Abstract
The present study was carried out to examine salt-induced modulation in growth, photosynthetic characteristics and antioxidant system in two cultivars of Brassica juncea Czern and Coss varieties (Varuna and RH-30). The surface sterilized seeds of these varieties were sown in the soil amended with different levels (2.8, 4.2 or 5.6 dsm−1) of sodium chloride under a simple randomized block design. The salt treatment significantly decreased growth, net photosynthetic rate and its related attributes, chlorophyll fluorescence, SPAD value of chlorophyll, leaf carbonic anhydrase activity and leaf water potential, whereas electrolyte leakage, proline content, and activity of catalase, peroxidase and superoxide dismutase enzymes increased in both the varieties at 30 d stage of growth. The variety Varuna was found more resistant than RH-30 to the salt stress and possessed higher values for growth, photosynthetic attributes and antioxidant enzymes. Out of the graded concentrations (2.8, 4.2 or 5.6 dsm−1) of sodium chloride, 2.8 sm−1 was least toxic and 5.6 dsm−1 was most harmful. The variation in the responses of these two varieties to salt stress is attributed to their differential photosynthetic traits, SPAD chlorophyll value and antioxidant capacity, which can be used as potential markers for screening mustard plants for salt tolerance.
Keywords: Antioxidant system, Leaf water potential, Photosynthesis, Salt stress
1. Introduction
Plants throughout their life cycle experience various types of environmental stresses (such as drought, salinity, high temperature, cold, heavy metal and other similar stresses) due to their sessile nature. Among these stresses, salinity stress has become the limiting factor for the productivity of agricultural crops by affecting germination, plant vigor and finally crop yield (Munns and Tester, 2008; Zhang et al., 2011). There are various effects of salinity stress on plants such as ion toxicity, water stress, oxidative stress, nutritional imbalances, alterations in metabolic processes, disorganization of membranes, reduction in division and expansion of cells, and genotoxicity (Hasegawa et al., 2000; Zhu, 2007). These adverse effects collectively lead to the reduction in plant growth, development and finally biological yield.
During the onset and development of salt stress within the plant, all major processes such as photosynthesis, protein synthesis and lipid metabolism are affected (Parida and Das, 2005; Hasanuzzaman et al., 2012; Rahdari et al., 2012). Photosynthesis is severely affected during salinity stress which is mediated through a decrease in stomatal conductance (Parida et al., 2004; Yan et al., 2012), internal CO2 partial pressure and gaseous exchange through stomata (Iyengar and Reddy, 1996). The decrease in photosynthesis under saline conditions is considered as one of the most important factors restricting plant growth and productivity (Manikandam and Desingh, 2009). Salinity reduces plant productivity first by reducing plant growth during the phase of osmotic stress and subsequently by inducing leaf senescence during the phase of toxicity when excessive salt is accumulated in transpiring leaves (Munns, 2002). Many plants have evolved various mechanisms either to exclude salt from their cells or to tolerate its presence within the cells. They counteract the toxic effects of the stress through the production of osmolytes or by an increasing activity of antioxidant enzymes (Ashraf and Foolad, 2007; Ahmad et al., 2010). Antioxidant enzymes such as superoxide dismutase, peroxidase and catalase etc. help plants to withstand harmful effects of the environmental stress. In plants, superoxide dismutase scavenges superoxide anions (O2−) and converts them to hydrogen peroxides (Alscher et al., 2003). Catalase, the second line of defense, converts these lethal hydrogen peroxides to water and molecular oxygen. The effectiveness of oxidative defense system in plants can be measured by the activity of antioxidant enzymes and level of non-enzymatic antioxidants such as proline (Geebelen et al., 2002; Ahmad et al., 2012).
Brassica juncea (L.) Czern and Coss is an important oil-seed crop, which often experiences saline stress as it is grown extensively in the arid and semi-arid regions of the world (Singh et al., 2001). India ranks second in the world with regard to the production of Brassicas (Afroz et al., 2005) and supplies nearly 7% of the world’s edible oil (Khan et al., 2002). A number of biotic and abiotic stresses contribute to yield losses and this low economic yield is related to the crop’s susceptibility. There is a greater need to improve crop plants for salinity tolerance, however Brassica has a considerable potential to grow in salt-affected areas. One of the approaches is the improvement of salt tolerance of the cultivated species. The identification of tolerant genotype provides an initial germplasm base for breeding salt-tolerant crops. The present study was therefore carried out to examine the salt-induced modulation in growth, photosynthetic characteristics, chlorophyll pigments, leaf fluorescence, antioxidant enzymes and levels of non-enzymatic antioxidants in two varieties of Brassica i.e. Varuna and RH-30. Such studies will facilitate the evaluation of the relative performance of varieties and characterization of mechanism of salt tolerance which in turn will be helpful in effective breeding for salt tolerance.
2. Material and methods
2.1. Plant material and treatment
The authentic and healthy seeds of B. juncea (L.) Czern and Coss cv. Varuna and RH-30 were procured from National Seed Corporation Ltd. New Delhi, India. Before sowing the seeds were surface sterilized with 0.01% mercuric chloride solution followed by rinsing with sterilized, double distilled water (DDW), at least thrice, to remove the traces of adhered mercuric chloride to the seed surface. The surface sterilized seeds of these two cultivars were sown in earthen pots (25 × 25 cm) containing soil amended with different levels (2.8, 4.2 or 5.6 dsm−1) of NaCl. Pots were amended with the recommended dose of fertilizers (nitrogen from urea, single superphosphate, and muriate of potash) added at rates of 40, 138 and 26 mgkg−1 of soil, respectively at the time of sowing. Thinning was done on the 7th day after sowing (DAS), leaving three plants per pot. Each treatment was represented by five pots. Irrigation was done with tap water as and when required. The plants were up-rooted at 30 DAS to assess the following parameters. The remaining plants were allowed to grow up to maturity and were harvested at 120 DAS to study the yield characteristics.
