | - | - | - |
EEfRT Trial Completion Rates
For both the MDD and control groups, all subjects chose a mix of HC/HR trials and LC/LR trials. There was no difference in the percentage of trials successfully completed by MDD patients (Mean = 99.4%, SD = 0.19%) or controls (Mean = 99.5%, SD = 0.15%) (t33 = 0.144, p = 0.89).
Results of GEE Models
We tested six independent models GEE models. Each model included all experimental task variables, including reward magnitude, reward probability, expected value, and trial number. Because of group differences in years of education, all between-group models included years of education as a covariate. For within-group, individual difference models, gender was added as a covariate.
Model 1 tested for main effects of Group on preference for HC/HR options, and found that compared to controls, MDD patients were significantly less likely to make HC/HR choices (b = −0.79, p < 0.001). The effect of group remained a significant predictor even when symptoms of psychomotor slowing—as assessed by SCID—were included as a covariate in the model (p < 0.001), indicating that the results were not explainable by depression-related differences in psychomotor speed.
In model 2, we tested for the presence of an interaction between Group and Reward Magnitude, and found a significant interaction (b = −0.379, p = 0.012). In follow-up within-group analyses, we found that while reward magnitude was a significant predictor of HC/HR choices for both groups, its effect was larger for controls (b = 0.694, p < 0.001) than for MDD patients (b = 0.437, p < 0.001). This suggests that the magnitude of the reward associated with the HC/HR option was more strongly predictive of choosing the HC/HR option in controls than in MDD patients.
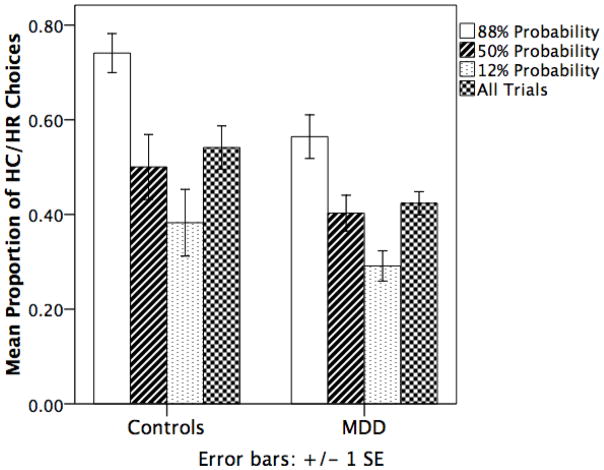
Model 3 tested for an interaction between Group and Probability level. We observed a significant interaction between MDD patients and controls (b = −0.23, p = 0.038) such that probability was a stronger predictor of choice behavior for controls (b = 0.484, p < 0.001) then for patients (b = 0.361, p < 0.001).
In model 4, we tested for an interaction between Group and expected value, but did not find evidence for an interaction (b = −0.17, p = 0.399). However, while this interaction term was not significant, the expected value predictor showed a similar pattern to both reward magnitude and probability, such that it was a stronger predictor for the control group (b = 1.44, p = 0.03) as compared to the MDD group (b = −0.61, p < 0.001).
In model 5, we performed an individual differences analysis within the MDD group to see if EEfRT performance was related to symptom severity (BDI-II) and course of illness. In an initial model, we found that duration of the current MDE predicted significantly fewer HC/HR choices (b = −0.014, p < 0.001), while BDI-II scores were predicted more HC/HR choices (b = 0.027, p < 0.001). These effects were both present when each of these predictors was included independently. We also note that sex was a significant predictor in this model, with men choosing more HC/HR choices than women.
Given the unexpected direction of the relationship between BDI-II scores and EEfRT choices, in model 6 we followed up with item-level analysis of BDI-II items related to reward anticipation (item 2) and reported enjoyment (item 4). We found that reduced anticipation was inversely associated with HC/HR choices (b = −0.15, p < 0.001), while the opposite was true for deficits in enjoyment (b = 0.51, p < 0.001), suggesting that specific MDD symptoms may be differentially associated with EEfRT performance (see Supplemental Information for additional figures).
Discussion
In the present study, we found evidence that patients with MDD show motivational and decisional anhedonia as indexed by an objective, translational cost/benefit decision-making task. Individuals with current MDD were less willing to expend effort for the opportunity to earn larger monetary rewards as compared to healthy controls. This supports a growing body of evidence suggesting that motivation may be an especially crucial aspect of altered reward processing in MDD (Clery-Melin et al., 2011; Sherdell et al., 2011). It may also help explain the success of behavioral activation treatments for MDD, which specifically target motivational symptoms (Dimidjian et al., 2006).
