Abstract
Background
The emergence of human monkeypox and the potential use of recombinant variola and monkeypox viruses as biological terrorist agents have necessitated the development of therapeutic and prophylactic therapies. The primary, or index, cases of smallpox and/or human monkeypox will likely be identified by a characteristic rash. Effective biomarkers will be required to monitor disease progression, guide the choice of therapeutic intervention strategies and evaluate their efficacies. To address this we have evaluated several biomarkers of disease in a lethal mousepox model.
Methods
The efficacy of a single dose of a hexadecyloxypropyl ester of cidofovir (CMX001) at 20, 25 and 30 mg/ kg doses administered on days 4, 5, 6 and 7 post-infection was evaluated in A/Ncr mice intranasally infected with low doses of ectromelia virus (<20 plaque-forming units). Mice were monitored for weight loss, blood interferon-γ levels, alanine aminotransferase (ALT), aspartate aminotransferase, viral DNA copies and neutrophilia levels to stage disease progression.
Results
We have used these biomarkers to establish the optimal dosing regimen for treatment and reveal that a single dose of 25 mg/kg of CMX001 can be efficacious at treating lethal mousepox when administered on days 4 or 5 post-infection. This dose significantly reduces ALT, interferon-γ and DNA copies found in the blood of infected animals.
Conclusions
A single dose regimen of CMX001 is efficacious at treating mousepox. Disease progression and antiviral efficacy can be monitored using several biomarkers that could readily be used in the case of a human monkeypox or smallpox outbreak.
Introduction
Variola virus, the etiological agent of smallpox, is considered by many governments as a practicable tool for bioterrorists and rogue nations. Furthermore, the monkeypox virus, which is infecting increasing numbers of humans in Africa (reviewed by [1]), has inherent qualities that could be exploited for bioterrorist activities. Vaccination to protect against these viruses largely ceased over 30 years ago, leaving a population with little or no natural protection [2]. Moreover, the re-introduction of vaccination with Dryvax™ or associated vaccines is a poor prophylactic and therapeutic option because of its incompatibility with immuno-compromised or immunosuppressed individuals, such as those with HIV/AIDS, those receiving organ trans-plants or those with eczema [3–6]. For these reasons, the availability of efficacious antivirals to treat orthopoxvirus infections is crucial. One such antiviral, CMX001 (the hexadecyloxypropyl ester of cidofovir [CDV]), has been demonstrated to be highly efficacious against several poxvirus diseases in animal models [7–10]. Many vertebrates are susceptible to orthopoxvirus infections, but the mousepox model is arguably the best small animal model for the evaluation of smallpox therapeutics because, in part, of the low dose of virus required to initiate a lethal infection [11]. Moreover, mousepox is thought to accurately reflect the progress of natural infection in the human.
CDV is a wide-spectrum antiviral with efficacy against many DNA viruses, including the orthopoxviruses [12]. CDV is of limited use because it must be administered intravenously and is associated with severe nephrotoxicity, thus making it a poor therapeutic option in the event of a bioterror or biowarfare attack [13]. Esterification of CDV with alkoxyalkanols decreases the poxviral 50% effective concentration (EC50) values by 24- to 910-fold compared with CDV [7,13–15]. Esterification allows the drug to be delivered orally without diminishing its efficacy and prevents accumulation of CDV in the kidneys [16]. CMX001 has been demonstrated to have a good balance between high efficacy and low toxicity [14].
We have previously shown that orally administered CMX001 is equal or superior to intraperitoneally administered CDV and is protective over a broad range of virus challenge doses [8]. We have also optimized the treatment regimen to provide complete protection against an ectromelia virus (ECTV) challenge when administered as late as 5 days following challenge, which is 3–4 days prior to the death of the untreated controls [8]. In this study, we elucidated several biomarkers that can be used to stage disease and monitor the efficacy of CMX001. These biomarkers could potentially be used to relate stages of mousepox with those of human orthopoxvirus disease and, in combination with pharmacokinetic and pharmacodynamic data, predict the efficacy of CMX001.
Methods
Cells and virus
African green monkey kidney cells (BSC-1; American Type Culture Collection [ATCC] CCL 26) were grown in Eagle’s minimum essential medium (MEM) containing 10% fetal calf serum (FCS; Hyclone III, Logan, UT, USA), 2 mM l-glutamine (GIBCO, Grand Island, NY, USA), 100 U/ml penicillin (GIBCO) and 100 µg/ ml streptomycin (GIBCO). A plaque-purified isolate of the MOS strain of ECTV (ATCC VR-1374), designated MOS-3-P2, was propagated in BSC-1 cells [17]. Virus was purified through a sucrose cushion as described else-where [18]. Virus infectivity was estimated as described previously [19]. Briefly, virus suspensions were serially diluted in phosphate-buffered saline plus 1% sera, absorbed to monolayers for 1 h at 37°C and overlaid with a suspension of 1% carboxyl methyl cellulose in Dulbecco’s modified Eagle’s medium plus 5% Fetal clone III. After 4 days at 37°C, virus plaques were visualized and virus inactivated by the addition of 0.5 ml (0.3% crystal violet/10% formalin) solution to each well.
Animals
Four- to six-week-old female A/Ncr mice were obtained from the National Cancer Institute (Frederick, MD, USA), housed in filter-top microisolator cages and fed commercial mouse chow and water ad libitum. The mice were housed in an animal biosafety level 3 containment area. Animal husbandry and experimental procedures were in accordance with the Public Health Service policy and approved by the Institutional Animal Care and Use Committee (St Louis University, MO, USA). Live mice were bled via the submadibular vein.
Antiviral compounds
CMX001 (Chimerix Inc., Durham, NC, USA) was prepared fresh prior to each experiment by dissolving the compound in sterile, distilled water and stored at 4°C over the course of the experiment.
Intranasal challenge
Mice were anaesthetized with 0.1 ml/10 g body weight of ketamine HCl (9 mg/ml) and xylazine (1 mg/ml) by intraperitoneal injections. Anaesthetized mice were laid on their dorsal side with their bodies angled so that the anterior end was raised 45° from the surface; a plastic mouse holder was used to ensure conformity. ECTV was diluted in phosphate-buffered saline without Ca2+ and Mg2+ to the required concentration and slowly loaded into each nare (5 µl/nare). Mice were subsequently left in situ for 2–3 min before being returned to their cages.
Statistics
An unpaired Student’s t-test was used to compare the means of the two groups of mice. P-values <0.05 were considered statistically significant. Mortality rates were compared using Fisher’s exact test.