2.2. Growth parameters
The plants were removed along with soil at 30 DAS and dipped in water to dislodge the adhering soil particles without injuring the roots. The length of the root and shoot was measured on a meter scale. The roots were then separated from the shoot and blotted. The roots and shoot were weighed separately to record their fresh mass and placed in an oven (80 °C for 72 h). The samples were weighed again to record the respective dry mass. Leaf area was ascertained by gravimetric method by tracing the outline of the leaf on graph sheet and counting the squares covered by it on graph paper.
2.3. Leaf water potential and electrolyte leakage
The leaf water potential (LWP) was measured by Psypro water potential system (Wescor Inc. USA). Total inorganic ions leaked out of the leaf were quantified by the method described by Sullivan and Ross (1979). Twenty leaf discs were taken in a boiling test tube containing 10 mL of DDW, and electrical conductivity was measured (ECa). The tubes were heated at 45 °C and 55 °C for 30 min in water bath, and electrical conductivity was measured (ECb). The contents were again boiled at 100 °C for 10 min, and electrical conductivity was recorded (ECc). The electrolyte leakage was calculated using the formula:
2.4. Carbonic anhydrase (CA) activity
The activity of CA was determined following the procedure described by (Dwivedi and Randhawav, 1974). The leaf samples were cut into small pieces and suspended in cysteine hydrochloride solution. The samples were incubated at 4 °C for 20 min and then filtered. The filtrate was transferred to the test tubes, containing phosphate buffer (pH 6.8) followed by the addition of alkaline bicarbonate solution and bromothymol blue. The samples were incubated at 4 °C for 20 min. The reaction mixture was titrated against 0.05 N HCl after the addition of 0.2 mL of methyl red indicator.
2.5. SPAD value of chlorophyll and photosynthetic attributes
SPAD chlorophyll meter (Minolta 502) was used to assess the SPAD value of chlorophyll in the intact leaves. The photosynthetic attributes [net photosynthetic rate (PN), stomatal conductance (gs), internal CO2 concentration (Ci), and transpiration rate (E)] were measured by using portable photosynthetic system (LICOR-6400, Lincoln, NE, USA). These measurements were recorded on the uppermost fully expanded leaf of the main branch between 11:00 and 13:00 h, under bright sunlight. The atmospheric conditions during measurements were: photosynthetically active radiation 1016 ± 6 μmol m−2 s−1, relative air humidity 60 ± 3%, atmospheric temperature 22 ± 1 °C, and atmospheric CO2 360 μmol mol−1. The ratio of atmospheric CO2 to intercellular CO2 concentration was constant.
2.6. Maximum quantum yield of PSII
The maximum quantum yield of PSII (Fv/Fm) was measured on the adaxial surface of the intact leaf using portable photosynthesis system (LICOR-6400, Lincoln NE, USA). Prior to measurements, plants were left for 30 min in dark at room temperature. The chlorophyll molecules were excited for 10 s by actinic light with a photon flux density of 40 μmol m−2 s−1.
2.7. Antioxidant enzymes
Fresh leaves (0.5 g) were homogenized with 5 mL of 50 mM phosphate buffer (pH 7.0) containing 1% PVP (polyvinylpyrrolidone). The homogenate was centrifuged at 10080g for 10 min. The supernatant was collected and used as a source for enzyme assay. This whole extraction process was carried out at 4 °C. The assay of peroxidase (POX) and catalase (CAT) was done by adapting the method of Chance and Maehly (1956). Activity of CAT was measured by titrating the reaction mixture [phosphate buffer (pH 6.8), 0.1 M H2O2, enzyme extract and 2% H2SO4] against 0.1 N KMnO4. The activity of POX was measured by observing the change in the absorbance of reaction mixture [pyragallol phosphate buffer (pH 6.8), 1% H2O2 and enzyme extract], due to catalytic conversion of pyragallol to purpurogallin at an interval of 20 s for 2 min at 420 nm. A control set was prepared by using DDW instead of enzyme extract.
The activity of superoxide dismutase (SOD) was assayed by measuring its ability to inhibit the photochemical reduction of NBT by using the method of Beauchamp and Fridovich (1971). The reaction mixture [50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 μM nitroblue tetrazolium (NBT), 2 μM riboflavin, 0.1 mM EDTA and 0–50 μl enzyme extract] in tubes was placed under 15 W fluorescent lamps for starting the reaction. After 10 min, the reaction was stopped by switching off the light. Non-illuminated reaction mixture was used as a blank. The absorbance was measured at 560 nm and the SOD activity was expressed as unit g−1 fresh mass. One unit of SOD activity was defined as the amount of enzyme that inhibited 50% of NBT photo-reduction.
2.8. Leaf proline content
The proline content in fresh leaf was determined by the method given by Bates et al. (1973). The samples were extracted in sulphosalicylic acid. To the extract, an equal volume (2 mL) of glacial acetic acid and ninhydrin solutions was added. The sample was heated at 100 °C, to which 5 mL of toluene was added after cooling in ice bath. The absorbance by toluene layer was read at 528 nm, on a spectrophotometer (Spectronic-20D, Milton Roy, USA).
2.9. Statistical analysis
Treatment means were compared by the analysis of variance using SPSS (SPSS ver. 17, Chicago, United States). Least Significant Difference (LSD) was calculated at the 5% level of probability. Standard error between the replicates was also calculated.
3. Results
3.1. Growth characteristics
The length, fresh and dry mass of the shoot and root of both the varieties showed a marked decrease on being subjected to different levels of NaCl (2.8, 4.2 or 5.6 dsm−1), applied through soil (Figs. 1a–f). Out of the different levels of NaCl, lowest concentration (2.8 dsm−1) proved least toxic. However, the highest concentration of NaCl (5.6 dsm−1) generated severe damage and caused maximum per cent decrease in the shoot length of Varuna and RH-30 (39.3% and 47.1%) and that of root length by 55.0% and 61.2%, compared with their respective controls. Moreover, NaCl (5.6 dsm−1) also caused a maximum decrease (47.0% and 58.0%, compared with the controls) in the leaf area of Varuna and RH-30 (Fig. 2a).The damage was more prominent in RH-30 than in Varuna.
