Commensal bacteria in the lower intestine of mammals are tenfold more numerous than the body’s cells. Here we show that there is a flexible continuum between innate and adaptive immune function in containing the commensal microbiota. Mice deficient in critical innate immune functions such as Toll-like receptor signalling or oxidative burst production spontaneously produce high-titer serum antibodies against their commensal microbiota. These antibody responses are functionally essential to maintain host-commensal mutualism in vivo in the face of innate immune deficiency. Spontaneous hyper-activation of adaptive immunity against the intestinal microbiota, secondary to innate immune deficiency, may clarify the underlying mechanisms of inflammatory diseases where immune dysfunction is implicated.
Innate recognition of just a few thousand bacteria in the blood and peripheral tissues potently instructs the development of an inflammatory response, and subsequently both cell-mediated and humoral adaptive immunity (1). Paradoxically, our many trillions of intestinal bacteria do not normally induce spontaneous pathological inflammatory responses or high-titer serum antibody responses (2, 3). Many layers of defense compartmentalize commensal bacteria within the gut lumen and highly specialized mucosal immune compartment (Lamina propria, Peyer’s patches and mesenteric lymph nodes) (3). Thus the paradigm emerged that minimizing microbial recognition within the specialized intestinal microenvironment is crucial to establishing mutualism (4) and consequently the ability of animals with deficient innate immunity to contain their microbiota was unsurprising. Here we present data that challenge this view. Instead, we propose that innate and adaptive immunity can function not only in a sequential manner (5), but also in a complimentary manner, and intrinsic crosstalk translates the relative functionality of the two systems into a set point permissive for mutualism with the microbiota.
To study how mammals can adapt to commensal intestinal bacteria in the absence of signaling through Toll-like receptors (TLRs), a major family of innate immune sensors, it was necessary to maintain mice deficient in the TLR adaptor molecules MyD88 and TRIF (also known as Ticam-1) (Myd88−/− Ticam-1−/−) and control animals with different loads of intestinal commensal bacteria. Using specialized animal technology, we generated germ-free mice, who can be supposed to have never before encountered a live bacterium, “clean SPF” mice containing a very limited intestinal microbiota known to be free of both Enterococcus faecalis and Escherichia coli, and “conventional SPF” mice reared in standard clean animal house conditions and thus free of all monitored mouse pathogens (See supplementary materials and methods for details).
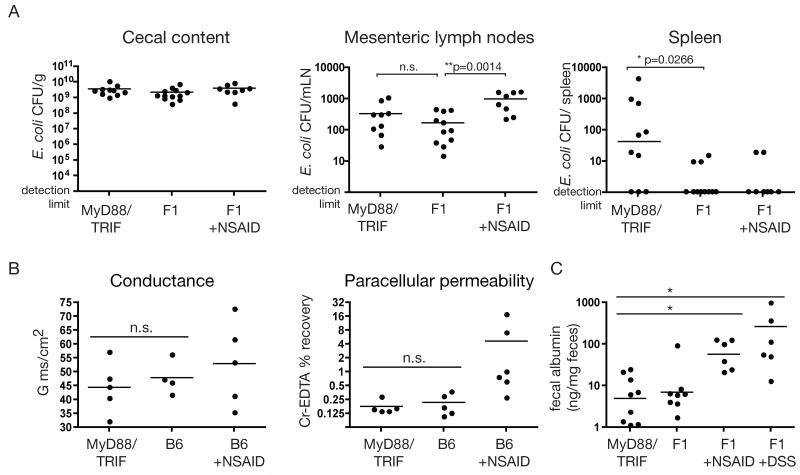
To address whether mucosal containment of the microbiota was normal in TLR-deficient mice, we challenged “clean SPF” Myd88−/− Ticam1−/− mice intragastrically with high doses of a commensal bacterium to which they had never previously been exposed (E. coli K-12). As previously reported (6), because of dendritic cell sampling at the intestinal surface, both Myd88−/− Ticam1−/− and control mice had similar loads of culturable E. coli K-12 in the mesenteric lymph nodes 18 hours after challenge (Fig. 1A). In contrast, and in agreement with a recent publication from Vaishnava et al. (7) the density of live bacteria recovered from the spleen was significantly higher in Myd88−/− Ticam1−/− mice (Fig. 1A). Identical data was obtained when MyD88TRIF mice were naturally colonised by co-housing with “conventional SPF” mice (Fig. S1A), ruling out any complications due to gavage, such as transient unphysiological bacterial loads in the small intestine, or mechanical damage. These data demonstrate a dramatic increase in escape of live intestinally delivered bacteria into the systemic circulation in Myd88−/− Ticam1−/− mice; i.e. a failure to compartmentalize the intestinal microbiota within the mucosal immune compartment.
