Abstract
The aim of the present study was to evaluate the cytotoxicity and antioxidant activity of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one (DMBPO) extracted from marine Streptomyces VITSVK5 spp. The strain was isolated from sediment samples collected at the Marakkanam coast of Bay of Bengal, India. Systematic screening of isolates for anti-Aspergillus activity resulted in the identification of Streptomyces species designated as Streptomyces VITSVK5 spp. Bioactivity guided extraction and purification yielded a compound 5-(2,4-dimethylbenzyl)pyrrolidin-2-one (DMBPO) and was tested for cytotoxicity and antioxidant activity. The structure of the extracted compound was established by spectroscopic studies and identified as 5-(2,4-dimethylbenzyl)pyrrolidin-2-one (DMBPO). DMBPO exhibited cytotoxic activity on HEP 2 and Hep G2 cell lines with the IC50 value of 2.8 μg/ml and 8.3 μg/ml, respectively, as compared to Vero cell line (22.6). DMBPO showed the hemolytic EC50 value of 288 μg/ml on human erythrocytes. DMBPO treatment showed fewer (31.7%) aberrations, gaps and chromatid breaks as compared to untreated controls (27.8%) of human chromosomes. DMBPO also exhibited significant (44.13% at 5 μg/ml DMBPO) DPPH radical scavenging activity and total antioxidant activity (50.10% at 5 μg/ml DMBPO). The results of this study showed that DMBPO is cytotoxic to cancer cells and possesses antioxidant property.
Keywords: Streptomyces VITSVK5 spp.; 5-(2,4-dimethylbenzyl)pyrrolidin-2-one; Cytotoxic activity; Radical scavenging activity; Total antioxidant activity
1. Introduction
Over the past 75 years, natural product derived compounds have led to the discovery of many drugs to treat numerous human diseases (Grabley and Thiericke, 1999). Natural products are chemical compounds derived from living organisms e.g. plants, animals and microorganisms. They can be defined as chemical compounds isolated or derived from organisms as primary or secondary metabolites. By employing sophisticated techniques under various screening programs, the rate of discovery of natural compounds exceeded 1 million so far (Pimentel-Elardo et al., 2010). Out of which 22,500 biologically active compounds that have been extracted are from microbes, 45% are produced by actinobacteria, 38% by fungi and 17% by unicellular bacteria (Demain and Sánchez, 2009). The oceans cover more than 70% of earth surface and little is known about the microbial diversity of marine sediments, which is an inexhaustible resource that has not been fully exploited. Marine extremophiles serve as valuable natural resource for novel products such as antibiotics, antitumor agents and other therapeutic substances (Amador et al., 2003). Microbial secondary metabolites have been known as one of the immense reservoir of natural chemical diversity with potent biological activity (Bush and Macielag, 2000). Most bacterial secondary metabolites are generated through a unique, multi-step biosynthetic process with specific enzymes for each complex structure formation. Their encoding genes are normally clustered within the genome of the organism and the precursors for the biosynthesis are derived from primary metabolites. Marine actinomycetes are potential providers of novel bioactive metabolites and have been currently emerging as an important source for natural products with unique chemical diversity. Members of the class actinobacteria especially Streptomyces spp. have long been recognized as prolific sources of useful bioactive metabolites, providing more than 85% of naturally occurring antibiotics discovered to date and continuing as a rich source of new bioactive metabolites (Berdy, 2005).
