Abstract
A leaf spotting disease of an ornamental variety of Ophiopogon japonicus was discovered at several locations in northern Thailand. In all cases a species of Phyllosticta was associated with the lesions. Phyllosticta ophiopogonis sp. nov. is distinguished from Phyllosticta species from Liliaceae in conidia size, mucilaginous sheath and appendage thus the species is introduced as new in this paper. The new species which causes unsightly lesions on this ornamental plant is described, illustrated and compared with other similar Phyllosticta species.
Keywords: Coelomycetes, Botryosphaeriaceae, Dothideales, Leaf spot, Pathogen, Taxonomy
1. Introduction
The genus Phyllosticta had been relatively well-studied worldwide and a monograph has been published with details of the excepted species (van der Aa and Vanev, 2002). Five species have been introduced since the publication of van der Aa and Vanev (2002), mainly based on morphology and host occurrence. Four new species were introduced from Japan by Motohashi et al. (2008), while Phyllosticta citriasiana, Wulandari, Crous and Gruyter, from the peel of the fruit Citrus maxima was introduced from China and Thailand (Wulandari et al., 2009). Three new species of the sexual state of Phyllosticta have also recently been introduced and include Guignardia musicola, Wulandari, Cai and Hyde, Guignardia bispora Wulandari and Hyde, and Guignardia ellipsoidea, Wulandari and Hyde from the palms in northern Thailand (Wulandari et al., 2010a,b, 2011). Glienke et al. (2011) also introduced Phyllosticta bifrenariae Pereira, Glienke and Crous on orchids, Phyllosticta citribraziliensis Glienke and Crous on Citrus and Phyllosticta brazilianiae, Stringri, Glienke and Crous on Mangifera indica and epitypified Phyllosticta capitalensis Henn. and Phyllosticta citricarpa (McAlpine) Aa.
Ophiopogon japonicus (L.f.) Ker Gawl is an ornamental plant grown in gardens and parks throughout northern Thailand. During field surveys we repeatedly came across severely diseased plants, with symptoms ranging from numerous leaf spots to severe blighting (Fig. 1). The separation of Phyllosticta species is presently mainly based on morphological characters, although molecular data has recently helped to differentiate some taxa, e.g. P. bifrenariae, P. brazilianiae, P. citriasiana, P. citribraziliensis (Wulandari et al., 2009; Glienke et al., 2011). However, despite the use of molecular data, we still need to rely on morphological data (Hyde et al., 2010; Udayanga et al., 2011).
Figure 1.
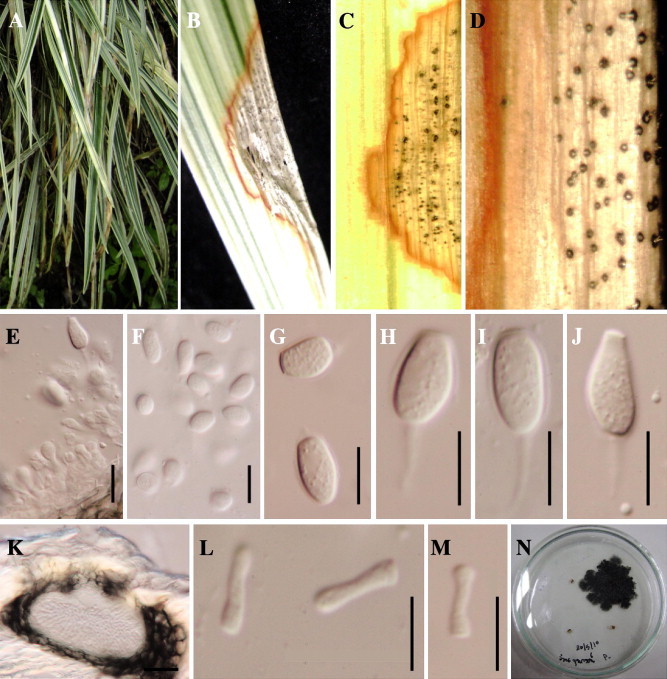
(A–N) Phyllosticta ophiopogonis (MFLU11 0027, holotype). (A) Symptom of disease. (B–D) Pycnidia growing on infected leaf of Liliaceae. (E) Conidiogenous cell. (F–J) Conidia Cross section through (K) Leptodothiorella state; scale bar = 50 μm. (L and M) Spermatia; scale bar = 10 μm. (N) Upper of cultures after 5 weeks.