Fig. 1.
Increased bacterial penetration in Myd88−/− Ticam1−/− mice is not dependent on increased intestinal permeability. (A) “Clean SPF” Myd88−/− Ticam1−/− (MyD88TRIF), F1 control mice, and F1 mice treated with 7.5 mg/kg Indomethacin 24 hours earlier (F1 + NSAID) were gavaged with 1010 ampicillin-resistant E. coli K-12. After 18 hours, the density of ampicillin-resistant E. coli in the cecal content, mesenteric lymph nodes and spleen was determined by selective plating (*P < 0.027, **P < 0.002). Data are pooled from 3 independent experiments. n.d. Not detectable. (B) Ussing chamber measurements of conductance and paracellular permeability, as assessed by serosal 51Cr-EDTA recovery, of jejunum from Myd88−/− Ticam1−/− (MyD88TRIF) mice, co-housed C57BL/6 control mice (B6), and positive control (C57BL/6) mice treated with 7.5 mg/kg Indomethacin (NSAID). Each data point represents an individual mouse and all collected data is shown. (C) ELISA for albumin presence in the feces of Myd88−/− Ticam1−/− mice, F1 control mice, NSAID-treated control mice or DSS-treated control mice (*P<0.05). Each data point represents an individual mouse and all collected data is shown.
Alterations in epithelial proliferation and increased susceptibility to intestinal pathogens and chemical damage have been reported in the Myd88−/− strain (8, 9). We therefore addressed whether altered intestinal barrier function was responsible for increased microbial translocation to the spleen in colonised Myd88−/− Ticam1−/− mice. Treatment of “clean SPF” control mice with non-steroidal anti-inflammatory drugs (NSAIDs) to increase small intestine permeability (10), before administering high numbers of E. coli K-12 intragastrically resulted in increased recovery of live E. coli from mesenteric lymph nodes but not to from the spleen (Fig. 1A). This suggested that non-specific increases in intestinal permeability such as those caused by low-dose NSAIDs were not sufficient to cause high numbers of live intestinal bacteria to access the spleen.
We carried out three additional independent measurements to directly assess intestinal permeability in Myd88−/− Ticam1−/− mice. We used Ussing chamers to measure permeability in vitro (Fig. 1B and S1C), a novel ex vivo assessment of permeability between the intestinal lumen and the vasculature (Fig. S1C), and direct in vivo measurement of serum protein loss into the intestinal lumen (Fig. 1D). In each case, the values were similar in Myd88−/− Ticam1−/− and C57BL/6 mice, but were elevated where permeability is increased by prior pharmacological treatment of the animals with NSAIDs or dextran sodium sulfate. Histological examination of intestinal architecture and epithelial cell proliferation also revealed no discernable differences between Myd88−/− Ticam1−/− mice and controls (Fig. S1D and E). Thus although previous work demonstrated that MyD88 function is extremely important in determining susceptibility to DSS-induced (8) and pathogen-induced (9) inflammation in the intestine, we found no evidence for altered intestinal barrier function in Myd88−/− Ticam1−/− mice with a limited defined intestinal flora. Therefore, out data suggest that loss of mucosal compartmentalization can occur in the presence of normal intestinal barrier function, and in the absence of any measurable intestinal pathology.
Bacteria encountered in the blood and spleen induce strong adaptive immune responses (1). We therefore determined the serum titers of commensal bacteria-specific antibodies in “conventional SPF” Myd88−/− Ticam1−/− mice. In order to avoid detection of highly cross-reactive epitopes such as bacterial ribosomal proteins, we determined titres by surface staining of live bacteria and flow cytometric analysis (Fig. S2). We found that as expected (6), “conventional SPF” C57BL/6 mice and control mice had no detectable serum antibodies to intestinal commensal bacterial species (Fig. 2A). In contrast, commensal-specific IgGs were consistently present in “conventional SPF” unmanipulated Myd88−/− Ticam1−/− mice (Fig. 2A), at similar titers to those observed after experimental intravenous vaccinations of control mice with commensal bacteria (Fig. S4). Similar results were observed in IgM antibody titers (Fig. S3A). Furthermore, commensal-specific antibodies were also observed in “clean SPF” Myd88−/− Ticam1−/− mice, and in an entirely independent colony of Myd88−/− mice housed at the ETH Zürich, Switzerland (Table S1 and fig. S3A). MyD88 or TRIF single knock-out animals maintained under “conventional SPF” conditions, also had spontaneous induction of commensal bacteria-specific antibodies, although at lower titers than Myd88−/− Ticam1−/− littermates (Fig. S3B and C), suggesting an additive effect of the two genetic lesions.