Figure 1.
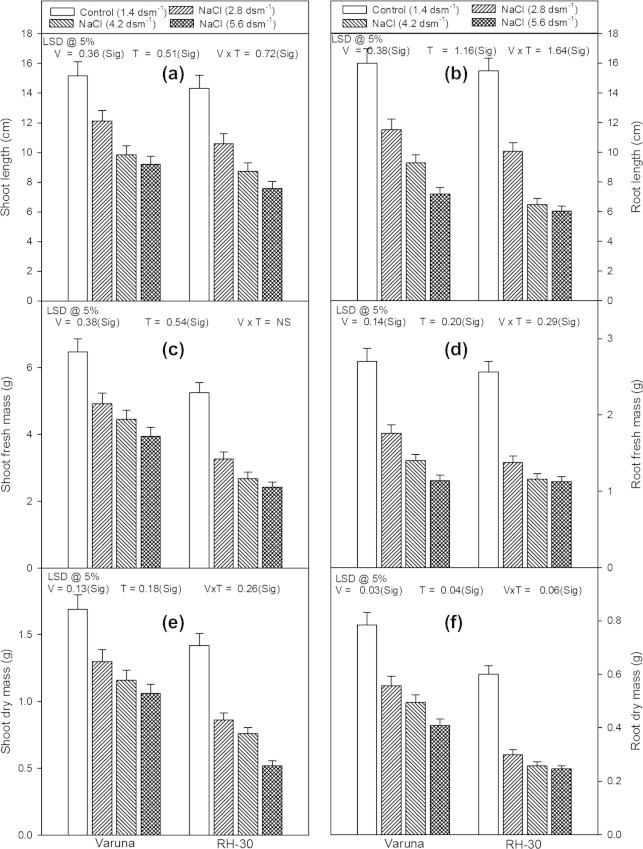
Effect of soil applied sodium chloride (NaCl; 2.8, 4.2 or 5.6 dsm−1) on length (cm), fresh mass (g) and dry mass (g) of shoot and root in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern and Coss at 30 DAS.
Figure 2.
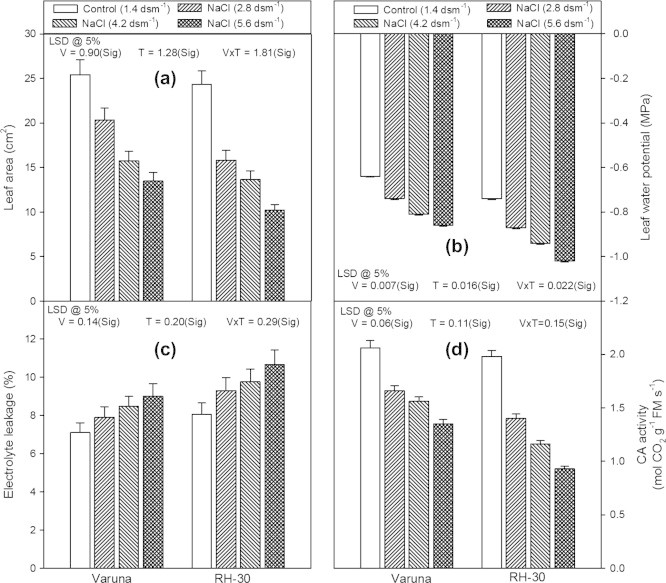
Effect of soil applied sodium chloride (NaCl; 2.8, 4.2 or 5.6 dsm−1) on (a) leaf area, (b) Leaf water potential, (c) electrolyte leakage, and (d) carbonic anhydrase (CA) activity in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern and Coss at 30 DAS.
3.2. Leaf water potential and electrolyte leakage
The variety RH-30 had lower values for leaf water potential than Varuna (Fig. 2b). The presence of NaCl in the soil decreased these values where the maximum loss was observed at 5.6 dsm−1 which was 34.3% and 37.8% less than the controls in Varuna and RH-30, respectively. However, the presence of NaCl in the soil caused a significant increase in the electrolyte leakage in both the varieties (Fig. 2c). The leakage increased as the concentration of NaCl was increased. The highest level (5.6 dsm−1) of NaCl increased the electrolyte leakage by 26.7% and 32.3% in Varuna and RH-30, respectively, as compared to their controls.
3.3. Carbonic anhydrase (CA) activity
Plants of both the varieties raised in the soil amended with different levels of NaCl (2.8, 4.2 or 5.6 dsm−1) possessed a lower activity of CA enzyme in comparison to their controls (Fig. 2d). The decrease was proportional to the soil NaCl level and therefore the highest salt concentration was most toxic and caused maximum inhibition (42.0% less than the control) in variety RH-30. The loss in the activity of the enzyme was more prominent in variety RH-30 than Varuna, at all the concentrations of the NaCl.
3.4. SPAD value of chlorophyll
The plants grown in the soil amended with varied concentrations of NaCl possessed significantly lower SPAD values of chlorophyll than unstressed control plants (Fig. 3a). Out of the NaCl concentrations 5.6 dsm−1 was most toxic and decreased the values by 41.9% and 50.0% in Varuna and RH-30 respectively, compared to the respective controls. RH-30 was more prone than Varuna to salt stress.
Figure 3.