A total of 10 mice were used to calculate each assay value. Mice were housed in two cages of five. Blood samples were collected via mandibular vein bleeds (50 µl) and pooled. The average assay value from each cage was plotted with the indicated sem
PCR assays
DNA from blood was isolated using the High Pure PCR Preparation Kit (Roche Diagnostics, Mannheim, Germany). Quantitative PCR was performed on eluted DNA using the Power SYBR Green Master mix (Applied Biosystems, Foster City, CA, USA) with primers SP028 (GTAGAACGACGCCAGAATAAGAATA, 5′ at 120,627 base pairs) and SP029 (AGAAGATAT-CAGACGATCCACAATC, 5′ at 120,462 base pairs), which amplified 165 base pairs of the EV107 gene. SP028 and SP029 were used to amplify DNA for cloning into a plasmid vector (pGEM-T; Promega, Madison, WI, USA) that was used as a standard control to estimate the copies of DNA in the blood.
Flow cytometry assays
Red blood cells were lysed with BD Pharm Lyse (BD Biosciences Pharmingen, Franklin Lakes, NJ, USA) and resuspended in RPMI-1640 (BioWhittaker, Walkers-ville, MD, USA) with 10% FCS (vida supra). A total of 1×106 cells were blocked for 10 min with Fc Block (BD Biosciences Pharmingen) and stained with the antibodies Gr-1-Pacific Blue 445, DX5-FITC, CD11b-PerCP Cy5.5, CD3-APC 660, CD4-Pacific Blue 445, CD1-PE 575, CD11c-PE-Cy7, CD8-APC-Cy5.5 and CD45-APC 660 (all from BD Biosciences Pharmingen). Cells were fixed with 10% ultrapure formaldehyde (Polysciences Inc., Warrington, PA, USA) and analysed using a BD LSR II flow cytometer (BD Biosciences Pharmingen). Interferon (IFN)-γ was detected using a Mouse IFN-γ Flex Set (BD Biosciences Pharmingen) and assayed on the BD LSR II.
Clinical chemistry assay
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) analysis was executed on a Cobas Mira Plus Chemistry Analyser (Roche Diagnostics). The samples were diluted as needed to provide ade-quate sample volume for analysis or to bring the results within the linear range of the analyser.
Results
Evaluation of biomarkers to stage mousepox disease CMX001 has been shown to be highly efficacious at therapeutically treating mousepox in the A/Ncr mouse strain [8,14]. However, the efficacy of this therapy is affected by the infectious dose and the age of the mice. Because the Food and Drug Administration’s animal efficacy rule permits licensure of therapeutics on the basis of animal efficacy data, it is important to fully understand the relationship of the animal model to human disease [20–22]. The discovery and validation of biomarkers that relate mousepox to human disease is a key aspect of this process. To address these challenges, we have investigated several biological markers of mousepox namely viral DNA load in the blood, ALT and AST levels in serum and IFN-γ levels in serum and blood neutrophilia.
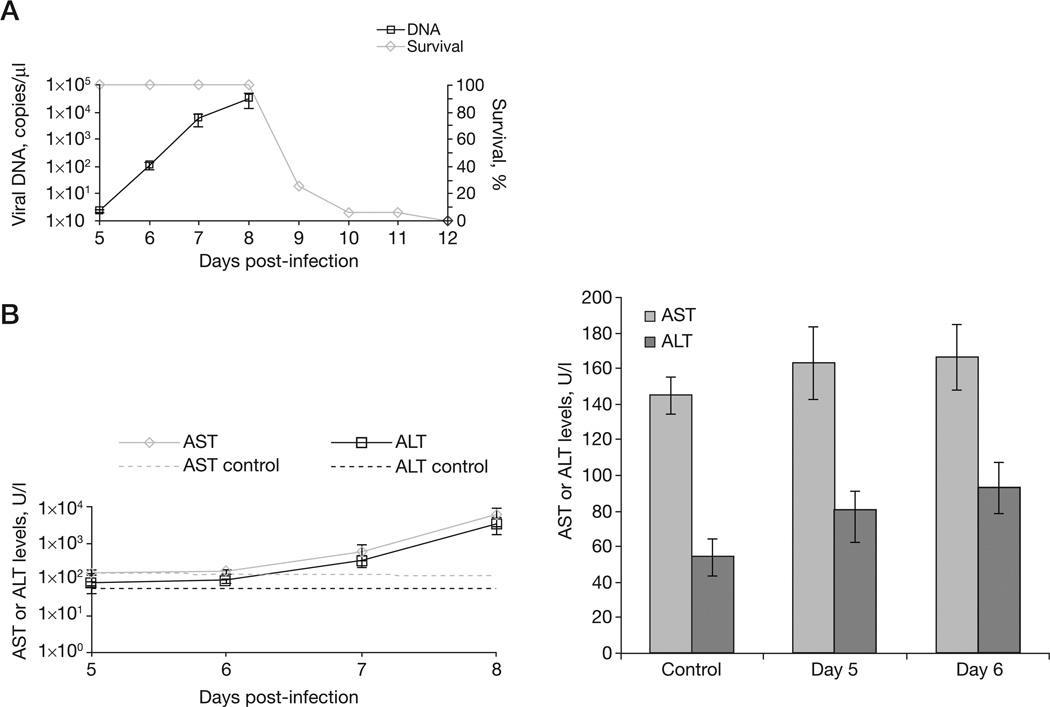
Groups of mice were intranasally infected with 5 plaque-forming units (PFU; 10× the lethal dose that kills 50% of the animals tested [LD50]) of ECTV and monitored for disease. By 10 days post-infection (p.i.) 90% of mice had died (Figure 1A). At 5 days p.i. we detected low levels of viral DNA in the blood (<5 copies/µl). Viral DNA levels increased exponentially to 1×104 copies/µl on day 8, this level coincided with a mortality of 80% (Figure 1A). AST and ALT levels were also monitored and increased from approximately 1×102 to 1×104 U/l between days 5 and 8 (P=0.06, P=0.06 and P=0.09 for ALT and P=0.35, P=0.12 and P=0.09 for AST on days 6, 7 and 8, respectively, compared with controls; Figure 1B). Statistically significant changes in blood ALT and AST levels between control and infected mice could not be detected before 5 days p.i. (data not shown). Blood samples were analysed by flow cytometry to assay for changes in lympocytes, B-cells, T-cells, monocytes, natural killer cells and neutrophils. The proportion of each cell type did not change compared with CMX001-naive blood, except for neutrophils (not shown). Neutrophilia was observed as early as 5 days p.i. (P=0.0058, P<0.001 and P<0.001 for days 5, 6 and 7, respectively, compared with the saline control) and increased with time. These data reveal that blood genome copies, ALT and AST levels, and white blood cell composition can be used as early biomarkers to monitor mousepox progression, but weight loss cannot (data not shown).