There is no report of Phyllosticta species on Ophiopogon sp. in northern Thailand or worldwide. We therefore compare the species from O. japonicus with the species from other members from Liliaceae. Phyllosticta ophiopogonis is however distinct. The aim of this paper is therefore to introduce the new species from O. japonicus based on morphology as it causes unsightly disease of this ornamental. The new species is compared with other Phyllosticta species described from Liliaceae in Table 1.
Table 1.
Phyllosticta spp. described from Liliaceae.
| Phyllosticta species | Host plant and family⁎ | Pycnidia size (μm) | Peridium thickness (μm) | Conidiogenous cells (μm) | Conidia (μm) | Sheath size (μm) | Appendage size (μm) | Reference |
|---|---|---|---|---|---|---|---|---|
| P. aspidistricola | Aspidistra elatior var. elatior Liliaceae | 61–118 × 86–110 | – | 7–12.5 × 1.2–2.5 | 9.5–12.5 × 8.5–10 | – | 17–24.5 | Motohashi et al. (2008) |
| P. cruenta var. discincta | Polygonatum latifolium, Liliaceae | 100–195 × 145 | 9–21 | 4–14 × 3–6 | 12–23 × 6.4–10 | 0.2 | 4–17 | Bissett (1979a) present study |
| P. crypta | Smilax sp., Liliaceae | 70–130 × 45–95 | 4–14 | 5–12 × 2–3.5 | 5.4–8.9 × 3.8–6.2 | 0.3–1.0 | 3–8 | Bissett (1979b) |
| P. cumminsii | Smilax sp., Liliaceae | 75–140 × 110 | 4–19 | 3.5–14 × 3–6 | 6.7–10.5 | 1–2 | 5–20 | Bissett (1979b) |
| P. discincta | Uvularia grandiflora, Liliaceae | 65–120 | 5–12 | 4–13 × 2.5–6 | 5–8.6 × 3.9–6.6 | Less than 0.8 | 4–14 | van der Aa and Vanev (2002) |
| P. hemerocallidis | Hemerocallis fulva, Liliaceae | 84–139 | – | – | 8–13 × 3–5 | – | 3–10 | van der Aa and Vanev (2002) |
| P. hypoglossi | Ruscus hypoglossum, Liliaceae | 120–250 | 12–30 | 4–10 × 2–3.5 | 8–15 (−18) × 6–10 | – | 10 up to 35 | Van der Aa (1973) |
| P. ophiopogonis | Ophiopogon japonicus, Liliaceae | 96–100 × 75–80 | 9–15 | 7–12 × 2–4 | 10–14 × 7–8 | 0.8–1 | 5–16 | Present study |
| P. subeffusa | Smilax herbacca, Liliaceae | 90–150 × 120–140 | 5–16 | 5–8 × 3–4.5 | 7–13 × 7–10 | – | 5–7 up to 15 | Van der Aa (1973) |
| P. yuccae | Yucca elephantipes, Liliaceae | 90–150 | 14–38 | 5.4–9.8 × 2.7–6 | 7.5–15.4 × 6–9.5 | 1 | 4–15 | Bissett (1986) |
They may now belong to other families.
2. Material and Methods
Diseased leaves of O. japonicus were collected from various sites in Chiang Rai Province, Thailand. Morphological characters were recorded using the methods described by Wulandari et al. (2010a,b). Single spore isolates were prepared using the method of Choi et al. (1999).
3. Results
Phyllosticta ophiopogonis Wulandari, Wikee and Hyde, sp. nov. MycoBank: MB 519321 (Figs. 1A–N and 2M–O).
Figure 2.
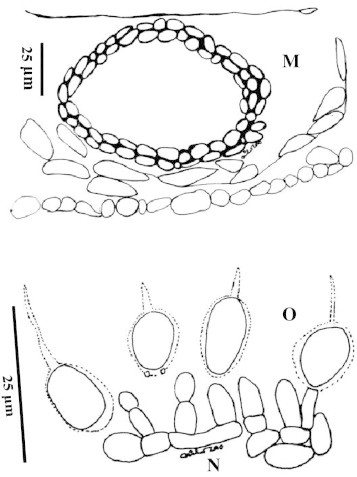
(M–O) Phyllosticta ophiopogonis (MFLU 10-0480) line drawing. (M) Section of pycnidia in the leaf (darkened area are plant cells – arrowed). (N) Conidiogenous cells. (O) Conidia with appendage and sheath.