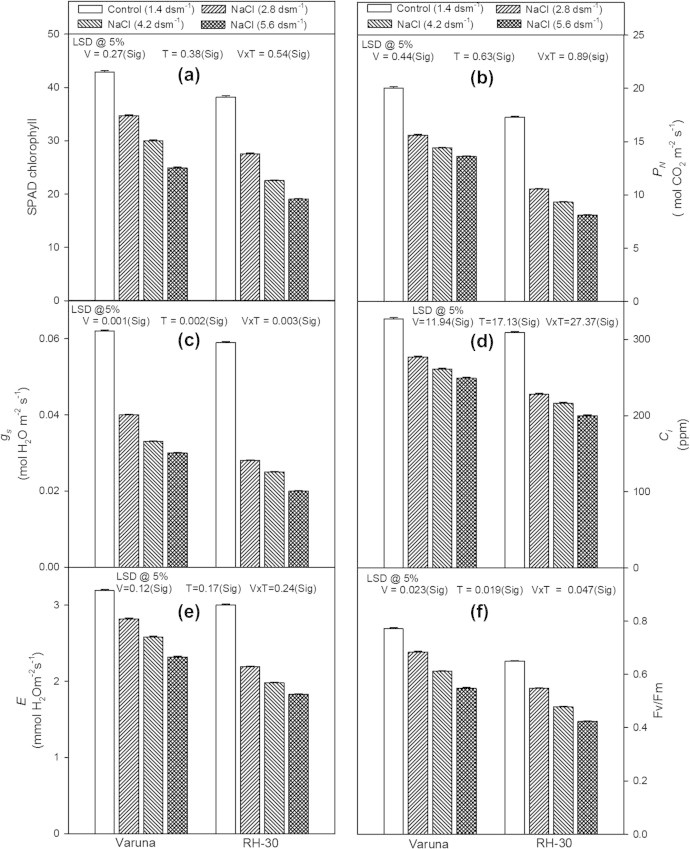
Effect of soil applied sodium chloride (NaCl; 2.8, 4.2 or 5.6 dsm−1) on (a) SPAD chlorophyll, (b) Net photosynthetic rate (PN), (c) stomatal conductance (gs), (d) internal CO2 concentration (Ci), (e) transpiration rate (E), and (f) maximum quantum yield of PSII (Fv/Fm) in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern and Coss at 30 DAS.
3.5. Photosynthetic attributes
The plants raised from the seeds sown in the soil fed with different levels (2.8, 4.2 or 5.6 dsm−1) of NaCl showed significant decrease in the net photosynthetic rate (PN) and its related attributes [stomatal conductance (gs), internal CO2 concentration (Ci) and transpiration rate (E)] in Varuna and RH-30 (Figs. 3b–e). The decrease was proportionate to the concentrations of the NaCl. The 5.6 dsm−1 of NaCl decreased PN, gs, Ci and E by 32.0%, 51.6%, 23.9% and 27.2% in Varuna and 53.0%, 66.1%, 35.3% and 39.0% in RH-30, respectively when compared to their control plants. Moreover, the variety RH-30 was more sensitive to salt stress than Varuna.
3.6. Maximum quantum yield of PSII
As depicted in Fig. 3f, the maximum quantum yield of PSII (Fv/Fm) showed a linear decrease with the increase in the concentration of NaCl in both the varieties (Varuna and RH-30). The maximum loss was recorded at the highest concentration (5.6 dsm−1) which was 28.9% and 34.8% in Varuna in RH-30 respectively, compared to their controls. The variety Varuna possessed higher values for Fv/Fm than RH-30.
3.7. Antioxidant enzyme activities
Unlike the other parameters, the activity of antioxidant enzymes [catalase (CAT), peroxidase (POX) and superoxide dismutase (SOD)] showed completely different response (Figs. 4a–c). The data revealed that the antioxidant enzyme activity increased in response to the concentrations of NaCl in the soil in both the varieties (Varuna and RH-30). The plants raised in the soil amended with the highest NaCl level (5.6 dsm−1) possessed maximum values for antioxidant enzymes in both the varieties. The values for CAT, POX and SOD activity increased by 39.8%, 57.0% and 96.8% in Varuna and 22.9%, 34.9% and 80.6% in RH-30, respectively compared to their respective control plants.
Figure 4.
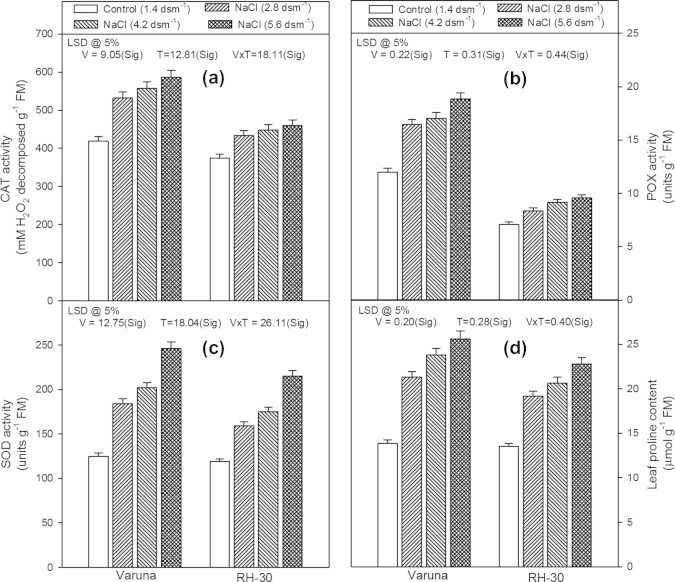
Effect of soil applied sodium chloride (NaCl; 2.8, 4.2 or 5.6 dsm−1) on (a) catalase (CAT) activity, (b) peroxidase (POX) activity, (c) superoxide dismutase (SOD) activity, and (d) leaf proline content in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern and Coss at 30 DAS.
3.8. Leaf proline content
The leaf proline content was higher in the plants that were fed with NaCl (Fig. 4d). The values increased with an increase in the concentration of the salt. Maximum values were found in the plants which were fed with 5.6 dsm−1 of NaCl through the soil in both the varieties and the increase was 84.9% and 68.9% in Varuna and RH-30, respectively, over the respective controls.
3.9. Yield characteristics
Yield characteristics (number of pods per plant, number of seeds per pod, 100 seed mass and seed yield per plant) were significantly affected and exhibited a linear decrease in their values in response to the NaCl present in the soil in both the varieties (Figs. 5a–d). The maximum reduction in the values of all the above yield characteristics was noticed in RH-30 at the highest level of NaCl (5.6 dsm−1). The number of pods per plant, number of seeds per pod, 100 seed mass and seed yield decreased by 25.0%, 17.9%, 10.3% and 34.0% in Varuna and 30.3%, 33.0%, 10.2% and 46.9% in RH-30 at 5.6 dsm−1 of NaCl respectively, as compared to their control plants.
