Abstract
Polygonum aviculare (Polygonaceae) is an herb commonly distributed in Mediterranean coastal regions in Egypt and used in folkloric medicine. Organic and aqueous solvent extracts and fractions of P. aviculare were investigated for antimicrobial activities on several microorganisms including bacteria and fungi. Phytochemical constituents of air-dried powered plant parts were extracted using aqueous and organic solvents (acetone, ethanol, chloroform and water). Antimicrobial activity of the concentrated extracts was evaluated by determination of the diameter of inhibition zone against both Gram-negative and Gram-positive bacteria and fungi using paper disc diffusion method.
Results of the phytochemical studies revealed the presence of tannins, saponins, flavonoids, alkaloids and sesquiterpenes and the extracts were active against both Gram-negative and Gram-positive bacteria. Chloroform extract gave very good and excellent antimicrobial activity against all tested bacteria and good activity against all tested fungi except Candida albicans. Structural spectroscopic analysis that was carried out on the active substances in the chloroform extract led to the identification of panicudine (6-hydroxy-11-deoxy-13 dehydrohetisane).
Evaluation of the antimicrobial activity of panicudine indicated significant activity against all tested Gram-negative and Gram-positive organisms. Panicudine displayed considerable activity against the tested fungi with the exception of C. albicans. Antimicrobial activity of the extracts was unaffected after exposure to different heat treatments, but was reduced at alkaline pH. Studies of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of panicudine on the tested organisms showed that the lowest MIC and the MBC were demonstrated against Salmonella paratyphi, Bacillus subtilis and Salmonella typhi and the highest MIC and MBC were against Staphylococcus aureus.
Keywords: Polygonum aviculare, Antimicrobial activity, Phytochemical analysis, Minimum inhibitory concentration, Minimum bactericidal concentration
1. Introduction
Polygonum aviculare L. (Polygonaceae family) is an annual prostrate herb with small elliptic lanceolate leaves is widely distributed in the Mediterranean coastal strip in Egypt (Täckholm, 1974). Plants belonging to this family are known to produce a large number of biologically important secondary metabolites, such as flavonoids, anthraquinones, alkaloids and steroids (Baraka, 1985, Marjorie, 1999). P. aviculare was selected because of the special pharmaceutical attributes which have been reported on some taxa of the family Polygonaceae. Some plants of this family were reported to be active as tumour inhibitors, including Rheum officinale (Belkin and Fitzgerald, 1966) and Rumex crispus (Shimada, 1966). Some Polygonum spp. contains alkaloids which have been used in traditional African system medicine (Iwu et al., 1999).
Use of plants as traditional health remedies is very popular and important for 80% of the world’s population in African, Asian, Latin America and Middle Eastern Countries. Their use is reported to have minimal side effects (Bibitha et al., 2002, Maghrani et al., 2005, Doughari, 2006). In recent years, pharmaceutical companies have spent considerable time and money in developing therapeutics based upon natural products extracted from plants (Ben Sassi et al., 2007, Coruh et al., 2007). The rising incidence of multidrug resistance amongst pathogenic microbes has further necessitated the need to search for newer antibiotic sources (Cody et al., 2000, Veronika et al., 2006). Because of its abundant and widespread availability, this study set out to investigate the antimicrobial activity of P. aviculare extracts and to determine the effect of temperature and pH on the efficacy of these extracts, and undertakes to identify one of the active constituents.
2. Materials and methods
Samples of plant materials were collected from wetted soil in the Mediterranean coastal region of Egypt, identified and authenticated at the Botany Department, Faculty of Science, Cairo University, Egypt.