Etymology named after its host plant, O. japonicus.
Leaf spots on lamina and apex of the leaf, at first minute, later becoming large spots with a dark reddish border, eventually coalescing to form blights on the leaves, centre of lesions pale brown with numerous pycnidia (Fig. 1A–C). Pycnidia 96–99 μm diameter, 75–81 μm high, on the surface of leaves, black, globose to pyriform, immersed in plant tissues, coriaceous, solitary to clustered, ostiolate, ostioles as black dots in the centre (Fig. 1D). Conidiogenous cells 7–12 × 2–4 μm , holoblastic, determinate, discrete, hyaline, sometimes rarely integrated, with cylindrical to doliiform cells lining the pycnidial locule (Fig. 1E). Conidia 10–14 × 7–8 μm , hyaline, 1-celled, coarse-guttulate, smooth-walled, globose, ellipsoidal, clavate or obclavate, with an obtuse apex, sometimes truncate at the base, surrounded by 0.8–1 μm thick mucilaginous sheath which persists at maturity and with 5–16 μm a single, hyaline, curved or straight appendage (Fig. 1H–J).
Leptodothiorella state, 60–80 μm in length, 40–50 μm in wide and thick 22 μm. Spermatia are produced from spermatiogenous cells, cylindrical and globose at two ends 6–8 μm long, 6.7–8.3 × 1.3–1.6 μm.
Colonies black, fimbriate, black in reverse, reaching 3–5 cm in diameter after 21 days incubation at 28 °C of on half strength PDA.
Habitat: On living leaves causing leaf spots.
Host: O. japonicus (Lilliaceae).
Known distribution: Thailand (Chiang Rai).
Material examined: Thailand, Chiang Rai Province, Khun.
Korn Waterfall, on the leaves of O. japonicus, 10 November 2010, Wikee, WK 10 (MFLU11-0027, holotype); culture ex-type MFLUCC11-0057; Wieng Chiang Rung, Houi Mae Sak Waterfall, on the leaves of O. japonicus, 10 September 2010, S. Wikee, WK 12 (MFLU11-0028), culture MFLUCC11-0059; Nang Lae, Pasangwiwat, on the leaves of O. japonicus, 10 December 2010, Wikee, WK 17 (MFLU11-0029), culture MFLUCC11-0063; ibid. 11 November 2010, Wikee, WK 23 (MFLU11-0030), culture MFLUCC11-0069; Weing Kan, on the leaves of O. japonicus, 6 January 2011, Wikee, WK 26 (MFLU11-0031), culture MFLUCC10-0132. Mae Fah Luang University, on the leaves of O. japonicus, 30 June 2010, Wulandari, NFW 330 (MFLU10-0480); ibid., 06 August 2010, Wulandari, NFW 332 (MFLU10-0482); ibid., Hue Pui Temple, on the leaves of O. japonicus, 28 October 2010, Wulandari, NFW 341 (MFLU10-0980); ibid., Hue Pui Temple, on the leaves of O. japonicus, 17 August 2010, Wulandari, NFW 343 (MFLU10-0982).
Notes: Van der Aa (1973) and van der Aa and Vanev (2002) distinguished nine species of Phyllosticta on Liliaceae (Table 1).
Of the Phyllosticta spp. that occurs on Liliaceae, P. cruenta var. discincta is the most similar. The conidia of P. ophiopogonis differ as they are smaller than P. cruenta var. discincta (10–14 × 7–8 μm versus 12–23 × 6.4–10 μm) and the pycnidia are also smaller in P. ophiopogonis (96–99 μm diameter, 75–81 μm high versus 100–195 μm diameter 145 μm high). P. ophiopogonis also differs from Phyllosticta hypoglossi in having shorter appendages, 5–16 μm versus 10 up to 35 μm long (van der Aa, 1973).
4. Discussion
There is a move towards use of one name for a single biological species instead of different names for different morphs (Hyde et al., 2011). In this study, the sexual Guignardia state did not form in any of the collections made or in culture. We choose to use the oldest name Phyllosticta as compared to Guignardia for this taxon as Phyllosticta species usually cause serious disease. If the teleomorph is found later it can be described under P. ophiopogonis. This disease is important as it causes unsightly spots and blights on this commonly used ornamental (Fig. 1A).