Figure 5.
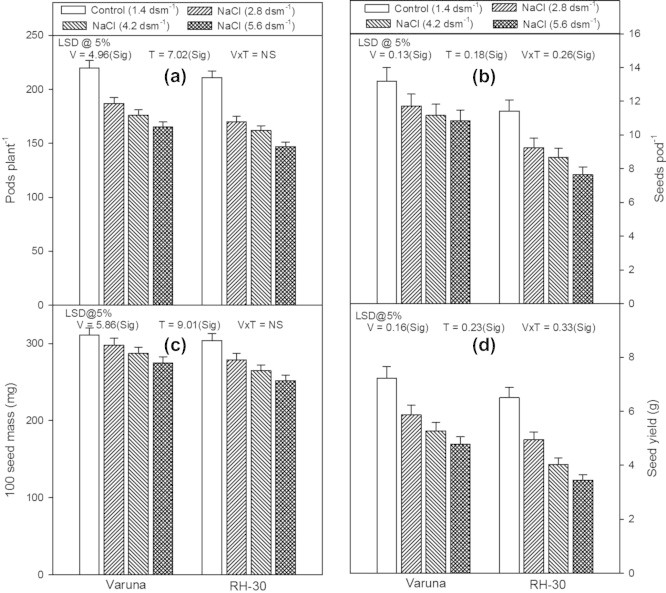
Effect of soil applied sodium chloride (NaCl; 2.8, 4.2 or 5.6 dsm−1) on (a) pods plant−1, (b) seeds pod−1, (c) 100 seed mass and (d) seed yield in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern and Coss at harvest (120 DAS).
4. Discussion
Salt stress (2.8, 4.2 or 5.6 dsm−1 of NaCl added to the soil) considerably decreased growth of the plants in both the varieties (Varuna and RH-30) as reflected by reduced length, fresh mass and dry mass of roots and shoot and leaf area (Figs. 1a–f and 2a). These observations are in conformity with tomato (Hayat et al., 2010a), sunflower (Akram and Ashraf, 2011), mulberry (Ahmad and Sharma, 2010), okra (Saleem et al., 2011), mustard (Hayat et al., 2011) and proso millet (Sabir et al., 2011). Out of the two cultivars, as also reported by Hayat et al. (2011), Varuna is likely more salt tolerant. This varied growth response of the two varieties of mustard could possibly be due to differential regulation of the processes related to growth at their genetical, biochemical and physiological levels. The salt stress is known to cause reduction in cell division and elongation (Pitann et al., 2009) which is mainly due to salt induced alterations in the nutrient uptake, induced formation of reactive oxygen species (Ashraf, 2009), inhibition of cytoplasmic enzymes, turgor loss (Pitann et al., 2009) and hormonal imbalance (Ashraf et al., 2010) which will naturally impair plant growth and finally the yield (Fig. 5a–d).
The SPAD value of chlorophyll decreased significantly in the stressed leaves of plants of both the varieties (Fig. 3a). The reason behind this loss is that the salinity either inhibits synthesis and/or accelerates the degradation of existing chlorophyll molecules (Iyengar and Reddy, 1996). These results are in conformity with Hayat et al. (2011), Ahmad et al. (2012), Ghogdi et al. (2012) and Heidari (2012).
Leaf carbonic anhydrase (CA) enzyme catalyzes the reversible hydration of CO2 and maintains its constant supply to Rubisco, at the level of the grana of the chloroplast (Price et al., 1994). In the present study the CA activity decreased as the NaCl concentration increased (Fig 2d). Since NaCl inhibits the activity of the key enzymes (Rubisco and PEP carboxylase) of photosynthesis (Soussi et al., 1998), decrease in the activity of CA could be due to similar reasons. Moreover, NaCl induced regulation of genes for CA synthesis (Liu et al., 2012) could be another reason for the observed decrease in the activity of enzyme. The decrease in CA activity is further corroborated by the findings of Hayat et al. (2011), Idrees et al. (2012) and Liu et al. (2012).
Plants exposed to the increasing levels of NaCl in the soil showed a diminished net photosynthetic rate (PN) accomplished by a significant decrease in the stomatal conductance (gs), internal CO2 concentration (Ci) and transpiration rate (E) (Figs. 3b–e). The reduction in the level of photosynthetic capacity under salinity might be largely due to the stomatal closure, brought about by salt-induced ABA accumulation, which will limit automatically the photosynthetic CO2 assimilation (Saleem et al., 2011). The poor PN values, under salt stress, were noted to be positively related to the observed decrease in gs and Ci (Lu et al., 2009). Speeding up of the senescence of plant organs and shift in the activity of enzymes induced by the modifications in cytoplasmic structures and negative feedback of diminished sink activity associated with slow transport of photosynthates are other possible reasons for the salinity induced decrease in PN and its related attributes (Iyengar and Reddy, 1996). The observed decrease in SPAD value of chlorophyll (Fig. 3a) and CA activity (Fig. 2d) are also the other reasons for low PN in salt stressed plants. Similar observations have also been made by others (Ahmad et al., 2012; Eisa et al., 2012; Wang et al., 2012; Wu et al., 2012).
The NaCl decreases the photochemical efficiency which has been ascribed with the suppression of PSII activity (Mehta et al., 2010). In the present study, it has been observed that NaCl applied through the soil caused a significant reduction in the values of quantum yield of PSII (Fv/Fm) (Fig. 3f) suggesting the salt stress induced perturbations in electron transport of PSII (Megdiche et al., 2008). Salinity blocks the electron transfer from the primary acceptor, plastoquinone (QA) to the secondary acceptor, plastoquinone (QB) at the acceptor side of PSII which leads to the decrease in Fv/Fm (Shu et al., 2012) These results are in conformity with the studies in Triticum aestivum (Kanwal et al., 2011), Vigna radiata (Hayat et al., 2010b), Brassica napus (Naeem et al., 2010), Solanum melongena (Wu et al., 2012), Cucumis sativus (Shu et al., 2012), B. juncea (Ahmad et al., 2012), exposed to salt stress.