Figure 1. Disease biomarkers to monitor mousepox progression.
A/Ncr mice were intranasally infected with 5 plaque-forming units of ectromelia virus. (A) Mice were monitored for mortality for 42 days. Viral DNA copies were measured from blood on days 5, 6, 7 and 8 post-infection. (B) Mortality correlated with increased levels of asparate aminotransferase (AST) and alanine aminotransferase (ALT) that indicated liver damage.
Optimization of the loading dose of CMX001
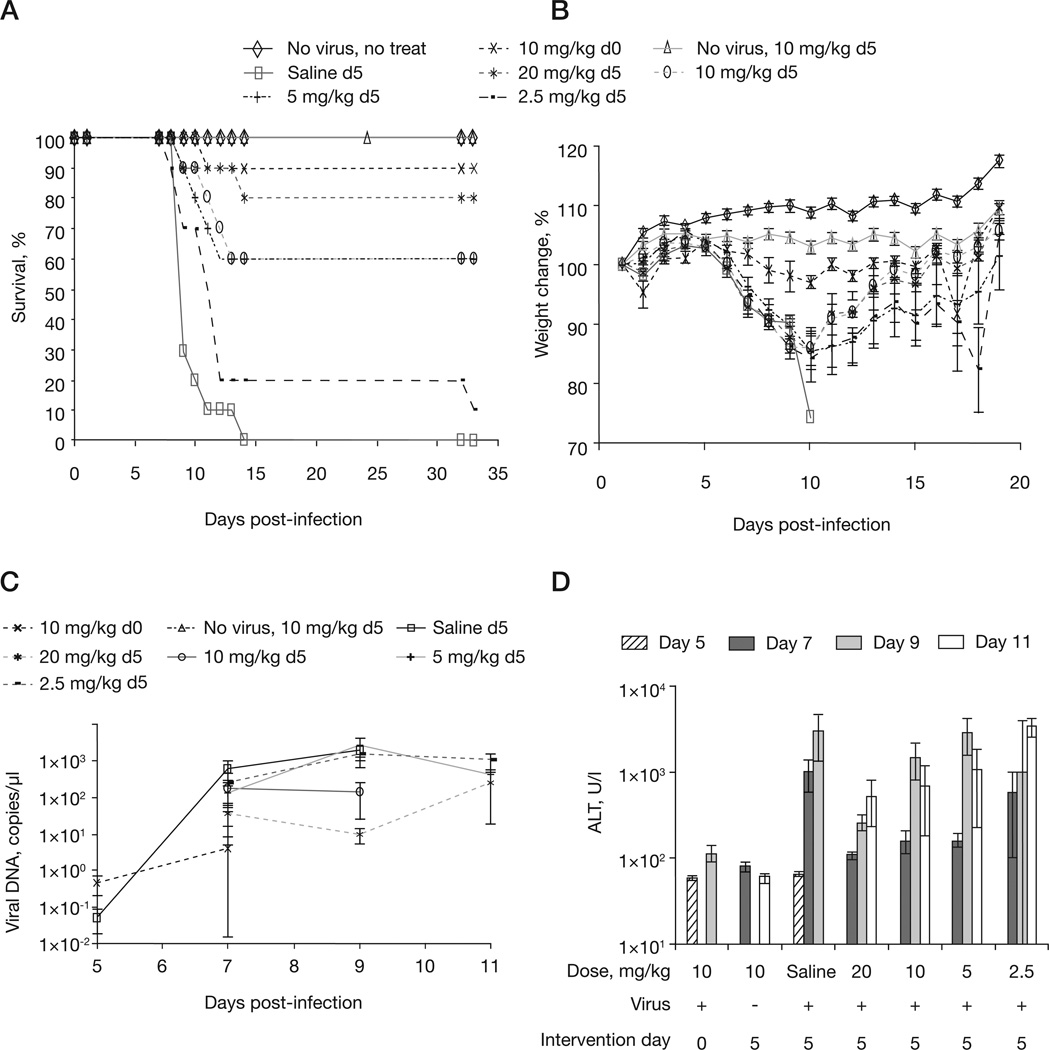
We have previously shown that a 5-day delayed loading dose of 10 mg/kg followed by every-other-day dosing with 2.5 mg/kg of CMX001 is sufficient to provide 100% protection for mice infected with 5 PFU (10× LD50) of ECTV [8]. However, loading doses and maintenance doses had not been extensively evaluated under conditions of therapeutic intervention. To further optimize this treatment regimen for therapeutic use, mice were intranasally infected with 5 PFU of ECTV (10× LD50) and treatment was initiated at 5 days p.i. according to five regimens: gavage of saline every other day following an initial saline gavage on day 5 p.i., 20 mg/kg loading dose, 10 mg/kg loading dose, 5 mg/kg loading dose and 2.5 mg/kg loading dose. The regimens 20 mg/ kg loading dose, 10 mg/kg loading dose, 5 mg/kg loading dose and 2.5 mg/kg loading dose were followed by every-other-day maintenance doses of 2.5 mg/kg following the loading dose. We found that a 20 mg/kg loading dose followed by the 2.5 mg/kg maintenance dose provided the highest level of delayed protection to mice treated at 5 days p.i. (Figure 2A). Over the first 8 days p.i., mice that were treated with delayed dosings lost similar amounts of body weight and mice treated with loading doses of 20 mg/kg and 10 mg/kg regained weight rapidly after day 10 p.i. (Figure 2B). Blood viral DNA levels indicated that for ≥80% survival the level must remain <1×103 copies/µl (Figure 2C). Mice receiving saline or loading doses of 5 mg/kg or 2.5 mg/kg all had >1×103 copies/µl in their blood at 9 days p.i.; they also had the highest mortality of the treated groups. Infected, mock-treated controls had a mean of 1,893 copies/µl of ECTV in blood on day 9 versus <10 copies/µl in mice treated with 20 mg/kg on day 9 (P=0.0191). These data suggest that CMX001 reduces viral load and that this reduction is directly related to mortality.
Figure 2. Optimizing the loading dose of CMX001 to treat mousepox.