Actinomycetes represent one of the most studied and exploited classes of bacteria for their ability to make a wide range of biologically active metabolites (Ikeda et al., 2003). The actinobacteria plays a very important role among the marine bacterial communities, because of its diversity and ability to produce novel chemical compounds of high commercial value (Hopwood, 2007, Amador et al., 2003). The compounds isolated from marine Streptomyces, 2-allyoxyphenol and streptopyyrolidine have been reported to possess antioxidant and no cytotoxic activity (Arumugam et al., 2010, Shin et al., 2008). The studies on marine actinomycetes with respect to antioxidant and cytotoxic activity are very limited in the Indian sub-continent and most of the actinomycetes isolated were yet to be screened for bioactive secondary metabolites. Hence a study was carried out to extract the active compound from marine Streptomyces VITSVK5 spp. and to study its hemolytic activity on human red blood cells, cytotoxic effect on normal and selected cancer cells, DPPH free radical scavenging, and total antioxidant activity. In this study the cytotoxicity and antioxidant activity of a novel compound 5-(2,4-dimethylbenzyl)pyrrolidin-2-one extracted from Streptomyces VITSVK5 spp. was reported.
2. Materials and methods
2.1. Strain
The strain Streptomyces VITSVK5 spp. was isolated from the salt pans of Marrakanam coast of Bay of Bengal, India. The strain was selectively isolated using Starch casein agar, ISP No. 1 medium and the nutritional and cultural conditions for the growth were optimized. Isolation and characterization of the strain was previously reported (Saurav and Kannabiran, 2010).
2.2. Extraction and purification of the compound
Well grown slant culture of the potential isolate was used for the preparation of seed culture. The seed culture was inoculated in 50 ml medium containing the optimized production medium prepared with sea water 50%, distilled water 50%, pH 8.2 and incubated for 2 days in a rotary shaker (200 rpm) at 30 °C. The inoculums (10%) were transferred into 200 ml production medium in 1 l Erlenmeyer flasks and kept for fermentation for a week. After fermentation, the broth was centrifuged at 4000 rpm for 10 min at 10 °C and the supernatant was separated and filtered in 0.2 μm membrane filter. The supernatant was extracted twice with n-butanol (400 ml) and washed with 500 ml water. After separation, the organic phase was dried over Na2SO4 (anhydrous). The extract was then concentrated in a rotary vacuum and lyophilized using a freeze drier (Thermo, USA) at 5 °C for 5 h. The crude extracts were stored at −20 °C. The butanol layer was concentrated and the residual suspension (750 mg) was chromatographed over silica gel column and eluted with chloroform:MeOH (10:0, 9.5:0.5, 9:1, 8.5:1.5, 8:2, 7.5:2.5, 7:3). The active fractions were collected, concentrated and further separated by preparative TLC on silica gel with chloroform:MeOH (8:2) and the purity of the compound was analyzed.
2.3. Structure elucidation
The UV spectra of the compound were measured using UV–Visible spectrophotometer (Techcomp, Hong Kong). In order to investigate the presence of various functional groups in bioactive compound, the sample was lyophilized and mixed with KBr (1:20; 0.02 g of sample with KBr at a final weight of 0.4 g) and then grounded, desorbed at 60 °C for 24 h and pressed to obtain IR-transparent pellets. Infrared spectra of the compound were obtained using a Fourier Transform Infrared Spectrometer (FT/IR-AVATAR 330). The spectra were collected within a scanning range of 400–4000/cm. The FT-IR was first calibrated for background signal scanning with a control sample of pure KBr, and then the experimental sample was scanned. The spectra obtained was analyzed for various functional groups.
The proton NMR (1H NMR) and carbon NMR (13C NMR, V Bruker Avance III 500 MHz (AV 500)) spectra of the compound were obtained by using a dimethyl sulfoxide d6 (DMSO-d6) as solvent. It was further evaluated with DEPT-135. It was further confirmed by mass spectroscopy (HR-MS, Jeol GCMATE II). The structure of the compound was established with the help of spectral data obtained from spectroscopic techniques. The 3D structure of the compound was obtained by using chemdraw software (Ultra 8.0).