Fig. 2.
Myd88−/− Ticam1−/− mice lose systemic ignorance to their commensal flora. (A and B). The commensal bacteria Enterococcus faecalis and Staphylococcus xylosus were stained with whole serum from Myd88−/− Ticam1−/− and control mice, followed by PE-anti-mouse IgG1 and analyzed by flow cytometry. Titrations of anti-S. xylosus, anti-E. faecalis and anti-Salmonella typhimurium IgG1 reactivity from 24 week old Myd88−/− Ticam1−/− or F1 control mice housed in the indicated conditions. Each line represents an individual mouse. Data is representative of n>30 mice. (C) 12-week old germ-free Myd88−/− Ticam1−/− and Myd88+/− Ticam1−/− mice were monocolonized by co-housing with an E. coli K-12 monocolonized sentinel for the indicated amount of time. Serum was taken weekly to follow the development of E. coli K-12 IgG1 antibody responses by bacterial flow cytometry and ELISA. Each line represents an individual mouse. Representative data of two independent experiments are shown. (D) E. coli K-12-specific IgA titers as determined by bacterial surface staining with cleared intestinal lavage from day 28 E. coli-monocolonized mice. Data shown are representative of two experiments.
Commensal bacteria-specific antibodies were shown to be a consequence of colonization with intestinal bacteria, because germ-free Myd88−/− Ticam1−/− mice had no detectable anti-commensal IgG (Fig. 2B). Further, this phenomenon occurred independently of opportunistic pathogens in the microbiota, as we detected high titres of anti-E. coli IgG in the serum of of Myd88−/y Ticam1−/− mice monocolonized with the non-pathogen (11) E. coli K-12 by co-housing (fig. 2C). Interestingly, “clean SPF” Myd88−/− Ticam1−/− mice vaccinated systemically with peracetic acid-killed bacteria required higher doses of killed bacteria to induce robust IgG1 antibody responses than control mice (Fig. S4), suggesting that colonised Myd88−/− Ticam1−/− mice must experience profoundly elevated systemic commensal bacterial-antigen loads. Intestinal and serum IgA was present at normal concentrations in “clean SPF” and monocolonised MyD88−/− Ticam1−/− mice (Fig. S5), and with normal or elevated titers specific for colonizing organisms (Fig. 2D and fig. S5). These results demonstrate that induction of commensal-specific antibodies occurs in a microbiota-dependent manner, but is independent of pathogenicity of the microbiota, and is not secondary to a defect in IgA production. Furthermore, and similar to previous observations (5, 12-15), although TLR signalling increases the sensitivity of the host for induction of bacteria-specific antibodies, TLR-signalling is not essential for the induction of anti-bacterial IgG1 responses. This is presumably due to contributions of other innate immune detection pathways such as the NOD-like receptors, which still operate in the absence of TLRs, but with lower sensitivity.
Studies of bacterial infection in MyD88−/− Ticam1−/− mice imply that systemic spread of intestinal bacteria and subsequent serum antibody production in MyD88−/− Ticam1−/− mice may be secondary to a deficiency in bacterial clearance (9, 13, 15-19), involving both hematopoietic and/or stromal cells. Indeed, intravenous injection of an apathogenic E. coli K-12 resulted in dramatically higher numbers of culturable bacteria in the spleens of “clean SPF” MyD88−/− Ticam1−/− mice than in spleens of “clean SPF” control mice at both 4 and 24 hours post-injection (Fig. S6). In addition we examined mice deficient in inducible nitric oxide synthase (NOS2) and CYBB (gp91 phox). These mice clear bacteria poorly due to a profound defect in phagocyte oxidative burst generation (20). Nos2−/− Cybb−/− mice displayed serum antibodies specific for some of their commensal microbiota, whereas co-housed C57BL/6 controls did not (Fig. 3). These data suggested that production of commensal bacteria-specific IgG in serum is a broad phenomenon associated with impaired bacterial clearance.