A/Ncr mice were intranasally infected with 5 plaque-forming units of ectromelia virus and treated with CMX001 at 5 days post-infection. The maintenance dose remained constant at 2.5 mg/kg every other day. The loading dose administered was 20, 10, 5 or 2.5 mg/kg. Control mice were either uninfected and untreated (no virus, no treat), infected and treated with a 10 mg/kg loading dose on the day of infection (10 mg/kg d0) or uninfected and treated with 10 mg/kg on day 5 (no virus, 10 mg/kg d5) followed by the maintenance dose. Untreated, infected mice were dosed with saline on day 5 followed by every-other-day dosings (saline d5). Mice were monitored for (A) morbidity and (B) weight loss for day 42 post-infection. (C) Viral DNA levels and (D) alanine aminotransferase (ALT) levels were monitored in blood samples taken at days 5, 7, 9 and 11 post-infection.
ALT levels in untreated control mice were significantly increased by day 7 p.i. (Figure 2D). This increase is reduced in mice treated with 20, 10 or 5 mg/kg loading doses. However, by day 9 p.i. the mice treated with 10, 5 and 2.5 mg/kg loading doses had ALT levels that were similar to those observed in the untreated control mice. This suggests that liver damage, as indicated by ALT levels, decreases with increasing CMX001 loading dose and correlates with delayed day of death and mortality in susceptible animals (Figure 2A).
Optimization of the maintenance dose of CMX001
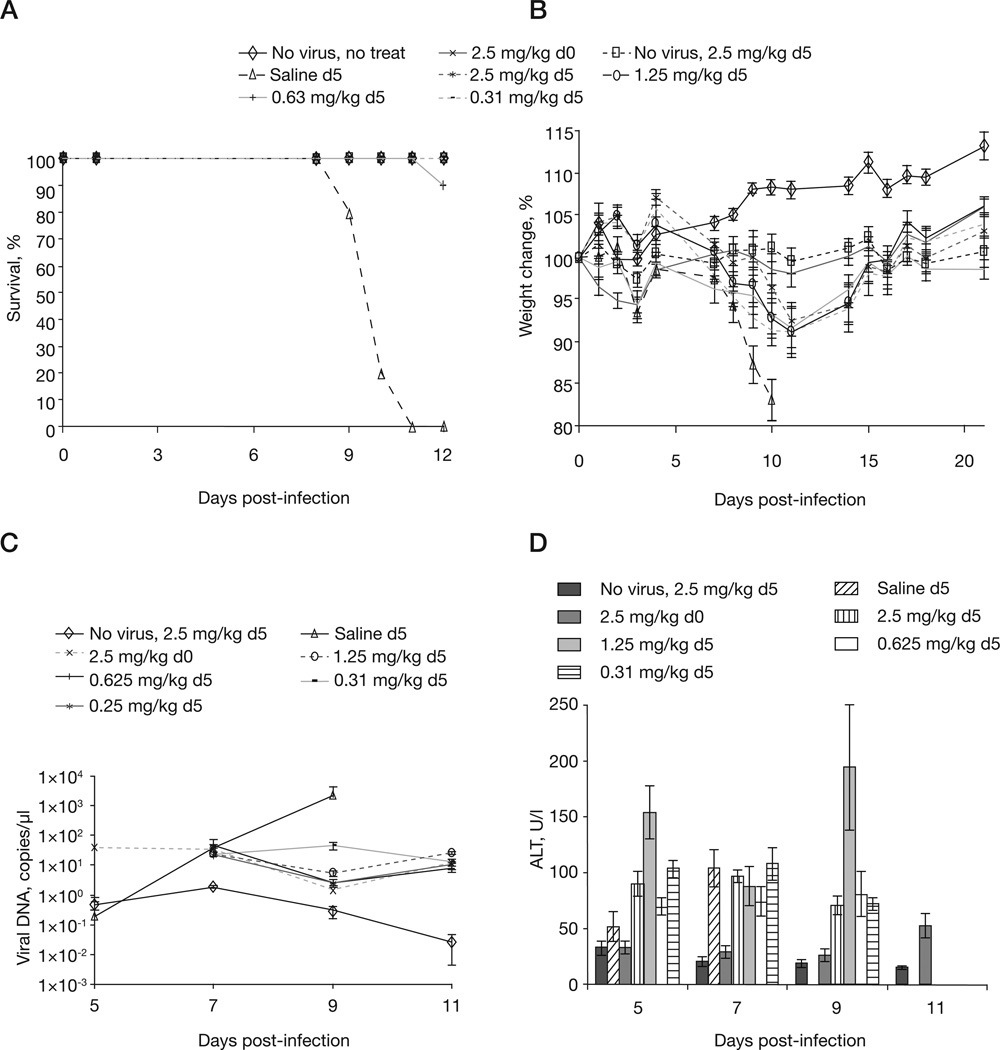
To determine the therapeutic value of the maintenance dose we treated 5 PFU infected mice with a standard 20 mg/kg loading dose on day 5 p.i. followed by one of four different maintenance dose regimens of 2.5 mg/ kg, 1.25 mg/kg, 0.63 mg/kg and 0.31 mg/kg. Data from Figures 3A and 3B show that the maintenance dose is of minimal significance to recovery; furthermore, the data shows that mice receiving the higher maintenance doses lose weight at the same rate as mice receiving the lower maintenance dose (Figure 3B). Surviving mice consistently had similar viral loads regardless of the maintenance dose (compare day 9 saline mean of 2,221.6 copies/µl with the mean from lowest maintenance dose of 0.31 mg/kg, which is 44.1 copies/µl, P=0.0088; Figure 3C). Untreated control mice (receiving saline) had a significant rise in ALT levels between days 5 and 7 (means 52 and 104, respectively, P=0.0407; Figure 3D). In treated groups, the level of ALT did not increase significantly (except for the 1.25 mg/kg group) between days 5 and 9, indicating that CMX001 provides protection against viral-induced liver damage and that the maintenance dose is of limited value. Thus, ALT can be used as a biomarker of disease progression from day 5 p.i. onwards. Taken together, these data indicate that a maintenance dose is of limited benefit when mice receive a loading dose of 20 mg/kg up to 5 days p.i. with 5 PFU.
Figure 3. Optimizing the maintenance dose of CMX001 to treat mousepox.