Acknowledgements
The Global Research Network for Fungal Biology and King Saud University are thanked for supporting this research. Nilam Wulandari is grateful to the Mushroom Research Foundation for a scholarship to carry our studies towards a Ph.D. and the Graduate School, Chiang Mai University, Thailand for the financial support and the School of Science, Mae Fah Luang University for the laboratory facilities. MFLU awarded Grant No. 53101020017 to study the genus Phyllosticta in northern Thailand and the National Research Council of Thailand awarded Grant No. 54201020004 to study the genus Phyllosticta in Thailand. The Thailand Research Fund in the Royal Golden Ph.D. Jubilee Program agreement No. Ph.D. /0198/2552 in 2.B.M.F./52/A.1.N.XX to study the taxonomy and phylogeny of Phyllosticta is acknowledged. Crous, CBS, the Netherlands is thanked for partially funding this research. Wara Asfiya (LIPI) and Samantha Karunarathna (MFLU) are thanked for valuable references on Phyllosticta species.
References
- Bissett J. Discochora yuccae sp. nov. with Phyllosticta and Leptodothiorella synanamorphs. Canadian Journal of Botany. 1986;64:1720–1726. [Google Scholar]
- Bissett J. Coelomycetes on Liliales: the Genus Phyllosticta. Canadian Journal of Botany. 1979;57:2082–2095. [Google Scholar]
- Bissett J. Coelomycetes on Liliales: Dothiorella smilacinae and Stagonospora smilacis. Canadian Journal of Botany. 1979;57:2071–2081. [Google Scholar]
- Choi Y.W., Hyde K.D., Ho W.H. Single spore isolation of fungi. Fungal Diversity. 1999;3:29–38. [Google Scholar]
- Glienke C., Pereira O.L., Stringri D., Fabris J., Kava-Cordeiro V., galli-terasawa L., Cunnington J., Shivas R.G., Groenewald J.Z., Crous P.W. Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia. 2011;26:47–56. doi: 10.3767/003158511X569169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde K.D., Abd-Elsalam K., Cai L. Morphology: still essential in a molecular world. Mycotaxon. 2010;114:439–451. [Google Scholar]
- Hyde K.D., KoKo T.W., McKenzie E.H.C. Towards incorporating anamorphic fungi in a natural classification – Checklist and notes for 2010. Mycosphere. 2011;2(1):1–88. [Google Scholar]
- Motohashi K., Araki I., Nakashima C. Four new species of Phyllosticta, one new species of Pseudocercospora, and one new combination in Passalora from Japan. Mycoscience. 2008;49(2):138–146. [Google Scholar]
- Udayanga D., Liu X.Z., McKenzie E.H.C., Chukeatorate E., Bahkali H.A., Hyde K.D. The genus Phomopsis: biology, species concepts, future and names of important phytopathogens. Fungal Diversity. 2011;50:189–225. [Google Scholar]
- Van Der Aa H.A. Studies in Phyllosticta 1. Studies in Mycology. 1973;5:1–110. [Google Scholar]
- Van der Aa H.A., Vanev S. Centraalbureau voor Schimmelcultures, Utrech; The Netherlands: 2002. A Revision of the Species Described in Phyllosticta. pp. 1–49. [Google Scholar]
- Wulandari N.F., To-anun C., Hyde K.D., Duong L.M., De Gruyter J., Meffert J.P., Groenewald J.Z., Crous P.W. Phyllosticta citri-asiana sp. nov, the cause of Citrus tan spot of Citrus maxima in Asia. Fungal Diversity. 2009;34:23–39. [Google Scholar]
- Wulandari N.F., To-anun C., Cai L., Abd-Elsalam K., Hyde K.D. Guignardia/Phyllosticta species on banana. Cryptogamie Mycologie. 2010;31(4):403–418. [Google Scholar]
- Wulandari N.F., To-anun C., Hyde K.D. Guignardia morindae frog eye-leaf spotting disease of Morinda citrifolia (Rubiaceae) Mycosphere. 2010;1(4):325–331. [Google Scholar]
- Wulandari N.F., To-anun C., McKenzie E.H.C., Hyde K.D. Guignardia bispora and G. ellipsoidea spp. nov. and other Guignardia species from palms (Arecaceae) Mycosphere. 2011;2(2):115–118. [Google Scholar]