Under stress conditions (such as drought and salinity) plant cells accumulate osmolytes in order to reduce the osmotic potential which increases the water absorption capacity, maintain turgor pressure at a certain extent and protects the cell growth. This whole phenomenon is called osmotic adjustment (Wang et al., 2012). In the present study the water potential decreased with an increase in the salt concentration (Fig. 2b) indicating that the leaves of B. juncea have evolved certain mechanisms to adjust its survival, under salt stress. Moreover, the varied range of increase in the ion concentration in the leaves caused varied degree of reduction in water potential under salt stress (Liu, 2004). These results are in conformity with Eisa et al. (2012), Wang et al. (2012) and Naeem et al. (2010). The cell membranes under various environmental stress are subjected to changes, like the loss of integrity and increase in permeability (Blokhina et al. 2003) which cause an increase in the electrolyte leakage as observed in the present study (Fig. 2c).
Plants possess complex antioxidative defense system comprising of non-enzymatic (such as proline) and enzymatic components (such as CAT, POX, SOD) to scavenge reactive oxygen species (ROS) produced during stress. The production and scavenging of ROS occurs in different cell organelles such as chloroplasts, mitochondria and peroxisomes, however, pathways are well coordinated (Pang and Wang, 2008). Under normal conditions, ROS are generated at very low levels and a homeostasis is maintained between production and quenching of these molecules. This balance could be disturbed by the environmental stress, giving rise to a rapid increase in intercellular ROS levels which induce oxidative damage to lipids, proteins, and/or nucleic acids (Sharma et al., 2010). In order to cope with the oxidative damages under stress, plants raise the level of endogenous enzymes (CAT, POX and SOD) and the non-enzymatic component such as proline (Sharma et al., 2010 and Figs. 4a–d). Similar observations have been reported earlier in different crops such as Kochia scoparia (Nabati et al., 2011), proso millet (Sabir et al., 2011), wheat (Ashraf et al., 2010), safflower (Siddiqi, 2010), tomato (Hayat et al., 2010a) and B. juncea (Hayat et al., 2011; Ahmad et al., 2012). Under water or salinity stress the increase in proline content (Fig. 4d) may be due to the surpassing of the rate of protein hydrolysis over that of its synthesis (Irigoyen et al., 1992). Moreover, a higher proline content may be the result of its slower rate of breakdown or the diversion of protein synthesis so as to accumulate more proline. Similar observations have also been reported earlier in response to salt stress in B. juncea (Hayat et al., 2011; Ahmad et al., 2012), V. radiata (Hayat et al., 2010b) and sugar beet (Farkhondeh et al., 2012). It seems that the increased level of proline has a protective role in plants, exposed to stress (Hayat et al., 2010b). Therefore, in the present study, variety Varuna, a salt tolerant, possessed a higher proline content and the activity of CAT, POX and SOD enzymes than RH-30, a salt-sensitive. Such plant responses, differing in salt tolerance have been studied earlier in which salt tolerant varieties possessed better antioxidative defense system than the salt sensitive varieties (Sabir et al., 2011; Hayat et al., 2011). The yield characteristics (number of pods per plant, number of seeds per pod, 100 seed mass and seed yield per plant) decreased significantly with an increase in NaCl level of the soil (Figs. 5a–d). However, variety Varuna performed better and showed a lesser loss than RH-30. This reduction in seed yield and its other related parameters, with an increase in the level of NaCl could be attributed to poor plant growth (Figs. 1a–f), resulting from the reduced rate of photosynthesis (Fig. 3b; Chen et al., 2009). These results are in conformity with those of Ali et al. (2007) and Asgari et al. (2012). Under salt stress, the thickness of the pathway elements conducting assimilates gets reduced (Aldesuquy and Ibrahim, 2001), at the same time the leaves start behaving as sink rather than source (Arbona et al., 2005) the cumulative effect being the inhibition of assimilate movement toward the developing reproductive organs leading to their poor growth and seed setting (Figs. 5a–d).
5. Conclusion
From the present study, it can be concluded that sodium chloride in a concentration (2.8, 4.2 or 5.6 dsm−1) dependent manner through soil significantly retarded plant growth, pace of photosynthesis and ultimately the seed yield in the B. juncea (L.) Czern and Coss cv. Varuna and RH-30, even though the plants exhibited a higher antioxidant enzyme activity and an accumulation of proline (the protective mechansims). The variety RH-30 was more sensitive to the salt stress than Varuna.