2.4. Assay of hemolytic activity
Hemolytic effect of the lead compound on human erythrocytes was evaluated by using washed human erythrocytes (RBCs). For the preparation of human erythrocytes the method of Malagoli (2007) was followed. The human erythrocyte was obtained from the peripheral blood (B+) of a healthy volunteer. The blood was used within 24 h after bleeding. The erythrocyte fraction was washed thrice with saline and resuspended in 10 ml PBS. The hemolytic activity of the compound was tested as reported earlier under in vitro conditions in 96-well plates. Each well received 100 μl of 0.85% NaCl solution containing 10 mM CaCl2. The first well that served as negative control contained only water, and in the second well, 100 μl of compound in different concentrations (5–500 μg/ml) was added. The osmolarity of extract was adjusted with 10X PBS to prevent osmotic lysis of erythrocytes. The last well served as positive control containing 20 μl of 0.1% Triton X-100 in 0.85% saline. Then, each well received 100 μl of human erythrocytes (2% suspension) in 0.85% saline containing 10 mM CaCl2. After 2 h of incubation, the cell suspension was centrifuged and the absorbance of supernatant was obtained at 540 nm. The average value was calculated from triplicate assay.
2.5. Assay of cytotoxicity
The cytotoxicity of the DMBPO (0–25 μg/ml) was tested on Vero (Green monkey kidney), HEP 2 (laryngeal carcinoma cells) and Hep G2 (Hepatocellular carcinoma) cell lines by MTT cell proliferation assay. Cells were cultured routinely in 75 cm2 culture flasks and maintained in RPMI 1640/DMEM (Himedia/Gibco, Mumbai, India) medium supplemented with 10% FBS (v/v) and 100 mg/l streptomycin and 100 IU/ml penicillin (Himedia, India), at 37 °C in 5% carbon dioxide. Cell lines were quantified (1 × 105 cells/well) as per user manual (CellQuanti MTT assay kit, Bioassay systems, CA). Briefly, the cells were cultured (80 ml per well) in a clear bottom 96-well tissue culture plate and incubated till confluence. Test compounds were added with cells and incubated for various time periods. After incubation, 15 ml (per 80 μl cell culture) of CellQuanti-MTTTM reagent was added per well and incubated for 4 h at 37 °C. Then 100 ml of the solubilization solution was added and kept in an orbital shaker for one hour at room temperature. The optical density was measured at 570 nm for each well on a multi well plate reader (Bio Rad). The test was carried out in triplicates. The wells containing only culture medium or treated with 0.1% of DMSO served as control. The average of the blank (controls) was determined and subtracted from the absorbance values. The graph was plotted with cell viability against the time period with various concentrations of the compound.
2.6. Assay of chromosomal aberrations
The effect of DMBPO on healthy human chromosome was studied to evaluate chromosomal aberrations if any. A total of 10 healthy volunteer donors who have no record of smoking and any history of chemical or radiation exposure were included in this study. Venous blood was drawn in sterile condition. Tests were performed in triplicates by adding 0.6 ml of heparinized blood to 6 ml of RPMI 1640 medium (Gibco, India), supplemented with 1.2 ml of fetal bovine serum (Himedia, India), antibiotics (penicillin and streptomycin, Himedia, India) lymphocytes were stimulated with 4% phytohemagglutinin (Invitrogen, USA) and the cultures were incubated at 37 °C for 72 h. Varying concentration of DMBPO (20–500 μg/ml) was added in G2 phase of the cell cycle. One hour prior to harvest, 0.4 mg/ml of colchicine (Sigma–Aldrich, USA) was added to arrest the cells at metaphase.
2.7. Slide scoring analysis
A total of 50 well-spread metaphases were scored for each individual on coded slides. The metaphases were scored for a chromosome number and for number and type of structural chromosomal aberrations. Breaks (lesions in which there is a clear misalignment) and gaps (an achromatic lesion in which there is a minimal misalignment) were considered. In addition other structural aberrations like dicentrics and ring chromosomes were also scored. The mitotic index (MI) was expressed as the number of cells in the mitotic division stage per 1000 cells. Mean chromosomal aberrations per cell for each subject was calculated and group mean values for patients and control individuals were compared for a statistical significance using Student’s t-test.