2.1. Preparation of extracts
Extraction of plant tissues was carried out as described by Predrag et al. (2005) with slight modification. Either the freshly collected stem or fresh mature leaves were chopped into estimate size and state pieces and shade dried for 5 days at room temperature (30–35 °C) to constant weight. Fifty grams of each of the plant parts were coarsely powdered using a mortar and pestle and were further reduced to powder using an electric blender. The powdered fractions were transferred into separate closed containers. Twenty-five grams of powdered air-dried plant material was extracted with either 100 ml of water, acetone, ethanol or chloroform in a conical flask, with shaking at 120 rpm for 30 min, followed by storage for 24 h in the fridge. After 24 h, each of the extracts was filtered through four layers of gauze, and then filtrates were passed through a Whatman No. 1 filter paper. The resulting double filtrates were then concentrated in a rotary evaporator until eventual lyophilization. The yield of powder was 41% wt/wt from water extracts, 27% wt/wt from acetone, 17% wt/wt from ethanol and 15% wt/wt from chloroform extracts for the stem, while the respective values of 32%, 30%, 20% and 18% wt/wt were obtained for the leaves.
2.2. Phytochemical analysis
Freshly prepared extracts were subjected to standard phytochemical analyses to ensure the presence of the following phytoconstituents tannins, saponins, alkaloids, flavonoids and sesquiterpenes (Hess et al., 1995, Aida et al., 2001). The residue obtained from a chloroform extract (15% wt/wt) was fractioned on a basic Al2O3 column (5 × 70 cm) eluting with a gradient of EtOH up to 100%. The elutes were chromatographed on TLC results (10 mg) which revealed the presence of compound and obtained recrystallized. The structure of the compound isolated from chloroform extract was established by infrared spectroscopy, Mass and 1H and 13C NMR spectral analysis.
2.3. Test organisms
Bacterial isolates used in this study were: Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, S. paratyphi and Shigella flexneri for Gram-negative bacteria and Staphylococcus aureus, Bacillus subtilis, and Streptococcus pyogenes for Gram-positive bacteria. All strains were clinical isolates obtained from the Microbiological Laboratory, Botany Department, Faculty of Science, Zagazig University, Egypt. Fungal isolates from the same source were used, these being Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger and the yeast C. albicans.
All bacterial strains were cultivated in nutrient agar medium (NA), and incubated at 37 °C for 24 h, while yeast (C. albicans) and fungi were cultivated in potato dextrose agar (PDA).
2.4. Determination of antimicrobial activity by disc diffusion assay
Antimicrobial activity of the aqueous and organic extracts of plant samples were evaluated by the paper disc diffusion method (Brantner and Grein, 1994, Ali-Shtayeh et al., 1997). For determination of antibacterial activity, bacterial cultures were adjusted to 0.5 McFarland turbidity standards and inoculated onto 15 cm diameter nutrient agar (oxoid) plates. For the determination of antimycotic activity, all the fungal isolates including C. albicans were firstly adjusted to the concentration of 106 cfu/ml. Cultures of C. albicans were suspended in sterile solution of 0.9% normal saline and the spores of the other filamentous fungi were suspended in Tanquay buffer. All the cultures were inoculated onto Sabouraud Dextrose Agar plates. Sterile filter paper discs (diameter: 6 mm for bacteria and fungi) impregnated with 20 μl of reconstituted extract in minimum amount of solvent at concentrations of 10 mg/ml were placed on the culture plates previously seeded with the 0.5 McFarland and 106 cfu/ml cultures of bacteria and fungi, respectively. Bacterial cultures and those of C. albicans were then incubated at 37 °C for 24 h, while the other fungal cultures were incubated at room temperature (28 °C) for 48 h. Paper discs impregnated with 20 μl of a solution of 10 mg/ml of cotrimoxazole and chloramphenicol (for bacteria) and streptomycin (for fungi) as standard antimicrobials were used for comparison. Antimicrobial activity was determined by measurement of inhibition zone around each paper disc. For each extract three replicate trials were conducted against each organism.
2.5. Determination of antimicrobial activity of different concentration of isolated compound
Different concentrations of isolated panicudine dissolved in chloroform (400, 40, 4, 0.4 mg/g) were added to tested bacteria and fungi. Antimicrobial activity was determined by measurement of inhibition zone. For each concentration three replicate trials were conducted against each organism.