A/Ncr mice were intranasally infected with 5 plaque-forming units of ectromelia virus and treated with CMX001 at 5 days post-infection. The loading dose remained constant at 20 mg/kg at 5 days post-infection. The maintenance dose was administered every other day after the loading dose at 2.5, 1.25, 0.63 or 0.31 mg/kg. Control mice were either uninfected and untreated (No virus, no treat), treated with a 20 mg/kg loading dose on the day of infection followed by a 2.5 mg/kg maintenance dose every other day (2.5 mg/kg d0) or uninfected and treated with 20 mg/kg followed by a 2.5 mg/kg maintenance dose every other day (No virus, 2.5 mg/kg d5). Untreated infected mice were dosed with saline on day 5 (Saline d5) followed by every-other-day dosing of saline. Mice were monitored for (A) mortality and (B) weight loss for day 42 post-infection. (C) Viral DNA levels and (D) alanine aminotransferase (ALT) levels were monitored in blood samples taken at day 5, 7, 9 and 11 post-infection.
Intervention with a single dose of CMX001
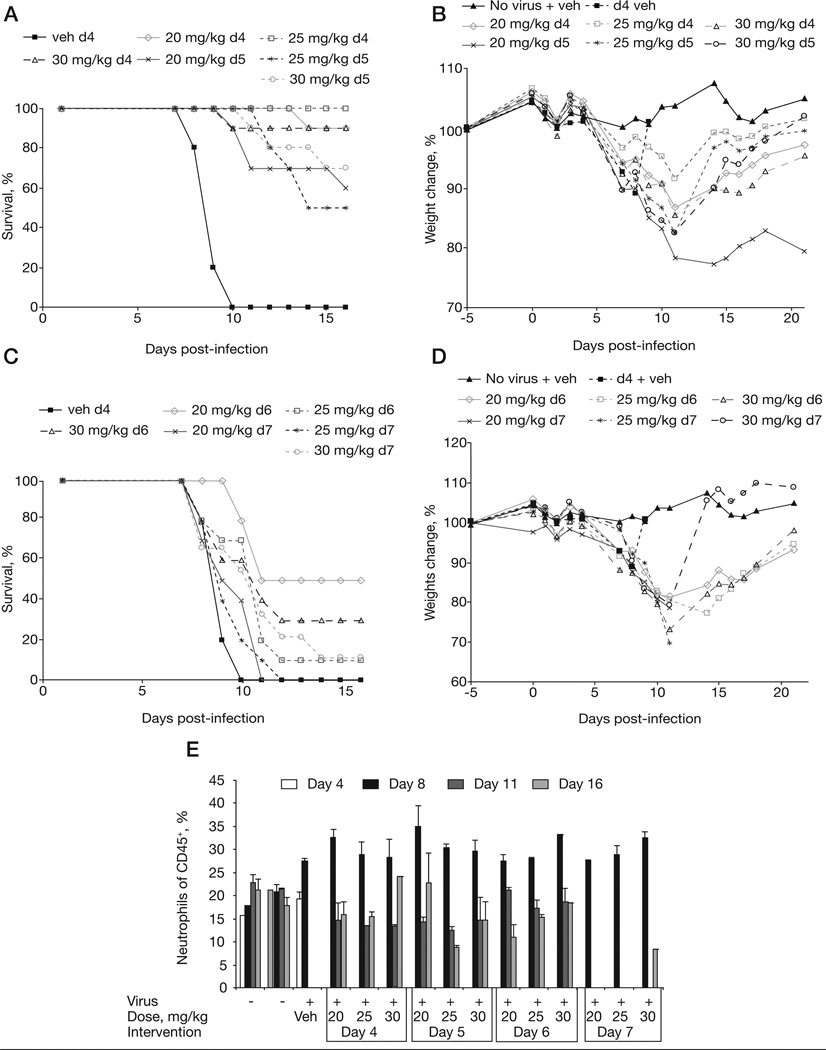
In light of the discovery that the maintenance dose appeared to be of little value, we investigated if mice could be protected with a single dose treatment. We infected mice with 20 PFU (40× LD50) of ECTV and intervened with CMX001 on days 4, 5, 6 or 7 p.i. Treatment doses of 20, 25 or 30 mg/kg were used. We found that when intervention was initiated on day 4 p.i., we could save ≥90% of animals with any of the three doses (Figures 4A & 4B). Delaying intervention until day 5 reduced the survival rate to 70, 50 and 60% when mice were treated with a single 30, 25 or 20 mg/kg dose, respectively. Mice receiving treatment on day 4 p.i. lost less weight between days 6 and 11 compared with mice treated on day 5 p.i. (Figure 4B). Intervening at days 5 and 6 p.i. resulted in ≤50% survival and significant weight loss (Figures 4C & 4D). We also observed that mice receiving treatment on day 4 p.i. lost more weight when treated with the highest dose of 30 mg/kg. This observation is consistent with previous experiments (not shown). Therefore, a dose between 20–25 mg/kg might be optimal from a weight loss perspective. These results suggest that a single dose of CMX001 is efficacious if intervention occurs within 4 days following an infection of ≤20 PFU.
Figure 4. Treatment of mousepox with a single dose of CMX001.
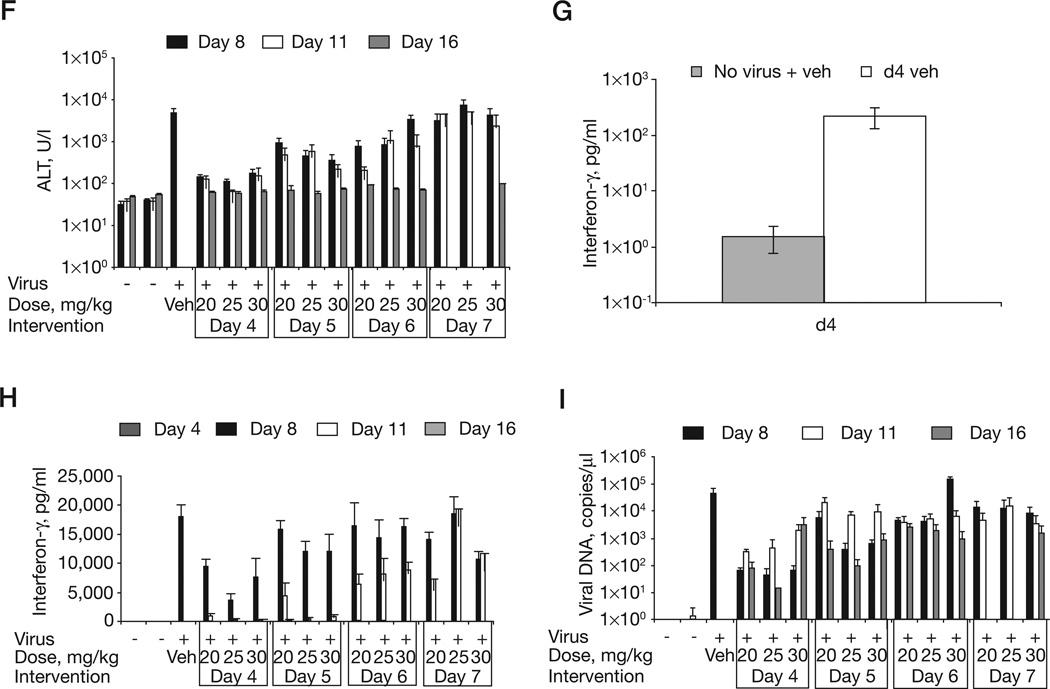
A/Ncr mice were intranasally infected with 20 plaque-forming units of ectromelia virus and dosed with a single CMX001 oral gavage at days 4, 5, 6, or 7 post-infection (indicated as d4, d5, d6 or d7). CMX001 was administered at a 20, 25 or 30 mg/kg dose on the indicated day. Infected control mice received a day 4 vehicle (d4 veh) gavage. Mice dosed on days 4, 5, 6 or 7 post-infection were monitored for (A & B) mortality and (C & D) weight loss. Blood was taken on days 4, 8, 11 and 16 post-infection and analysed for the (E) percentage of neutrophils, (F) levels of alanine aminotransferase (ALT) in the blood, (G) interferon-γ levels in the blood at day 4 post-infection and (H) days 8, 11 and 16 post-infection and (I) viral DNA copies/µl of blood.