In addition to differences in willingness to expend effort for rewards, we found that patients showed less sensitivity to information about the reward magnitude and probability of a win when making their choices. Prior studies have found associations between depression and sensitivity to reward probability (Forbes, Shaw, & Dahl, 2007; Gradin et al., 2011; Pizzagalli, Iosifescu, D., Hallett, L. A., Ratner, K. G., & Fava, M., 2008). This reduced capacity for integrating information about reward probability when making effort-related choices may be related to previously reported cognitive vulnerabilities regarding the prediction and expectancy of positive future events in MDD (Abramson, Metalsky, & Alloy, 1989). Despite these interactions with both probability and reward-magnitude, there was no interaction between group and expected value. That said, while the interaction term was not significant, the expected value predictor did follow a similar pattern, such that it was a stronger predictor for controls as compared to patients.
Within the MDD group, we observed several associations between the EEfRT and clinical variables. First, duration of the current MDE predicted fewer HC/HR choices, even when controlling for current symptom severity. This may suggest that motivational deficits are associated with a poorer course of MDE. Although the causal direction of this relationship remains to be elucidated, it is interesting to note that cognitive vulnerability models of depression have posited that helplessness and hopelessness are causally associated more pronounced motivational deficits as well as a longer course of illness (Abramson et al., 1989; Abramson, Seligman, & Teasdale, 1978). Unexpectedly, we additionally observed an overall positive association with current MDE symptom severity as indexed by the BDI-II and HC/HR choices. Using an item-level analysis, we found that reduced anticipation of positive future events was associated with less willingness to work for rewards, while the opposite was true for deficits in reward consummation. This may suggest that effort-mobilization is primarily linked to symptoms related to reward expectancy—consistent with prior reports (Sherdell et al., 2011)—and highlights the presence of distinct sub-components of anhedonia. However, given limitations in the reliability of individual items, this analysis should be interpreted with caution. Replication studies will be required to further clarify the relationship.
The present study possesses several limitations. First, the requirement of speeded button-presses could affect choice behavior in some patients with psychomotor slowing. However, this seems unlikely given that patients and controls showed equal completion rates and controlling for psychomotor retardation did not alter the results. A second limitation of the current study is the inclusion of depressed individuals who were not free of antidepressant medications. Given known interactions between serotonin and DA, it is possible that SSRI medications may have influenced the current results. However, preclinical studies of SSRI effects on reward processing are mixed, with evidence to suggest that SSRIs both potentiate (Deslandes, Pache, Buckland, & Sewell, 2002; Muscat, Papp, & Willner, 1992) and attenuate (Hoebel, Hernandez, Schwartz, Mark, & Hunter, 1989) reward function, and that these effects may depend on whether an animal is in a depressive state (Markou, Harrison, Chevrette, & Hoyer, 2005). Given these inconsistent findings it is unlikely that medication status alone could explain group differences in EEfRT task performance. Moreover, our results are consistent with significant prior evidence that SSRI treatments fail to address symptoms related to motivation and anhedonia in MDD (Nutt et al., 2007; Shelton & Tomarken, 2001). Finally, our control sample was screened to rule out high-levels of trait anhedonia, which may limit the specificity of our findings to MDD, as opposed to anhedonic traits.
In sum, the current findings demonstrate that reduced motivation and altered cost/benefit decision-making may be a crucial aspect of anhedonic symptoms. Additionally, the success of this translational approach highlights the importance of incorporating preclinical models of reward processing into the conceptualization and assessment of clinical symptoms. Such measures may ultimately facilitate the development of a more objective nosology of reward-related deficits in MDD.
Supplementary Material
Figure 2.
Bar graph of mean proportions of HC/HR choices for control subjects and patients with MDD across levels of probability. See results section and table 2 for inferential statistical analysis using GEE models.
Acknowledgments
This research was funded by the National Institute of Mental Health (R21 MH092751) to DHZ and (F31MH087015) to MTT. The authors wish to thank the Vanderbilt Institute for Clinical and Translational Research (VICTR) for additional support.
Footnotes
Please see supplemental materials for a discussion of EEfRT performance and co-morbid anxiety.
The authors report no financial interests or conflicts of interest.
Contributor Information
Michael T. Treadway, Department of Psychology, Vanderbilt University, Nashville, Tennessee, 37203
Nicholas Bossaller, Department of Psychiatry, Vanderbilt University, Nashville, Tennessee, 37203.