2.6. Determination of MIC and MBC
The minimum inhibitory concentration (MIC) of the extracts was determined for each of the test organisms in triplicates (Ali-Shtayeh et al., 1997). To 0.5 ml of varying concentrations of the extracts (20.0, 18.0, 15.0, 10.0, 8.0, 5.0, 1.0, 0.5, 0.05 and 0.005 mg/ml), 2 ml of nutrient broth was added and then a loopful of the test organism previously diluted to 0.5 McFarland turbidity standard for (bacterial isolates) and 106 cfu/ml (for fungal isolates) was introduced to the tubes. The procedure was repeated on the test organisms using the standard antibiotics (cotrimoxazole and chloramphenicol for bacteria and streptomycin for fungal isolates). A tube containing nutrient broth only was seeded with the test organisms as described above to serve as control. Tubes containing bacterial cultures were then incubated at 37 °C for 24 h, while tubes containing fungal spore cultures were incubated for 48 h at room temperature (28 °C). After incubation the tubes were then examined for microbial growth by observing turbidity.
To determine the MBC, for each set of test tubes in the MIC determination, a loopful of broth was collected from those tubes which did not show any growth and inoculated on sterile nutrient agar (for bacteria) and sabouraud dextrose agar (for fungi) by streaking. Nutrient agar and sabouraud agar only were streaked with the test organisms, respectively, to serve as control. Plates inoculated with bacteria were then incubated at 37 °C for 24 h, while those inoculated with fungi were incubated at room temperature (28 °C) for 48 h. After incubation, the lowest concentration at which no visible growth was noted as the minimum bacterial concentration.
2.7. Effect of temperature and pH on antimicrobial activity of chloroform extracts
Five milliliters of aliquots of 100 mg/ml of chloroform extracts were added in test tubes and treated at 4, 30, 60 and 100 °C in a water bath for 30 min and subsequently tested for antimicrobial activity.
To determine the effect of pH, chloroform extracts were treated at pH ranges of 2.5–10 using either 1 N HCl or 1 N NaOH solutions, respectively, in a series of test tubes for 30 min. After 30 min of treatment, each of the treated extracts were neutralized (pH 7) using either 1 N HCl or 1 N NaOH as the required, and then tested for antimicrobial activity.
3. Results
Phytochemical constituents present in the plant extract included tannins, saponnins, alkaloids, flavonoids and sesquiterpenes. Results of the plant extracts (Table 1) show that they were effective against most of the tested microorganisms. The highest activity (diameter of inhibition zone 28 mm) was demonstrated by the chloroform extracts of stem against P. mirabilis, while the lowest (diameter of inhibition zone 2 mm) was demonstrated by the acetone extracts against S. aureus. The leaf extracts generally showed lower activity against the test organisms compared to the stem extracts.
Table 1.
Antimicrobial activity of extracts of Polygonum aviculare.