2.8. Assay of DPPH scavenging activity
The DPPH free radical scavenging activity was determined by the method of Madhumitha and Saral (2009) with some modifications. Each sample at different concentration in ethanol (2 ml) was mixed with 2 ml of ethanolic solution containing 1 mM DPPH. The mixture was shaken vigorously, and then left to stand for 30 min in the dark. The absorbance was measured at 517 nm. The absorbance of the control was obtained by replacing the sample with ethanol. DPPH radical scavenging activity of the sample was calculated as follows:
2.9. Assay of total antioxidant activity
The total antioxidant activity of DMBPO was determined according to the method of Prieto et al., (1999). Briefly, 0.3 ml of sample was mixed with 3.0 ml reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). Reaction mixture was incubated at 95 °C for 90 min under water bath. Absorbance of all the sample mixtures was measured at 695 nm. The total antioxidant activity was expressed as the number of equivalents of ascorbic acid.
2.10. Statistical analysis
All the experiments were performed in triplicates and the data obtained were expressed as mean ± standard error. P-values < 0.05 were considered as statistically significant. The IC50 and MIC50 values and their respective 95% confidence intervals were calculated by non-linear regression analysis using the data analysis software (Prism).
3. Results and discussion
Microorganisms from extreme environments have gained considerable attention in the recent years because of their diversity and biological activities, mainly due to their ability to produce novel chemical compounds of high commercial value. Microbial sources serve as a template for the isolation of many bioactive compounds. Streptomyces being the largest genus of the actinomycetes; is exclusively important in the production of pharmaceutically useful compounds including antibacterials antifungals, antitumor agents and immunosuppressants. The process of bioactive guided extraction and purification resulted in the isolation of active compound from the culture broth (10 l) of Streptomyces VITSVK5 spp. and the yield of pure compound was 112.3 mg. The assessment of purity of the compound by TLC and visualization of spot by iodine reagent and sulfuric acid resulted in identification of single spot with Rf value of 0.43 (chloroform:methanol, 8:2). The spectral data obtained for the compound were used to establish the structure of the compound. UV/vis (MeOH) λmax 290 nm; FT-IR cm−1 3436 (–NH), 2928, 1729 (C O); 1H NMR (DMSO-d6 500 MHz): 0.871 (s, J = 64 Hz, –CH3), 1.23–1.39 (m, J = 44 Hz, 2 × CH2), 3.37 (t, 1H), 4.29 (s, 2H), 7.25 (s, 1H), 7.65–7.69 (t, 2H), 8.17 (s, 1H); 13C NMR (DMSO-d6 500 MHz): 13.80 (CH3), 18.60 (CH3), 34.65(2 × CH2), 60.34 (CH2), 64.98 (CH), 128.62, 131.4, 133.6, 134.3, 136.7, 144.7, 166.9. DEPT; HRMS; m/z (found/cal.): 203.1325/203.1310. Based on the spectral data, the structure of the compound was identified as 5-(2,4-dimethylbenzyl)pyrrolidin-2-one (DMBPO) and the molecular formula was determined as C13H17NO. The structure of the compound is illustrated in Fig. 1. It is soluble in methanol, chloroform and DMSO. DMBPO showed significant anti-Aspergillus activity (unpublished data) against drug resistant Aspergillus clinical isolates and in order to know its toxicity on human cells the following studies were carried out.
Figure 1.
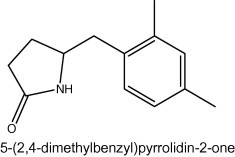
Structure of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one.