Fig. 3.
Cybb−/− Nos2−/− mice exhibit serum antibodies directed against their commensal flora. Serum and feces were collected from 8-week-old Cybb−/− Nos2−/− mice and co-housed C57BL/6 controls. Isolates of E. faecalis, Staphylococcus saprophyticus and Stentrophomonas maltophilia were obtained by aerobic culture from feces and pure cultures were stained with serum from Cybb−/− Nos2−/− and control C57BL/6 mice. Anti-bacterial IgG1 was quantified by flow cytometry. n=5 C57BL/6 controls and 5 CybbNOS2 mice. Representative data from two independent experiments are shown.
To ask whether these spontaneously induced anti-commensal bacteria antibodies were of functional importance, we determined whether responses of Myd88−/− Ticam1−/− mice to colonisation with commensal bacterial species could correct bacterial clearance defects. Adult Myd88−/− Ticam1−/− and C57BL/6 mice were monocolonized with the typical commensal Enterococcus faecalis by co-housing. Four weeks after colonization, Myd88−/− Ticam1−/−, but not C57BL/6 mice had mounted a robust serum IgG response to the colonising organism that required the presence of CD4+ T cells (Fig. 4A), but did not mount a response to the unrelated bacterium E. coli K-12 (Fig. 4B). We then infected these mice intravenously with E. faecalis and E. coli K-12 and determined the number of culturable bacteria in the spleens three hours after injection. As expected, in germ-free conditions Myd88−/− Ticam1−/− mice had significantly higher densities of culturable E. coli and Enterococcus faecalis in their spleens than C57BL/6 germ-free mice (Fig. 4C). If challenged after four weeks of monocolonisation with Enterococcus faecalis, however, Myd88−/− Ticam1−/y mice that had mounted a robust anti-E. faecalis IgG response still failed to efficiently clear E. coli but now could clear Enterococcus faecalis from their spleens as efficiently as either germ-free or monocolonized C57BL/6 mice (Fig. 4C). Those Myd88−/− Ticam1−/− mice that were depleted of CD4 T cells and had not mounted strong IgG responses remained unprotected (Fig. 4C). This is not due to the absence of CD4 T cells during the challenge as depletion of CD4 T cells just prior to challenge does not negatively influence protection (Fig. S7A-C). Further, we demonstrate that coating E. faecalis with serum antibodies 28 d monocolonized Myd88−/− Ticam1−/− mice is sufficient to correct the bacterial clearance defect in Myd88−/− Ticam1−/− mice (Fig. S7E). These data imply that adaptive immune responses, particularly high titre T cell-dependent antibody responses spontaneously induced to the microbiota, can functionally compensate for the defect in bacterial clearance in Myd88−/− Ticam1−/− mice.
Fig. 4.
Serum antibodies successfully protect Myd88−/− Ticam1−/− mice from bacteraemia. 12-week old germ-free Myd88−/− Ticam1−/− and C57BL/6 mice were monocolonized with E. faecalis for four weeks by co-housing with monocolonized sentinel mice, with and without continous CD4 T cell depletion. (A) Serum antibodies against E. faecalis were quantified by bacterial flow cytometry at days 0 and 28 of colonization. (B) Serum antibodies against E. coli K-12 were quantified by bacterial flow cytometry at day 28 post-colonization. (C) 32 day monocolonized and germ-free mice, which had been T cell depleted, or mock-depleted, as indicated, were injected intravenously with 107 CFU of nalidixic acid-resistant E. faecalis and 108 CFU of chloramphenicol-resistant E. coli K-12. Spleens were recovered at three hours post-injection and selectively plated (*P < 0.01). Data are representative of two independent experiments. (D) Total live mice of the indicated genotypes found at weaning (male and female mice). (E) Representative mice and weights of female mice at week 4 (seven days after weaning). (F) Protein-losing enteropathy quantified by measuring fecal albumin concentrations at four weeks of age. Total mice analysed n=97, including n=3 MyD88−/−JH−/− mice.