| No. | Organism | Diameter of zone of inhibition (mm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem extracts |
Leaf extracts |
Antibiotics |
||||||||||
| WE | AE | CL | EE | WE | AE | CL | EE | Ct | Ch | St | ||
| 1 | Escherichia coli | 22 | 18 | 27 | 23 | 9 | 7 | 11 | 7 | 31 | 17 | 16 |
| 2 | Proteus mirabilis | 24 | 15 | 28 | 23 | 8 | 5 | 8 | 4 | 29 | 19 | 18 |
| 3 | Pseudomonas aeruginosa | 21 | 19 | 23 | 22 | 7 | 5 | 9 | 5 | 28 | 20 | 19 |
| 4 | Salmonella typhi | 22 | 14 | 24 | 23 | 8 | 5 | 11 | 7 | 28 | 10 | 9 |
| 5 | Salmonella paratyphi | 23 | 15 | 23 | 20 | 5 | 7 | 12 | 8 | 29 | 12 | 11 |
| 6 | Shigella flexneri | 19 | 16 | 21 | 16 | 6 | 4 | 11 | 6 | 30 | 13 | 12 |
| 7 | Staphylococcus aureus | 22 | 17 | 24 | 21 | 7 | 2 | 10 | 8 | 26 | 4 | 8 |
| 8 | Bacillus subtilis | 25 | 20 | 25 | 22 | 5 | 5 | 8 | 7 | 34 | 24 | 23 |
| 9 | Streptococcus pyogenes | 18 | 15 | 23 | 20 | 4 | 8 | 12 | 9 | 31 | 8 | 7 |
| 10 | Aspergillus flavus | 14 | 10 | 17 | 13 | 6 | 6 | 10 | 9 | 22 | 9 | 8 |
| 11 | Aspergillus fumigatus | 15 | 8 | 18 | 14 | 3 | 4 | 9 | 8 | 19 | 7 | 6 |
| 12 | Aspergillus niger | 13 | 7 | 14 | 15 | 2 | 5 | 8 | 6 | 18 | 6 | 5 |
| 13 | Candida albicans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Key: WE, water extract; AE, acetone extract; CL, chloroform extract; EE, ethanol extract; Ct, 10 mg/g cotrimoxazole; Ch, 10 mg/g chloramphenico; St, 10 mg/g streptomycin; and 0, no measurable zone.
Chloroform extract gave very good and excellent antimicrobial activity against all tested bacteria and good activity against all tested fungi except C. albicans. Results showed that chloroform extract generally has antimicrobial activity higher than that caused by water extract against all tested organisms.
Results of the effect of temperature and pH on the antimicrobial activity of chloroform extract showed that various temperature ranges of 4, 30, 60 and 100 °C had no effect on the antimicrobial activity of the chloroform extract (Fig. 1). Activity slightly increased at acidic pH (2–6). While at alkaline pH the activity of the plant extracts was reduced (Fig. 2).
Figure 1.
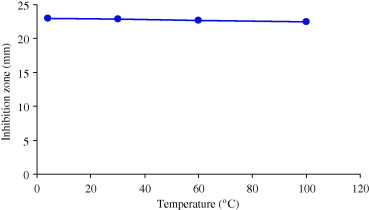
Effect of temperature on antimicrobial activity of 100 mg/ml chloroform extract from Polygonumaviculare.
Figure 2.
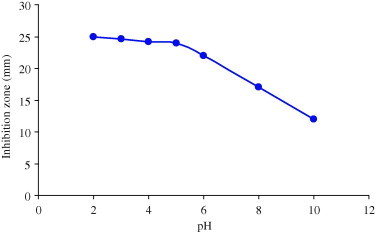
Effect of pH on antimicrobial activity of 100 mg/ml of chloroform extract from Polygonum aviculare.
The crystalline compound isolated from chloroform extract of the aerial parts of P. aviculare L. had a melting point of 213–215 °C. The IR spectrum showed absorption bands at (cm−1) 3405 (OH), 1718 (C O) and 1650 (C C). The 13C NMR spectrum showed the presence of six quarternary carbon singlets, six methine proton doublets, seven methylene and one methyl carbon atoms (Table 2). A singlet at 144.9 and a triplet at 110.3 confirmed the presence of an exomethylene group and the absence of substituent at C-15. A singlet signal at 99.7 was related to a carbinolamine carbon atom (Zang et al., 1990), and consequently there was a tertiary hydroxy group at C-6. The remaining carbonyl and hydroxyl groups could be present on the C-1, C-2, C-3, C-7, C-11 and C-13 positions.
Table 2.