3.1. Hemolytic activity
Hemolytic assay was performed to ascertain the effect of DMBPO on membrane disruption. DMBPO exhibited a weak membranolytic activity on erythrocyte membrane with EC50 value of 288 μg/ml (Fig. 2). The total hemolysis of erythrocytes (positive control) was achieved with 20 μl of Triton X-100 (0.1%) after 1 h incubation. The EC50 and 95% confidence interval (CI 95%) were obtained by non-linear regression analysis. Evaluation of membrane stability during exposure of newer drugs is important and that to erythrocytes represent a good model for the study of membrane stability. The effect of various bioactive compounds on mechanical stability of the erythrocytic membrane serves as good indicator of membrane stability. The results of the present study showed that the DMBPO is non-hemolytic at low concentrations (5–50 μg/ml).
Figure 2.
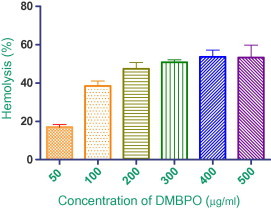
Effect of DMBPO (50–500 μg/ml) on hemolysis of human RBCs. Values of mean of three independent experiments with standard error bar.
3.2. Chromosomal aberrations
The effect of different concentrations of DMBPO on chromosomes was studied and it was observed that the most frequently observed aberrations were gaps and chromatid breaks in DMBPO (200 μg/ml) treated (31.7% total cells scored) cells and as compared to control (27.8%). The mean frequency of aberrant cells was found to be significantly low (2.05±0.76) as compared to controls (1.69±0.71) at 200 μg/ml where P > 0.01. The aberration was not increased up to 500 μg/ml of DMBPO. Induction of aberration by a DMBPO in donors was found to be significantly low as compared to the normal control (Fig. 4). Cytogenetic analysis of chromosomal aberrations has been suggested to be a useful tool to determine the safe maximum allowable concentration (MAC) of any drug (Šrám, 1981). The MAC of a chemical is defined as the maximum concentration which does not have any adverse affect on the human health on exposure. Chromosomal aberrations in human peripheral lymphocytes are recognized as a valuable biomarker to study the effect of the drug.
Figure 4.
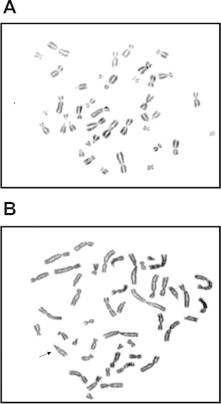
Effect of DMBPO on human chromosomal aberrations. (A) Metaphase spread for the untreated sample. (B) Metaphase spread for the treated sample with DMBPO at 200 μg/ml and arrow indicating chromosomal gap.
3.3. Cytotoxicity
The cytotoxic effect of different concentrations of DMBPO on normal Vero cell lines, Hep G2 and HEP2 cell lines were evaluated by MTT assay in 96-well plates. This assay is often used to measure viable cell where MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) is reduced to purple formazan in living cell. For determination of IC50, cells were assessed for proliferation inhibition and the IC50 values were calculated as 22.6, 8.3 and 2.8 μg/ml for Vero, HepG2 and HEP2 cell lines, respectively (Fig. 3). No morphological variations were observed in DMBPO treated Vero cells. Different concentrations of DMBPO on the tested cells revealed that DMBPO exhibits concentration and time dependent inhibition. The observed IC50 value indicates that DMBPO was less toxic to normal cells when compared to the malignant cells. Streptopyrrolidine, a benzyl pyrrolidine derivative isolated from a marine derived Streptomyces spp. KORDI-3973 was reported to be an angiogenesis inhibitor (Shin et al., 2008). Daryamides, antifungal polyketides isolated from culture broth of a Streptomyces strain, CNQ-085 have been shown to exhibit moderate cytotoxicity against the human colon carcinoma cell line HCT-116 and moderate antifungal activities against Candida albicans (Asolkar et al., 2006). Similarly Chandrananimycins, isolated from marine Actinomadura spp. MO48 have been shown to exhibit antibacterial, antifungal and anticancer activity (Maskey et al., 2003). The assessment of cytotoxicity is very important and a crucial step in the development of new therapeutic drugs for clinical application.
Figure 3.