We also examined levels of neutrophils, ALT and IFN-γ in the blood. At 4 days p.i. there was a modest increase in neutrophil levels in infected vehicle-treated mice as compared with vehicle-treated uninfected controls. As expected, neutrophilia was recorded at 8 days p.i. in infected-treated and infected-untreated mice (Figure 4E). At 8 days p.i., treated and untreated animals had similarly increased levels of neutrophils. Furthermore, surviving treated mice had similar (that is, normal) neutrophil levels at day 11 p.i., indicating that the level of neutrophilia is independent of the dose of CMX001. However, mice that were recovering from infection (that is, day 16 p.i.) had neutrophil levels approaching normal. Levels of ALT at 8 days p.i. in infected vehicle-treated mice were significantly increased compared with control mice and mice whose treatment was initiated on day 4 of intervention (at day 8 mean values of 4,935, 146, 112 and 169 U/l were recorded for vehicle 20 mg/kg, 25 mg/kg and 30 mg/kg treatment on day 4, P=0.0013, P=0.0013 and P=0.0014, respectively; Figure 4F). Mice whose treatment was initiated on day 4 p.i had ≥90% survival and maintained low levels of ALT even at days 11 and 16 p.i. Interestingly, all surviving mice had normal ALT levels by 16 days p.i. indicating that their livers had begun to recover from infection. ALT levels on day 8 were similar between infected controls and mice whose treatment was delayed until 7 days p.i., thus indicating that significant liver damage had already occurred before mice received any treatment. Mice whose treatment was initiated on day 5 p.i also had significantly lower ALT on day 8 p.i. compared with the control (day 5 intervention mean values of 922 U/l for 20 mg/kg treatment [P=0.0159], 460 U/l for 25 mg/kg [P=0.0065] and 359 U/l for 30 mg/kg treatment [P=0.0056]). Mice whose treatment was delayed until 6 days p.i. also had significantly lower ALT levels at 8 days p.i. when treated with the 20 and 25 mg/kg regimen, but not the 30 mg/kg regimen (means of 795 U/l [P=0.0142], 837 U/l [P=0.0319] and 3,344 U/l [P=0.5359]).
Monitoring the level of IFN-γ in the blood was introduced to provide a robust biomarker to stage disease at early time points [23]. Blood from non-infected animals typically have very little detectable IFN-γ (Figure 4G). However, as early as 4 days p.i. the level of IFN-γ increased by approximately 2 logs to 100 pg/ml (mean values of 1.54 and 216 for non-infected and infected controls, respectively, P=0.0218). The level of IFN-γ continued to increase to approximately 18,000 pg/ml by 8 days p.i. (Figure 4H). Animals with CMX001 intervention at 4 days p.i. had significantly reduced levels of IFN-γ in blood drawn at 8 days p.i. (day 4 mean values of 18,012 pg/ml for the infected control, 9,600 pg/ml for 20 mg/kg treatment [P=0.0022], 3,781 pg/ml for 25 mg/ kg treatment [P=0.001] and 7,681 pg/ml for 30 mg/kg treatment [P=0.014]). By 11 days p.i. IFN-γ levels had returned to almost normal in mice whose treatment began at 4 days p.i. (Figure 5H). But mice whose treatment was delayed until 6 and 7 days p.i. had increased IFN-γ levels until 11 days p.i. This data establishes IFN-γ levels as a robust biomarker of early infection.
Finally we analysed the number of viral genome copies in the blood (Figure 4I). Negative control mice had <2 copies/µl throughout the time course. The positive control group that was infected and dosed with vehicle had a mean of 44,451 copies/µl at day 8 p.i. Samples were not taken from vehicle controls at days 11 or 16 because of mortality. We found mice dosed at the earliest time point of 4 days p.i. had significantly reduced viral loads of <1×102 copies/µl at day 8 p.i. compared with the control (day 4 intervention mean values of 63.9 copies/µl for 20 mg/kg treatment [P=0.0897], 43 copies/µl for 25 mg/kg treatment [P=0.0617] and 65 copies/µl for 30 mg/kg treatment [P=0.0618]). The only treated group with 100% survival was the group that received 25 mg/kg group on day 4, this group also had the lowest viral load at day 8 and 16 p.i. and the second lowest at day 11 p.i. In most treated groups viral loads were highest at the 11 day p.i. time point, coinciding with the highest mortality rates detected (Figure 4A). Moreover, almost no mice were lost from day 15 onwards, suggesting that death occurs approximately when the highest viral load is recorded. Groups of mice whose treatment was delayed until days 6 or 7 days p.i. had difficulty in reducing their viral loads after day 8 p.i., except for mice receiving the highest dose of 30 mg/kg. This indicates that the higher dose is reducing the viral load to <1×103 by day 16, but is unable to prevent mortality in these mice. Notably, viral load at days 8 and 11 p.i. were not different for these mice.