13C NMR spectral data (50 MHz, CDCl3) for compound isolated from chloroform extract.
| C atom | Compound |
|---|---|
| 1 | 34.9 |
| 2 | 66.1 |
| 3 | 43.3 |
| 4 | 37.7 |
| 5 | 62.5 |
| 6 | 99.7 |
| 7 | 44.4 |
| 8 | 44.2 |
| 9 | 49.7 |
| 10 | 49.7 |
| 11 | 23.4 |
| 12 | 54.0 |
| 13 | 210.8 |
| 14 | 61.9 |
| 15 | 34.0 |
| 16 | 144.9 |
| 17 | 110.3 |
| 18 | 32.0 |
| 19 | 61.9 |
| 20 | 70.2 |
Singlet signals at δ 37.7, 44.2 and 49.7 were assigned to the C-4, C-8 and C-10 quarternary carbon atoms. The absence of N-methyl, N-ethyl and methoxyl groups indicated that the isolated compound belonged to the hetisane type of C20 diterpene alkaloids. The C-11 and C-13 positions remained possible for the carbonyl group. The choice between the C-11 and C-13 positions for the keto group was made by 13C NMR spectra which indicated that the location of the group should be at C-13 (Atta Ur-Rahman, 1990). The 1H NMR of isolated compound (Table 3) showed a singlet at δ 3.49 (1H, H-20), doublets at δ 3.12 and 2.95 (J = 11.5 Hz, 2H-19) and doublets with triplet components at δ 2.52 and 2.22 (J = 18 and 1.5 Hz, H-15) showing the absence of substituents at C-19 and C-15, respectively. The positions of the substituents in the hetisane skeleton of compound were elucidated by an analysis of the 13C NMR spectrum in which signals from 20 carbon atoms were detected. Thus the isolated compound has the structure of 6-hydroxy-11-deoxy-13-dehydrohetisane. The mass spectrum exhibited a [M]+ at m/z 327 (C20H25NO3) and significant peaks at m/z 313 [M−CH2]+. The compound identified is designated as panicudine (Fig. 3).
Table 3.
1H NMR spectral data (200 MHz, CDCL3) for isolated compound.
| H | Compound |
|---|---|
| 1 | |
| 2 | 4.02 (1H, J = 10 Hz, H-2β) |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | 2.74 (1H, br, d, J = 4 Hz, H-12) |
| 13 | |
| 14 | 2.20 (1H, S, J = 5 Hz, H-14) |
| 15 | 2.22; 2.52 (dt, each 1H, J = 18 Hz and 1.5 Hz, 2H-15) |
| 16 | |
| 17 | 4.76; 4.87 (S, each 1H, J = 4 Hz, 2H-17) |
| 18 | 1.29 (3H, S, M-18) |
| 19 | 2.95; 3.12 (d, each 1H, J = 11.5 Hz, 2H-19) |
| 20 | 3.49 (1H, S, H-20) |
Figure 3.
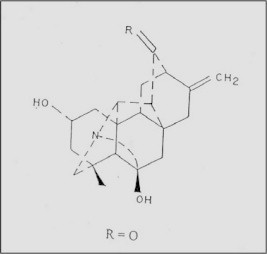
Chemical structure of isolated alkaloid from the aerial parts of Polygonum aviculareL.
The effective substance in chloroform extract was phytochemically precipitated, purified and identified. Different concentrations of the compound (panicudine) were tested against Gram-negative and Gram-positive bacteria and fungi. The effect of this compound (from greatest to lowest) against the tested microorganisms, followed the sequence: B. subtilis, P. mirabilis, S. paratyphi, S. typhi, E. coli, S. aureus, P. aeruginosa, S. flexneri, S. pyogenes, A. fumigatus, A. flavus and A. niger with no antimicrobial activity was observed against C. albicans (Table 4). Results of minimum inhibitory concentration (MIC) and minimum bacterial concentration (MBC) for general extracts are shown in Table 5. The results showed that S. aureus had the highest MIC (18 mg/ml) and MBC (20 mg/ml), while the lowest MIC and MBC (8 mg/ml) were shown by B. subtilis (Table 5). A. flavus had the highest values of MIC and MBC (8 and 10 mg/ml) for the leaf extract, while the lowest value of MIC and MBC (1 mg/ml) was shown by A. niger for steam extracts. The MIC and MBC values were generally higher for the leaf extracts against the test organisms compared to those of the stem extracts.