In order to determine if this mechanism had functional importance in unmanipulated mice we bred the Myd88−/− strain to the JH−/− strain, which lacks antibody production due to a deletion of the J segments of the immunoglobulin heavy chain locus. Both strains were on the C57BL/6 background, and were maintained in our “clean SPF” conditions. Despite a limited intestinal microbiota, Myd88−/−JH−/− offspring exhibited stunted growth and only half of the expected number survived to weaning (Fig. 4D and E), whereas Myd88−/− JH−/− offspring grew and survived normally. MyD88−/−JH−/− mice also displayed evidence of protein losing enteropathy at weaning (Fig. 4F). Protein-losing enteropathy is not observed when germ-free Myd88−/− Ticam1−/− mice are depleted of mature B cells using anti-mouse-CD20 monoclonal antibody (Fig. S8A and B) (21). These results suggest that antibody-mediated immunity is essential for TLR signalling-deficient mice to mutually co-exist with their microbiota.
In this paper we have shown that adaptive immunity is critical for successful mutualism in TLR-deficient mice. We conclude that TLR signalling is required for the normal elimination of low numbers of bacteria that are translocated from the intestinal lumen into the mucosa, but specific anti-commensal serum IgG responses, induced in response to “escaped” intestinal bacteria, can restore effective bacterial clearance to wild-type levels. Thus, innate and adaptive immune mechanisms can complement each other to establish and maintain mutualism, as illustrated by the severe phenotype of the MyD88−/−JH−/− mouse. We suggest that there is a flexible set-point between innate and adaptive immunity, determined by the functional performance of each system, which acts to protect the animal. It is interesting to note that mutations in innate immunity genes are commonly associated with autoinflammatory diseases, the best characterized of which is the association of the non-TLR microbial recognition receptor NOD2 and Crohn’s disease (22-24). Our work would clearly suggest that such patients would experience “escape” of a subset of the intestinal commensal flora, and the subsequent nature of immune responses induced may determine the presence or absence of disease.
Supplementary Material
Acknowledgments
We would like to thank Jorum Kirundi, Jennifer Jury and Jun Lu for their technical support. We also thank Emmanuel Denou, Matthias Heikenwälder, Caetano Reis e Sousa, Dan Stetson, Jayne Danska and Christoph Mueller for their helpful comments and editing of the manuscript.
Grant support: Canadian Institutes of Health Research, Crohn’s and Colitis Foundation of Canada, Genome Canada
Footnotes
Adaptive immunity spontaneously and functionally compensates for innate immune deficiency and this is essential for mutualism between the host and its intestinal microbiota.
References
- 1.Medzhitov R. Nature. 2007;449:819. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Whitman WB, Coleman DC, Wiebe WJ. Proc Natl Acad Sci U S A. 1998;95:6578. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller C, Macpherson AJ. Gut. 2006;55:276. doi: 10.1136/gut.2004.054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibolet O, Podolsky DK. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1469. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- 5.Pasare C, Medzhitov R. Nature. 2005;438:364. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 6.Macpherson AJ, Uhr T. Science. 2004;303:1662. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 7.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Proceedings of the National Academy of Sciences. 2008;105:20858. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Cell. 2004;118:229. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Gibson DL, et al. Cell Microbiol. 2008;10:618. doi: 10.1111/j.1462-5822.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 10.Porras M, et al. Inflamm Bowel Dis. 2006;12:843. doi: 10.1097/01.mib.0000231571.88806.62. [DOI] [PubMed] [Google Scholar]
- 11.Fux CA, Shirtliff M, Stoodley P, Costerton JW. Trends in Microbiology. 2005;13:58. doi: 10.1016/j.tim.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Gavin AL, et al. Science. 2006;314:1936. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. J Immunol. 2007;179:566. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Bahlburg A, Khim S, Rawlings DJ. J. Exp. Med. 2007;204:3095. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko H-J, et al. J Immunol. 2009;182:2305. doi: 10.4049/jimmunol.0801980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi O, Hoshino K, Akira S. J Immunol. 2000;165:5392. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 17.Kiyoshi Takeda SA. Cellular Microbiology. 2003;5:143. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 18.Archer KA, Roy CR. Infect. Immun. 2006;74:3325. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Bernuth H, et al. Science. 2008;321:691. [Google Scholar]
- 20.Shiloh MU, et al. Immunity. 1999;10:29. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi Y, et al. Journal of Immunology. 2005;174:4389. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 22.Hampe J, et al. The Lancet. 2001;357:1925. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 23.Ogura Y, et al. Nature. 2001;411:603. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 24.Hugot J-P, et al. Nature. 2001;411:599. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.