Acknowledgements
The authors are thankful to the Chairman, Department of Botany, Aligarh Muslim University, India for providing the necessary facilities for conducting the experimental work. The authors also thank University Grants Commission (UGC) for their financial support in terms of UGC Non-NET Fellowship.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afroz S., Mohammad F., Hayat S., Siddiqi M. Exogenous application of gibberellic acid counteracts the effect of sodium chloride in mustard. Turk. J. Biol. 2005;29:233–236. [Google Scholar]
- Ahmad P., Sharma S. Physio-biochemical attributes in two cultivars of mulberry (M. alba) under NaHCO3 stress. Int. J. Plant Prod. 2010;4:79–86. [Google Scholar]
- Ahmad P., Jaleel C.A., Sharma S. Antioxidative defence system, lipid peroxidation, proline metabolizing enzymes and biochemical activity in two genotypes of Morus alba L subjected to NaCl stress. Russ. J. Plant Physiol. 2010;57:509–517. [Google Scholar]
- Ahmad P., Hakeem K.U.R., Kumar A., Ashraf M., Akram N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L) Afr. J. Biotechnol. 2012;11(11):2694–2703. [Google Scholar]
- Akram N.A., Ashraf M. Pattern of accumulation of inorganic elements in sunflower (Helianthus annuus L) plants subjected to salt stress and exogenous application of 5-aminolevulinic acid. Pakistan J. Bot. 2011;43:521–530. [Google Scholar]
- Aldesuquy H.S., Ibrahim A.H. Interactive effect of seawater and growth bio-regulators on water relations, abscisic acid concentration, and yield of wheat plants. J. Agron. Crop. Sci. 2001;187:185–193. [Google Scholar]
- Ali B., Hayat S., Ahmad A. 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L) Environ. Exp. Bot. 2007;59:217–223. [Google Scholar]
- Alscher R.G., Erturk N., Heath L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2003;53:1131–1141. [PubMed] [Google Scholar]
- Arbona V., Marco A.J., Ijlesias D.J., Lopez-Climent M.F., Talon M., Gómez-Coudenas A. Carbohydrate depletion in roots and leavers of salt stressed potted Citrus clemtina L. Plant Growth Regul. 2005;46:153–160. [Google Scholar]
- Asgari H.R., Cornelis W., Van Damme P. Salt stress effect on wheat (Triticum aestivum L) growth and leaf ion concentrations. Int. J. Plant Prod. 2012;6(2):195–208. [Google Scholar]
- Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Ashraf M., Foolad M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. [Google Scholar]
- Ashraf M.A., Ashraf M., Ali Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: leaf lipid peroxidation and phenolic contents. Pakistan J. Bot. 2010;42:559–566. [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Sci. 1973;39:205–207. [Google Scholar]
- Beauchamp L.O., Fridovich I. Superoxide dismutase improved assays and assay applicable to acrylamide gels. Ann. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Blokhina O., Violainen E., Fagerstedt K.V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Maehly A.C. Assay of catalase and peroxidase. Methods Enzymol. 1956;2:764–775. [Google Scholar]
- Chen C., Huang D., Liu J. Functions and toxicity of nickel in plants: recent advances and future prospects. Clean. 2009;37:304–313. [Google Scholar]
- Dwivedi R.S., Randhawav N.S. Evaluation of rapid test for the hidden hunger of zinc in plants. Plant Soil. 1974;40:445–451. [Google Scholar]
- Eisa S., Hussin S., Geissler N., Koyro H.W. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of Quinoa (Chenopodium quinoa Willd.) as a potential cash crop halophyte. Aust. J. Crop Sci. 2012;6(2):357–368. [Google Scholar]
- Farkhondeh R., Nabizadeh E., Jalilnezhad N. Effect of salinity stress on proline content, membrane stability and water relations in two sugar beet cultivars. Int. J. Agrisci. 2012;2(5):385–392. [Google Scholar]
- Geebelen W., Vangronsveld J., Adriano D.C., Van Poucke L.C., Clijsters H. Effects of Pb-EDTA and EDTA on oxidative stress reactions and mineral uptake in Phaseolus vulgaris. Physiol. Plant. 2002;115:377–384. doi: 10.1034/j.1399-3054.2002.1150307.x. [DOI] [PubMed] [Google Scholar]
- Ghogdi E.A., Izadi-Darbandi A., Borzouei A. Effects of salinity on some physiological traits in wheat (Triticum aestivum L) cultivars. Indian J. Sci. Tech. 2012;5(1):1901–1906. [Google Scholar]
- Hasanuzzaman M., Hossain M.A., da Silva J.A.T., Fujita M. Plant responses and tolerance to abiotic oxidative stress: antioxidant defenses is a key factors. In: Bandi V., Shanker A.K., Shanker C., Mandapaka M., editors. Crop Stress and its Management: Perspectives and Strategies. Springer; Berlin: 2012. (pp. 261−316) [Google Scholar]
- Hasegawa P.M., Bressan R.A., Zhu J.K., Bohnert H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Hayat S., Yadav S., Wani A.S., Irfan M., Ahmad A. Response of tomato to two possible modes of salinity stress-a comparative analysis. J. Soil Salinity Water Quality. 2010;2(2):84–90. [Google Scholar]
- Hayat S., Hasan S.A., Yusuf M., Hayat Q., Ahmad A. Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ. Exp. Bot. 2010;69:105–112. [Google Scholar]
- Hayat S., Mir B.A., Wani A.S., Hasan S.A., Irfan M., Ahmad A. Screening of salt tolerant genotypes of Brassica juncea based on photosynthetic attributes. J. Plant Interact. 2011;6:53–60. [Google Scholar]
- Heidari M. Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L) genotypes. Afr. J. Biotech. 2012;11(2):379–384. [Google Scholar]
- Idrees M., Naeem M., Khan M.N., Aftab T., Khan M.M.A., Moinuddin Alleviation of salt stress in lemongrass by salicylic acid. Protoplasma. 2012;249:709–720. doi: 10.1007/s00709-011-0314-1. [DOI] [PubMed] [Google Scholar]
- Irigoyen J.J., Emerich D.W., Sanchez-Diaz M. Water stress induced changes in concentration of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992;84:55–64. [Google Scholar]
- Iyengar E.R.R., Reddy M.P. Photosynthesis in highly salt-tolerant plants. In: Pessaraki M., editor. Handbook of Photosynthesis. Marcel Dekker; New York: 1996. pp. 897–909. [Google Scholar]
- Kanwal H., Ashraf M., Shahbaz M. Assessment of salt tolerance of some newly developed and candidate wheat (Triticum aestivum L) cultivars using gas exchange and chlorophyll fluorescence attributes. Pakistan J. Bot. 2011;43:2693–2699. [Google Scholar]
- Khan N.A., Ansari H.R., Khan M., Samiullah M.R. Effect of phytohormones on growth and yield of Indian mustard. Indian J. Plant Physiol. 2002;7:75–78. [Google Scholar]
- Liu, Y., 2004. The study of mechanism of salt resistance in physiology and biochemistry of T. halophila and A. thaliana, Master’s thesis, University of Central family name.