Table 4.
Antimicrobial activity of different concentrations of panicudine recovered from chloroform extract of Polygonum aviculare.
| No. | Organism | Panicudine concentration (mg/ml) |
|||
|---|---|---|---|---|---|
| Diameter of zone of inhibition (mm) | |||||
| 400 | 40 | 4 | 0.4 | ||
| 1 | Escherichia coli | 23 | 20 | 15 | 10 |
| 2 | Proteus mirabilis | 25 | 21 | 16 | 11 |
| 3 | Pseudomonas aeruginosa | 22 | 19 | 14 | 9 |
| 4 | Salmonella typhi | 23 | 20 | 15 | 10 |
| 5 | Salmonella paratyphi | 24 | 19 | 18 | 10 |
| 6 | Shigella flexneri | 20 | 16 | 14 | 9 |
| 7 | Staphylococcus aureus | 23 | 20 | 15 | 10 |
| 8 | Bacillus subitilis | 26 | 22 | 19 | 12 |
| 9 | Streptococcus pyogenes | 19 | 15 | 13 | 8 |
| 10 | Aspergillus flavus | 15 | 11 | 8 | 6 |
| 11 | Aspergillus fumigatus | 16 | 12 | 9 | 7 |
| 12 | Aspergillus niger | 14 | 10 | 8 | 5 |
| 13 | Candida albicans | 0 | 0 | 0 | 0 |
Table 5.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of chloroform extracts of Polygonum aviculare.
| No. | Organism | Stem extracts |
Leaf extracts |
||
|---|---|---|---|---|---|
| MIC (mg/ml) | MBC (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | ||
| 1 | Escherichia coli | 15 | 15 | 18 | 18 |
| 2 | Proteus mirabilis | 15 | 18 | 20 | 20 |
| 3 | Pseudomonas aeruginosa | 15 | 15 | 18 | 20 |
| 4 | Salmonella typhi | 10 | 10 | 15 | 15 |
| 5 | Salmonella paratyphi | 8 | 8 | 15 | 15 |
| 6 | Shigella flexneri | 8 | 8 | 10 | 10 |
| 7 | Staphylococcus aureus | 18 | 20 | 20 | 20 |
| 8 | Bacillus subtilis | 8 | 8 | 18 | 18 |
| 9 | Streptococcus pyogenes | 10 | 10 | 15 | 15 |
| 10 | Aspergillus flavus | 5 | 8 | 8 | 10 |
| 11 | Aspergillus fumigatus | 1 | 5 | 5 | 8 |
| 12 | Aspergillus niger | 1 | 1 | 5 | 5 |
| 13 | Candida albicans | 0 | 0 | 0 | 0 |
4. Discussion
Phytochemical constituents such as tannins, saponins, flavonoids, alkaloids and several other aromatic compounds are secondary metabolites of plants that serve as defense mechanisms against predation by many microorganisms, insects and other herbivores (Afolayan and Meyer, 1997, Lutterodt et al., 1999, Marjorie, 1999, Bonjar et al., 2004). This can partially explain the demonstration of antimicrobial activity by the stem and leaf extracts of P. aviculare. Demonstration of antimicrobial activity against both Gram-positive and Gram-negative bacteria may be indicative of the presence of broad spectrum antibiotic compounds (Cichewicz and Thorpe, 1996, Srinivasan et al., 2001).
Results showed that stem extracts are more effective than leaf extracts. This may be due to the fact that the stem was more developed and mature than leaves and may contain fewer pigments and other phenolics which have been reported to interfere with the antimicrobial activity of the extracts or could it be simply that they contain different concentrations/types of active antimicrobial constituents.