Discussion
Eradication of smallpox at the end of the 20th century was facilitated by the availability of cheap but stable vaccines, a largely immobile population and the lack of an animal reservoir to support the variola virus. In the 21st century we are faced with the pros-pect of an increasing incidence of human monkeypox, an expanding host range for the monkeypox virus, and the use of natural or recombinant variola virus and/or monkeypox virus as biological weapons. The latter concern has been demonstrated in the mousepox model by the incorporation of interleukin-4 into ECTV, resulting in a dramatic increase in virulence [24]. As a result of the cessation of vaccination in the 1970s, the human population lacks solid herd immunity to orthopoxviruses. Furthermore, the traditional smallpox vaccine, which protects against all orthopoxviruses, is not suitable for a growing percentage of the world’s population. Intramuscular administration of vaccinia immunoglobulin, a product derived from the pooled plasma of vaccinated individuals, is indicated as a suitable treatment of generalized vaccinia, progressive vaccinia, eczema vaccinatum and certain autoinoculations [4]. However, the efficacy of vaccinia immunoglobulin has not been demonstrated in a controlled clinical trial and its efficacy is doubtful. Thus, there is a need for an antiviral that is efficacious during frank disease. None are currently licensed.
CDV has been shown to be efficacious against most double-stranded DNA viruses and is approved for the treatment of cytomegalovirus retinitis in HIV/AIDS patients [13]. CDV is the only drug available for the treatment of smallpox under an investigational new drug application. However, its use during a large outbreak would be limited due to the inherent require-ment for rigorous clinical management because of the intravenous mode of administration and nephrotoxicity. CMX001 addresses these limitations by mimicking the structure of lysophosphatidylcholine and its uptake pathways, thereby allowing oral drug delivery and reduced secretion into the kidneys [25]. CMX001 is currently in Phase I/II of clinical trials
The threat posed by the intentional release of variola or monkeypox virus and the epizoonosis of monkeypox virus will require a capacity to rapidly diagnose the disease and treat its progression with therapeutic antivirals. Intervention is likely to take place during the diagnosis of the primary (incident) cases after onset of disease at about 7–14 days p.i. [26]. To address this we have investigated several biomarkers of disease and evaluated their effectiveness in a lethal mousepox model. Traditionally, weight loss has been used to evaluate the progression of disease in mice with mousepox. However, weight loss is a trailing indication of disease and cannot accurately be used to stage early disease progression or the likelihood of survival. In mousepox, we found that following infection we could detect changes in the blood biomarkers of ECTV including viral DNA in plasma, neutrophilia, ALT, AST and IFN-γ. Of these biomarkers, IFN-γ was detectable the earliest (at day 4 p.i.); other experiments suggest that IFN-γ changes might be detectable as early as day 2 p.i. (not shown).
It is difficult to accurately correlate the biomarkers we have evaluated in mousepox with those of smallpox because very few blood chemistry and laboratory parameters are established for smallpox. The most complete analysis of blood chemistry from a human orthopoxvirus infection undoubtedly comes from patients infected in the Midwestern United States monkeypox virus outbreak of 2003. This outbreak revealed that 50 and 59% of analysed individuals had abnormal levels of AST and ALT, respectively. Only the frequency of abnormal blood urea nitrogen level was greater, with 61% of patients having abnormal levels [27]. Furthermore, it has previously been shown that infecting non-human primates intravenously with the variola virus induces a rapid increase in IFN-γ that is detectable from as early as 1 day p.i. [28]. These data are consistent with our observations in mousepox and suggests that the biomarkers we have evaluated will correlate with the staging of both smallpox and human monkeypox virus diseases.
The drawback with the above mentioned biomarkers is that they are indirect readouts of disease that are not specific to orthopoxvirus infection. Thus, in a clinical setting an individual would need to be confirmed to have a variola virus or monkeypox virus infection for these indirect biomarkers to be useful. PCR can be used to distinguish between 120 different orthopoxvirus strains [29,30] as well as ruling out other pox-forming infections, such as chickenpox. Furthermore, it is likely that viral DNA in the blood, or possibly saliva, can be detected several days before the initiation of a rash, meaning that the rash is likely to be most useful as a diagnostic criterion in the smallpox or monkeypox index cases. Another limitation is that none of the biomarkers detect local infection at the portal of entry. The site of inhalation and exhalation should be investigated further as a potential biomarker region.
We have shown, by using quantitative PCR, that in untreated mice the blood viral load increases until death. We have also shown that viral load levels can be significantly reduced when intervention with CMX001 is initiated early enough. Figure 4I indicates that viral loads are similar between groups that were treated on the same day, regardless of the dose of CMX001. This suggests that early intervention is more important than intervention with higher doses of CMX001.
We found that the optimal loading dose was between 20 and 25 mg/kg when mice were infected with 10 PFU of virus and intervention was initiated 5 days p.i. At this dose a maximum of 90% of treated animals survived. The 30 mg/kg dose does not have any superior protective ability over the lower doses and it is associated with greater weight loss. That said, the higher dose did reduce blood viral DNA load between day 8 and day 16 more than the other doses when initiation of treatment was delayed until day 6 (P=0.0179), but not day 7 (P=0.1734; Figure 4I). Perhaps intervention at later time points (days 6 or 7 p.i.) with the 30 mg/kg dose would be more efficacious if the natural disease course of mousepox was longer, such as in the case of smallpox. This could be evaluated by using a mouse strain that is less sensitive to ECTV infection, such as the C57BL/6 and SKH-1 strains, which die 3–4 days later than A/Ncr mice when given a lethal innoculum. Alternatively, the lack of a detectable increase in efficacy with a 30 mg/ kg dose could be because of a saturation of the drug uptake and anabolic pathways responsible for generating CDV diphosphate in pertinent cells.
There is a great deal of evidence to suggest that a very small number of virions are capable of initiating infection. For example, low levels of infectivity were detected in oropharyngeal secretions of infectious smallpox patients and virus was virtually undetectable in the air of smallpox wards unless measured within a short distance of the patient’s mouth [31,32]. These observations are consistent to those of vaccinia and rabbitpox infections in rabbits [33]. In other experiments (not shown) we discovered that all mice can be saved when a realistic inoculum of 1–2 PFU (2–4× LD50) is used and intervention with approximately 20 mg/kg dose occurs relatively early. At a higher inoculum of 20 PFU (40× LD50; Figure 4A) a single 20 mg/kg dose only provides protection for 60% of the mice when treatment initiation is delayed until 5 days p.i., but protects 90% when administered at day 4 p.i. These observations confirm that the efficacy of CMX001 is a direct function of the infectious dose [8].
CMX001 shows promise as an oral antiviral to treat poxvirus disease. Another orally administered antiviral, ST-246, is in phase II clinical trials and is also highly efficacious against poxviruses and CDV-resistant poxviruses. Combination therapy with ST-246 and CMX001 is synergistic and this suggests combination therapy may retard the resistance to each drug, reduce the inherent efficacy variation in the outbred human population, permit the conservation of drug stocks and provide a more potent therapy for late stage disease [34].