- Liu W., Ming Y., Li P., Huang Z. Inhibitory effects of hypo-osmotic stress on extracellular carbonic anhydrase and photosynthetic efficiency of green alga Dunaliella salina possibly through reactive oxygen species formation. Plant Physiol. Biochem. 2012;54:43–48. doi: 10.1016/j.plaphy.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Lu K.X., Cao B.H., Feng X.P., He Y., Jiang D.A. Photosynthetic response of salt tolerant and sensitive soybean varieties. Photosynthetica. 2009;47:381–387. [Google Scholar]
- Manikandam K., Desingh R. Effect of salt stress on growth, carbohydrate and proline content of two finger millet varieties. Recent Res. Sci. Tech. 2009;1:48–51. [Google Scholar]
- Megdiche W., Hessini K., Gharbi F., Jaleel C.A., Ksouri R., Abdelly C. Photosynthesis and photosystem-2 efficiency of two salt-adapted halophytic seashore Cakile maritima ecotypes. Photosynthetica. 2008;46:410–419. [Google Scholar]
- Mehta P., Jajoo A., Mathur S., Bharti S. Chlorophyll-a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol. Biochem. 2010;48:16–20. doi: 10.1016/j.plaphy.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25(2):239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nabati J., Kafi M., Nezami A., Moghaddam P.R., Masomi A., Mehrjerdi M.Z. Effect of salinity on biomass production and activities of some key enzymatic antioxidants in kochia (Kochia scoparia) Pakistan J. Bot. 2011;43(1):539–548. [Google Scholar]
- Naeem M.S., Jin Z.L., Wan Z.L., Liu D., Liu H.B., Yoneyama K., Zhou W.J. 5-aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L) Plant Soil. 2010;332:405–415. [Google Scholar]
- Pang C.H., Wang B.S. Oxidative stress and salt tolerance in plants. In: Lüttge U., Beyschlag W., Murata J., editors. Progress in Botany. Springer; Berlin, Germany: 2008. pp. 231–245. [Google Scholar]
- Parida A.K., Das A.B. Salt tolerance and salinity effects on plants: a review. Ecotox. Environ. Saf. 2005;60(3):324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Parida A.K., Das A.B., Mittra B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees Struct. Funct. 2004;18:167–174. [Google Scholar]
- Pitann B., Schubert S., Mühling K.H. Decline in leaf growth under salt stress is due to an inhibition of H+ pumping activity and increase in apoplastic pH of maize leaves. J. Plant Nutr. Soil Sci. 2009;172:535–543. [Google Scholar]
- Price G.D., Von Caemmerer S., Evans J.R., Yu J.W., Lloyd J., Oja V., Kell P., Harrison K., Gallagher A., Bodger M.R. Specific reduction of chloroplast carbonic anhydrase activity antisense RNA in transgenic tobacco has a minor effect on photosynthetic CO2 assimilation. Planta. 1994;193:331–340. [Google Scholar]
- Rahdari P., Tavakoli S., Hosseini S.M. Studying of salinity stress effect on germination, proline, sugar, protein, lipid and chlorophyll content in Purslane (Portulaca oleraceae L) leaves. Stress Physiol. Bio. J. 2012;8(1):182–193. [Google Scholar]
- Sabir P., Ashraf M., Akram N.A. Accession variation for salt tolerance in proso millet (Panicum miliaceum L) using leaf proline content and activities of some key antioxidant enzymes. J. Agron. Crop Sci. 2011;197(5):340–347. [Google Scholar]
- Saleem M., Ashraf M., Akram N.A. Salt (NaCl) induced modulation in some key physio-biochemical attributes in okra (Abelmoschus esculentus L) J. Agron. Crop Sci. 2011;197:202–213. [Google Scholar]
- Sharma P., Jha A.B., Dubey R.S. Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In: Pessarakli M., editor. Handbook of Plant and Crop Stress. third ed. CRC Press; Florida, USA: 2010. pp. 89–138. [Google Scholar]
- Shu S., Guo S.R., Sun J., Yuan L.Y. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol. Plant. 2012;146:285–296. doi: 10.1111/j.1399-3054.2012.01623.x. [DOI] [PubMed] [Google Scholar]
- Siddiqi, E.H., 2010. Influence of salt stress on some physiological and biochemical attributes and oil composition of a potential oilseed crop safflower (Carthamus tinctorius L.), PhD thesis Department of Botany, University of Agriculture, Faisalabad.
- Singh H., Singh B.P., Prasad H. Weed management in Brassica species. Indian J. Agron. 2001;46:533–537. [Google Scholar]
- Soussi M., Ocana A., Lluch C. Effects of salt stress on growth, photosynthesis and nitrogen fixation in chickpea (Cicer arietinum L) J. Exp. Bot. 1998;49:1329–1337. [Google Scholar]
- Sullivan C.Y., Ross W.M. Selection for drought and heat tolerance in grain sorghum. In: Mussel H., Staples R.C., editors. Stress Physiology in Crop Plants. John Wiley & Sons; New York: 1979. pp. 263–281. [Google Scholar]
- Wang W.Y., Yan X.F., Jiang Y., Qu B., Xu Y.F. Effects of salt stress on water content and photosynthetic characteristics in Iris lactea var. Chinensis seedlings. Middle East J. Sci. Res. 2012;12(1):70–74. [Google Scholar]
- Wu X.X., Ding H.D., Zhu Z.W., Yang S.J., Zha D.S. Effects of 24-epibrassinolide on photosynthesis of eggplant (Solanum melongena L) seedlings under salt stress. Afr. J. Biotech. 2012;11(35):8665–8671. [Google Scholar]
- Yan K., Chen P., Shao H., Zhao S., Zhang L., Zhang L., Xu G., Sun J. Responses of photosynthesis and photosystem II to higher temperature and salt stress in Sorghum. J. Agron. Crop Sci. 2012;198:218–226. [Google Scholar]
- Zhang H.J., Dong H.Z., Li W.J., Zhang D.M. Effects of soil salinity and plant density on yield and leaf senescence of field-grown cotton. J. Agron. Crop Sci. 2011;198(1):27–37. [Google Scholar]
- Zhu J-K. Encyclopedia of Life Sciences. John Wiley & Sons Ltd; Chichester: 2007. Plant Salt Stress. [Google Scholar]