Out of the four solvents used for extraction, chloroform extracts showed the highest activity against the tested organisms, followed by water extracts, ethanol extracts and acetone extracts. Different solvents have been reported to have the capacity to extract different phytoconstituents depending on their solubility or polarity in the solvent (Marjorie, 1999). Chloroform extracts obtained in this study might have higher solubility for more of active antimicrobial phytoconstituents, consequently displaying the highest relative antimicrobial activity. Demonstration of antimicrobial activity by chloroform extracts provides the scientific basis for the use of these plants in the traditional treatment of diseases. Evaluation of the antimicrobial activity of panicudine against all Gram-negative and Gram-positive bacteria and fungi gave very good antimicrobial activity against all tested bacteria and good activity against fungi except C. albicans.
The tolerance of the chloroform extract to temperature may be an indication that the phytoconstituents are thermostable. This explains the traditional usage of very high temperature used to boil these plant parts to isolate active constituents. Antibacterial activity of the extracts slightly increased at acidic pH. An increase in activity of phytoconstituents in the presence of acidic medium has been reported previously (Molan, 1992, Dixon, 2001).
The highest MIC and MBC values obtained with S. aureus perhaps an indication that, chloroform extracts are less effective on some Gram-positive bacteria or the organism has the potential of developing antibiotic resistance, while the low MIC and MBC values for other bacteria and fungi are indication of plant extracts efficacy.
5. Conclusion
Demonstration of broad spectrum of antibacterial activity by various extracts of P. aviculare may help to discover new chemical classes of antibiotic substances that could serve as selective agents for infectious disease chemotherapy and control. This investigation has opened up the possibility of the use of this plant in antimicrobial drug development for human application. The effect of these extracts on other pathogenic organisms, more toxicological investigations and further purification to isolate the specific active constituents need to be carried out.
References
- Afolayan A.J., Meyer J.J. The antimicrobial activity of 3, 5, 7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J. Ethnopharm. 1997;57(3):177–181. doi: 10.1016/s0378-8741(97)00065-2. [DOI] [PubMed] [Google Scholar]
- Aida P., Rosa V., Blamea F., Tomas A., Salvador C. Paraguyan plants used in traditional medicine. Short communication. J. Ethnopharm. 2001;16:93–98. [Google Scholar]
- Ali-Shtayeh M.S., Al-Nuri M.A., Yaghmour R.M., Faidi Y.R. Antimicrobial activity of Micromeria nervosa from the Palestine area. J. Ethnopharm. 1997;58(3):143–147. doi: 10.1016/s0378-8741(97)00088-3. [DOI] [PubMed] [Google Scholar]
- Atta Ur-Rahman . Elsevier; Amsterdam: 1990. Handbook of Natural Products Data. Vol. 1. Diterpenoid and Steroidal Alkaloids. p. 241. [Google Scholar]
- Baraka, D.M., 1985. Ecological and Phytochemical Study on One Species of Polygonaceae. M.Sc. Thesis, Fac. Sci. Zagazig University (Benha) Egypt, p. 171.