Richard C. Shelton, Department of Psychiatry, Vanderbilt University, Nashville, Tennessee, 37203
David H. Zald, Department of Psychology, Department of Psychiatry, Vanderbilt University, Nashville, Tennessee, 37203
References
- Abramson LY, Metalsky GI, Alloy LB. Hoplessness depression: a theory-based sub-type of depression. Psychological Review. 1989;96(2):358–372. [Google Scholar]
- Abramson LY, Seligman ME, Teasdale JD. Learned helplessness in humans: cirtique and reformulation. Journal of Abnormal Psychology. 1978;87(1):49–74. [PubMed] [Google Scholar]
- Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biological Psychiatry. 1987;22(12):1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behavioral Neuroscience. 2009;123(2):242–251. doi: 10.1037/a0014625. 2009-04037-002 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and scizophrenic patients in comparison with healthy subjects. European Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. S0166223603002339 [pii] [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. S0272-7358(07)00162-6 [pii] [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chentsova-Dutton Y, Hanley K. The effects of anhedonia and depression on hedonic responses. Psychiatry Research. 2010;179(2):176–180. doi: 10.1016/j.psychres.2009.06.013. S0165-1781(09)00222-4 [pii] [DOI] [PubMed] [Google Scholar]
- Clery-Melin ML, Schmidt L, Lafargue G, Baup N, Fossati P, Pessiglione M. Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE. 2011;6(8):e23178. doi: 10.1371/journal.pone.0023178. PONE-D-11-05483 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179(3):587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Deslandes PN, Pache DM, Buckland P, Sewell RD. Morphine, cocaine and antidepressant induced motivational activity and midbrain dopaminergic neurotransmission. European Journal of Pharmacology. 2002;453(2–3):223–229. doi: 10.1016/s0014-2999(02)02451-2. S0014299902024512 [pii] [DOI] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depression and Anxiety. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. 2006-09621-003 [pii] [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structred Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (With Psychotic Screen): Biometrics Research Department 2005 [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33(8):1966–1979. doi: 10.1038/sj.npp.1301565. 1301565 [pii] [DOI] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61(5):633–639. doi: 10.1016/j.biopsych.2006.05.026. S0006-3223(06)00725-6 [pii] [DOI] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(Pt 6):1751–1764. doi: 10.1093/brain/awr059. awr059 [pii] [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Annals of the New York Academy of Sciences. 1989;575:171–191. doi: 10.1111/j.1749-6632.1989.tb53242.x. discussion 192-173. [DOI] [PubMed] [Google Scholar]
- Kazes M, Danion JM, Grange D, Pradignac A, Simon C, Burrus-Mehl F, et al. Eating behaviour and depression before and after antidepressant treatment: a prospective, naturalistic study. Journal of Affective Disorders. 1994;30(3):193–207. doi: 10.1016/0165-0327(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Liang KY, Beaty TH, Cohen BH. Application of odds ratio regression models for assessing familial aggregation from case-control studies. American Journal of Epidemiology. 1986;124(4):678–683. doi: 10.1093/oxfordjournals.aje.a114441. [DOI] [PubMed] [Google Scholar]
- Markou A, Harrison AA, Chevrette J, Hoyer D. Paroxetine combined with a 5-HT(1A) receptor antagonist reversed reward deficits observed during amphetamine withdrawal in rats. Psychopharmacology. 2005;178(2–3):133–142. doi: 10.1007/s00213-004-2008-2. [DOI] [PubMed] [Google Scholar]
- McFarland BR, Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depression and Anxiety. 2009;26(2):117–122. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Primary and secondary hypohedonia. Journal of Abnormal Psychology. 2001;110(1):188–193. doi: 10.1037//0021-843x.110.1.188. [DOI] [PubMed] [Google Scholar]
- Muscat R, Papp M, Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology. 1992;109(4):433–438. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21(5):461–471. doi: 10.1177/0269881106069938. 0269881106069938 [pii] [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. S0022-3956(08)00055-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot T. La psychologie des sentiment [The psychology of feelings] Paris: Felix Alcan; 1896. [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacology, Biochemistry and Behavior. 1994;49(1):25–31. doi: 10.1016/0091-3057(94)90452-9. 0091-3057(94)90452-9 [pii] [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatric Services. 2001;52(11):1469–1478. doi: 10.1176/appi.ps.52.11.1469. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. Journal of Abnormal Psychology. 2011 doi: 10.1037/a0024945. 2011-17872-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34(3):681–697. doi: 10.1038/npp.2008.121. npp2008121 [pii] [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. S0149-7634(10)00112-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic Mechanisms of Individual Differences in Human Effort-Based Decision-Making. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.6459-11.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan VV, Casey KF, O’Hara C, O’Loughlin J, Benkelfat C, Fellows LK, et al. Acute Phenylalanine/Tyrosine Depletion Reduces Motivation to Smoke Cigarettes Across Stages of Addiction. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.135. npp2011135 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping Up Effort: Effects of d-Amphetamine on Human Effort-Based Decision-Making. Journal of Neuroscience. 2011;31(46):16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. 31/46/16597 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.