Acknowledgements
This work was supported by a subcontract with Chimerix Inc., an NIAID grant NOI-AI-15436 and U54-AI-057160 from the NIAID to the Midwestern Regional Center of Excellence for Biodefense and Emerging Infectious Diseases. The CMX001 analogue of CDV was a gift from Chimerix Inc.
Footnotes
Disclosure statement
GP is president and CEO of Chimerix Inc. All other authors declare no competing interests.
References
- 1.Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Handley LM, Mackey JP, Buller RML, Bellone CJ. Orthopoxvirus vaccines and vaccination. In: Mercer AA, Schmid A, Weber O, editors. Poxviruses. Basel: Birkhauser; 2007. pp. 329–353. [Google Scholar]
- 3.Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57:13–23. doi: 10.1016/S0166-3542(02)00196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101–114. doi: 10.1016/s0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 5.Diepgen TL, Fartasch M. Recent epidemiological and genetic studies in atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1992;176:13–18. [PubMed] [Google Scholar]
- 6.Rosenthal SR, Merchlinsky M, Kleppinger C, Goldenthal KL. Developing new smallpox vaccines. Emerg Infect Dis. 2001;7:920–926. doi: 10.3201/eid0706.010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern ER, Hartline C, Harden E, et al. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob Agents Chemother. 2002;46:991–995. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker S, Touchette E, Oberle C, et al. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antiviral Res. 2008;77:39–49. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quenelle DC, Collins DJ, Wan WB, Beadle JR, Hostetler KY, Kern ER. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004;48:404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smee DF, Wong MH, Bailey KW, Beadle JR, Hostetler KY, Sidwell RW. Effects of four antiviral substances on lethal vaccinia virus (IHD strain) respiratory infections in mice. Int J Antimicrob Agents. 2004;23:430–437. doi: 10.1016/j.ijantimicag.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Esteban DJ, Buller RM. Ectromelia virus: the causative agent of mousepox. J Gen Virol. 2005;86:2645–2659. doi: 10.1099/vir.0.81090-0. [DOI] [PubMed] [Google Scholar]
- 12.De Clercq E. Therapeutic potential of cidofovir (HPMPC, Vistide) for the treatment of dna virus (that is, herpes-, papova-, pox- and adenovirus) infections. Verh K Acad Geneeskd Belg. 1996;58:19–47. [PubMed] [Google Scholar]
- 13.Safrin S, Cherrington J, Jaffe HS. Clinical uses of cidofovir. Rev Med Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Buller RM, Owens G, Schriewer J, Melman L, Beadle JR, Hostetler KY. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318:474–481. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Keith KA, Wan WB, Ciesla SL, Beadle JR, Hostetler KY, Kern ER. Inhibitory activity of alkoxyalkyl and alkyl esters of cidofovir and cyclic cidofovir against orthopoxvirus replication in vitro . Antimicrob Agents Chemother. 2004;48:1869–1871. doi: 10.1128/AAC.48.5.1869-1871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciesla SL, Trahan J, Wan WB, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59:163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Drillien R, Spehner D, Buller RM. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 1992;187:433–442. doi: 10.1016/0042-6822(92)90445-u. [DOI] [PubMed] [Google Scholar]
- 18.Moss B, Earl PL. Current Protocols in Molecular Biology. Wiley; 1998. Expression of proteins in mammalian cells using vaccinia virus vectors. Overview of the vaccinia virus expression system; pp. 16.15.1–16.15.5. [Google Scholar]
- 19.Wallace GD, Buller RM. Kinetics of ectromelia virus (mousepox) transmission and clinical response in C57BL/6j, BALB/CByj and AKR/J inbred mice. Lab Anim Sci. 1985;35:41–46. [PubMed] [Google Scholar]
- 20.Buller RL, Handley L, Parker S. Development of prophylactics and therapeutics against the smallpox and monkeypox biothreat agents. NIAD/NIH. Humana Press; 2008. [Google Scholar]
- 21.Gronvall GK, Trent D, Borio L, Brey R, Nagao L. The FDA animal efficacy rule and biodefense. Nat Biotechnol. 2007;25:1084–1087. doi: 10.1038/nbt1007-1084. [DOI] [PubMed] [Google Scholar]
- 22.Roberts R, Styrt B, McCune S. FDA perspective on antivirals against biothreats: communicate early and often. Antiviral Res. 2007;78:60–63. doi: 10.1016/j.antiviral.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Atrasheuskaya AV, Bukin EK, Fredeking TM, Ignatyev GM. Protective effect of exogenous recombinant mouse interferon-gamma and tumour necrosis factor-alpha on ectromelia virus infection in susceptible BALB/c mice. Clin Exp Immunol. 2004;136:207–214. doi: 10.1111/j.1365-2249.2004.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, Ramshaw IA. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol. 2001;75:1205–1210. doi: 10.1128/JVI.75.3.1205-1210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciesla SL, Trahan J, Wan WB, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59:163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 26.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organisation; 1988. [Google Scholar]
- 27.Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 28.Jahrling PB, Hensley LE, Martinez MJ, et al. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc Natl Acad Sci U S A. 2004;101:15196–15200. doi: 10.1073/pnas.0405954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Ropp SL, Zhao H, Damon IK, Esposito JJ. Orthopoxvirus pan-genomic DNA Assay. J Virol Methods. 2007;141:154–165. doi: 10.1016/j.jviromet.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Olson VA, Laue T, Laker MT, et al. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–1946. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downie AW, Meiklejohn M, St Vincent L, Rao AR, Sundara Babu BV, Kempe CH. The recovery of smallpox virus from patients and their environment in a smallpox hospital. Bull World Health Organ. 1965;33:615–622. [PMC free article] [PubMed] [Google Scholar]
- 32.Meiklejohn G, Kempe CH, Downie AW, Berge TO, St Vincent L, Rao AR. Air sampling to recover variola virus in the environment of a smallpox hospital. Bull World Health Organ. 1961;25:63–67. [PMC free article] [PubMed] [Google Scholar]
- 33.Westwood JC, Boulter EA, Bowen ET, Maber HB. Experimental respiratory infection with poxviruses. I. Clinical virological and epidemiological studies. Br J Exp Pathol. 1966;47:453–465. [PMC free article] [PubMed] [Google Scholar]
- 34.Quenelle DC, Prichard MN, Keith KA, et al. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007;51:4118–4124. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]