- Belkin M., Fitzgerald D.B. Distribution of antineoplastic activity in plants. J. Pharmaceut. Sci. 1966;55(3):139–148. [Google Scholar]
- Ben Sassi A., Barzallah-Skhiri F., Aouni M. Investigation of some medicinal plants from Tunisia for antimicrobial activities. Pharmaceut. Biol. 2007;15(5):421–428. [Google Scholar]
- Bibitha B., Jisha V.K., Salitha C.V., Mohan S., Valsa A.K. Antibacterial activity of different plant extracts. Short communication. Indian J. Microbiol. 2002;42:361–363. [Google Scholar]
- Bonjar G.H.S., Nik A.K., Aghighi S. Antibacterial and antifungal survey in plants used in indigenous herbal-medicine of south east regions of Iran. J. Biol. Sci. 2004;4(3):405–412. [Google Scholar]
- Brantner A., Grein E. Antibacterial activity of plant extracts used externally in traditional medicine. J. Ethnopharm. 1994;44(1):35–40. doi: 10.1016/0378-8741(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Cichewicz R.H., Thorpe P.A. The antimicrobial properties of Chile peppers (Capsicum species) and their uses in Mayan medicine. J. Ethnopharm. 1996;52(2):61–70. doi: 10.1016/0378-8741(96)01384-0. [DOI] [PubMed] [Google Scholar]
- Cody C.C., Alexis T.H., Robert H.W., Lester J.C., Joseph O.F. Identification and characteristics of Movel Burkholderia strain with broad-spectrum antimicrobial activity. Appl. Environ. Microbiol. 2000;66(9):4139–4141. doi: 10.1128/aem.66.9.4139-4141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruh I., Gornez A.A., Ercisli S. Total phenolics, mineral elements, antioxidant and antibacterial activities of some edible wild plants in Turkey. Asian J. Chem. 2007;19(7):5755–5762. [Google Scholar]
- Dixon R.A. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Doughari J.H. Antimicrobial activity of Tamarindus indica Linn. Tropical J. Pharm. Res. 2006;5(2):597–603. [Google Scholar]
- Hess S.C., Brum R.L., Honda N.K., Cruz A.B., Moretto E., Cruz R.B., Mess M.A., Ferrari F., Cechinel F.V., Yunes R.A. Antibacterial activity and phytochemical analysis of Vochysia divergens (Vochysiaceae) J. Ethnopharm. 1995;47(2):97–100. doi: 10.1016/0378-8741(95)01260-k. [DOI] [PubMed] [Google Scholar]
- Iwu M.W., Duncan A.R., Okunji C.O. In: Perspectives on New Crops and New Uses. Janick A.S., editor. ASHS Press; Alexandria, VA: 1999. New antimicrobials of plant origin; pp. 457–462. [Google Scholar]
- Lutterodt G.D., Ismail A., Bashear R.H., Baharudin H.M. Antimicrobial effects of Psidium guajava extracts as one mechanism of its antidiarrhoeal action. Malay. J. Med. Sci. 1999;6(2):17–20. [PMC free article] [PubMed] [Google Scholar]
- Maghrani M., Zeggwah N., Michel J., Eddouks M. Antihypertensive effect of Lepidium sativum in spontaeneously hypertensive rats. J. Ethnopharm. 2005;102(1–2):193–197. doi: 10.1016/j.jep.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Marjorie M.C. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molan P.C. The antibacterial activity of honey 1. The nature of antibacterial activity. Bee World. 1992;73:59–76. [Google Scholar]
- Predrag L., Hui S., Uri C., Hasswan A., Arieh B. The effects of aqueous extracts prepared from leaves of Pistacia lentiscus in experimental liver disease. J. Ethnopharm. 2005;100(1–2):198–204. doi: 10.1016/j.jep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Shimada H. Semipurified fractions from Rumex hymeno-sepalus Shoyakugaku Zasshi. J. Pharamceut. Sci. 1966;55(3):49–57. [Google Scholar]
- Srinivasan D., Perumalsamy L.P., Nathan S., Sures T. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J. Ethnopharm. 2001;49:217–222. doi: 10.1016/s0378-8741(00)00345-7. [DOI] [PubMed] [Google Scholar]
- Täckholm V. second ed. Cairo University, Cooperative Printing Company; Beirut: 1974. Students Flora of Egypt. [Google Scholar]
- Veronika S., John P.M., Maita L., Michael C., Adam C., Carol Y., Anne O. Dual effect of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2006;50(8):2732–2740. doi: 10.1128/AAC.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X., Snyder J.K., Josbi B.S., Glinski J.A., Pelletier S.W. Systematic identification of natural products. Heterocycles. 1990;31:1879–1899. [Google Scholar]