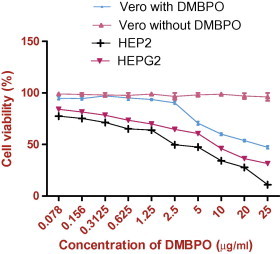
Effect of DMBPO on Vero, HepG2 and HEP2 cell lines determined by MTT assay. Cells treated with DMBPO at different concentration (0.078–25 μg/ml). Values are mean of three independent experiments standard error bar was shown in the figure.
3.4. Antioxidant activity
DPPH radical scavenging and total antioxidant activity of DMBPO was compared with ascorbic acid as control and are given in Fig. 5. The radical scavenging activity of DMBPO was concentration dependent and gradual increase of concentration increased the activity. At 5 μg/ml, this activity was 44.13% and at 10 μg/ml, the activity was increased to 57.83%. Total antioxidant activity was also concentration dependent. It was 50.10% (5 μg/ml) and 59.32% at 10 μg/ml of DMBPO concentration. Our results are in accordance with the report of Kumaqai et al. (1993), a compound PC-766 B isolated from marine actinomycetes Nocardia brasiliensis exhibited dose dependent antioxidant activity. Takamatsu et al. (2003) reported a Cymopo and avrainvilleol a meromonoterpene compound isolated from marine sponges exhibited antioxidant activity with the IC50 value of 4.0–6.1 μM. A superoxide scavenging inhibition and H2O2 induced DNA strand scission protection was observed with the compound strobilin–felixinin, sesterterpene isolated from marine sponges (Jiang et al., 2004).
Figure 5.
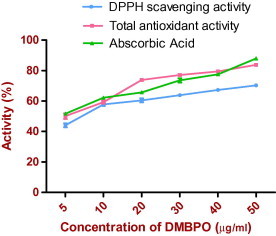
DPPH scavenging and total antioxidant activity of different concentration of DMBPO and was compared with control as Ascorbic acid. Values are mean standard error of three independent experiments.
The DPPH method is based on the reduction of DPPH, a stable free radical. With the odd electron, the free radical DPPH gives a maximum absorption at 517 nm by visible spectroscopy (purple color). As the odd electron of the radical becomes paired off in the presence of a hydrogen donor, e.g., a free radical scavenging antioxidant, the absorption strength is decreased, and the resulting decolorization (yellow color) is stoichiometric with respect to the number of electrons captured (Blois, 1958). This reaction has been widely used to investigate the free radical scavenging ability of pure compounds or act as hydrogen donors.
In conclusion the active compound DMBPO isolated from Streptomyces VITSVK5 spp. is less toxic to cells and possess antioxidant property which could be studied further by in vivo animal model studies.
Acknowledgment
Authors thank the management of VIT University for providing facilities to carry out this study.
References
- Amador M.L., Jimeno J., Ares L.P., Funes H.C., Hidalgo M. Progress in the development and acquisition of anticancer agents from marine sources. Ann. Oncol. 2003;14:1607–1615. doi: 10.1093/annonc/mdg443. [DOI] [PubMed] [Google Scholar]
- Arumugam M., Mitra A., Jaisankar P., Dasgupta S., Sen T., Gachhui R., Mukhopadhyay U.K., Mukherjee J. Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl. Microbiol. Biotechnol. 2010;86:109–117. doi: 10.1007/s00253-009-2311-2. [DOI] [PubMed] [Google Scholar]
- Asolkar R.N., Jensen P.R., Kauffman C.A., Fenical W. Daryamides A-C weakly cytotoxic polyketides from a marine derived actinomycete of the genus Streptomyces strain CNQ-085. J. Nat. Prod. 2006;69:1756–1759. doi: 10.1021/np0603828. [DOI] [PubMed] [Google Scholar]
- Berdy J. Bioactive microbial metabolites. A personal view. J. Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. [Google Scholar]
- Bush K., Macielag M. New approaches in the treatment of bacterial infections. Curr. Opin. Chem. Biol. 2000;4:433–439. doi: 10.1016/s1367-5931(00)00106-x. [DOI] [PubMed] [Google Scholar]
- Demain A.L., Sánchez S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabley S., Thiericke R. In: Drugs Discovery from Nature. Grabley S., Thiericke R., editors. Springer-Verlag; Berlin: 1999. The impact of natural products on drug discovery; pp. 1–37. [Google Scholar]
- Hopwood D.A. Therapeutic treasures from the deep. Nature Chem. Biol. 2007;3:457–458. doi: 10.1038/nchembio0807-457. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Ishikawa J., Hanamato A., Shinose M., Kikuchi H., Shiba T., Sakaki Y., Hattori H., Omura S. Complete genome sequence and comparative analysis of the industrial microorganism Stretomyces avermitilis. Nat. Biotechnol. 2003;21:526–531. doi: 10.1038/nbt820. [DOI] [PubMed] [Google Scholar]
- Jiang Y.H., Ryu S.H., Ahn E.Y., You S., Lee B.J., Jung J.H., Kim D.K. Antioxidant activity of (8E,13Z, 20Z)-strobilinin/(7E,13Z, 20Z)-felixinin from a marine sponge Psammocinia sp. Nat. Prod. Sci. 2004;10:272–276. [Google Scholar]
- Kumaqai K., Fukui A., Tanaka S., Ikemoto M., Moriquchi K., Nabeshima S. PC-766B, a new macrolide antibiotic produced by Nocardia brasiliensis II. Isolation, physico-chemical properties and structures elucidation. J Antibiot. (Tokyo) 1993;46:1139–1144. doi: 10.7164/antibiotics.46.1139. [DOI] [PubMed] [Google Scholar]
- Madhumitha G., Saral A.M. Free radical scavenging assay of Thevetia neriifolia leaf extracts. 2009;21:2468–2470. [Google Scholar]
- Maskey R.P., Li F.C.S., Qin H.H., Fiebig, Laatsch H. Chandrananimycins A–C: production of novel anticancer antibiotics from a marine Actinomadura spp. isolate M048 by variation of medium composition and growth conditions. J. Antibiot. 2003;56:622–629. doi: 10.7164/antibiotics.56.622. [DOI] [PubMed] [Google Scholar]
- Malagoli D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invert. Surviv. J. 2007;4:92–94. [Google Scholar]
- Pimentel-Elardo S.M., Kozytska S., Bugni T.S., Ireland C.M., Moll H., Hentsche U. Anti-parasitic compounds from Streptomyces spp. strains isolated from Mediterranean sponges. Mar. Drugs. 2010;8:373–380. doi: 10.3390/md8020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Saurav K., Kannabiran K. Antifungal activity of Streptomyces VITSVK5 spp. against drug resistant Aspergillus clinical isolates from pulmonary tuberculosis patients. J. Mycol. Med. 2010;20:101–107. [Google Scholar]
- Shin H.J., Kim T.S., Lee H-S., Park J.Y., Choi I-K., Kwon H.J. Streptopyrrolidine, an angiogenesis inhibitor from a marine-derived Streptomyces spp. KORDI-3973. Phytochemistry. 2008;69:2363–2366. doi: 10.1016/j.phytochem.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Šrám R.J. In: Industrial and Environmental Xenobiotics: Metabolism and Pharmacokinetics of Organic Chemicals and Methods. Gut I., Cikrt M., Plaa G.L., editors. Springer-Verlag; Berlin: 1981. Cytogenetic analysis of peripheral lymphocytes as a method for monitoring environmental levels of mutagens; pp. 187–193. [Google Scholar]
- Takamatsu S., Hodges T.W., Rajbhandari I., Gerwick W.H., Hamann M.T., Nagle D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003;66:605–608. doi: 10.1021/np0204038. [DOI] [PMC free article] [PubMed] [Google Scholar]


