Abstract
The intention of this investigation was to evaluate the free radical scavenging activity and erythrocyte protective activity of ethanolic extract of Crinumasiaticum (L) and lycorine. The ethanolic extract of C. asiaticum (L) and lycorine were found to have different levels of antioxidant properties in the test models. Both ethanolic extract of C. asiaticum (L) (0.5–2.5 mg/ml) and lycorine (0.010 mg–0.050 mg/ml) increases the percentage of lipid peroxidation inhibition (26.25 ± 0.23% and 19.25 ± 0.23%) and enhances the free radical scavenging activity (20.92 ± 0.22% and 20.52 ± 0.22%), scavenging of hydrogen peroxide (25.67 ± 0.17% and 23.07 ± 0.3%) superoxide anion scavenging activity (27.69 ± 0.16% and 16.09 ± 0.7%) at concentration of 2.5 and 0.050 mg of C. asiaticum (L) and lycorine, respectively. But compared with tocopherol (P < 0.05) less activity was observed by C. asiaticum (L) and lycorine. The ethanolic extract of C. asiaticum (L) and lycorine were normalized to reduce the level of glutathione and also to sustain the status of protein in erythrocytes during the peroxyl radical [2,2-azobis (2-amidinopropane) dihydrochloride (AAPH)] induced oxidative damage in ex vivo model. The present results of the investigations demonstrated that protective nature of the C. asiaticum (L) and lycorine will be considered as a significant natural antioxidant source.
Keywords: Crinumasiaticum (L), Lycorine, Antioxidant, AAPH, Free radical lipid peroxidation
1. Introduction
Free radicals have very short half life, high reactive damaging activity towards macromolecules like proteins, DNA and lipids (Evans and Halliwell, 1999). These free radicals may be either oxygen derived (reactive oxygen species ROS) or nitrogen derived (reactive nitrogen species RNS). The oxygen derived species include super oxide), HO• (hydroxyl), (Hydro peroxy), ROO• (peroxy), RO• (Alkoxy) as free radicals and Hydrogen Peroxide, Hydrochlorus acid, O3 (Ozone), (Singlet oxygen) as non radicals. Similarly, nitrogen derived oxidant species are Nitric Oxid (•NO), Peroxy nitrite (ONOO•), Nitrogen dioxide and nitrogen trioxide (N2O3) (Evans and Halliwell, 1999).
It is generally considered that the inhibition of lipid peroxidation by an antioxidant may be due to free radical scavenging activity. Lipids of biological membranes, especially, those in the spinal cord and brain containing highly oxidizable polyunsaturated fatty acids are affected (Braughler and Hall, 1989). Reactive oxygen mediated modification of DNA, proteins, lipids and small cellular molecules is associated with a number of pathological processes including atherosclerosis, arthritis, diabetes, cataractogenesis, muscular dystrophy, pulmonary dysfunction, inflammatory disorders, tissue damage and neurological disorders such as Alzheimer’s disease (Frlich and Riederer, 1995). The term of the antioxidant is defined as any substance which delays or inhibits oxidation of the substrate (Halliwell and Gutterridge, 1989). This may be illustrated by considering one of the many mechanisms by which oxidative stress can cause damage by stimulating the free radical chain reaction of lipid peroxidation.
The ethanobotanical use of Crinum as with many other medicinal plants can be explained on the basis of chemical and physiological studies. Most cases confirm the therapeutic value of the plants. However, where plants have been studied chemically a plant potential pharmaceutical value can be assumed, if it is used widely (Githens, 1949). The reason why Crinum is used for medicinal purposes in many countries for similar reason is possibly due to their alkaloid constituents (Waller and Nowacki, 1978). Lycorine is a natural alkaloid extracted from amaryllidaceae and it has various pharmacological and microbiological effects. Researchers had revealed that lycorine can inhibit protein synthesis in eukaryotic cells (Jimenez et al., 1976) and acetylcholinesterase activity (Hohmann et al., 2002). In the presence of calprotectin lycorine was shown to inhibit protein synthesis and cell apoptosis in MM46 cells (Elgorashi et al., 2004). Lycorine was found to posess potent antiviral activity against severe acute respiratory syndrome associated coronavirus (Li et al., 2005). Lycorine could effectively arrest the cell cycle at the G2/M phase and induce apoptosis in HL-60 cells (Liu et al., 2004). The present investigation is an endeavor to validate the scientific use of ethanolic extract of Crinum asiaticum (L) and lycorine against 2-amidinopropane induced oxidative damage in human erythrocytes.
2. Materials and method
2.1. Plant material
C. asiaticum (L) was collected in the month of November from the Cauvery river basin, Thanjavur, Tamilnadu, India and then transferred to PRIST University, Thanjavur, Tamilnadu, India. It was taxonomically identified and authenticated by Rev Dr. S. John Britto SJ, Director, The Rapinat Herbarium and Centre for Molecular Systematics, St. Joseph College (Autonomous), Tiruchirapalli, Tamilnadu, India. The voucher specimen was deposited at the Rapinat herbarium and the voucher number is RHCD BP15.
2.2. Preparation of extract
Fresh leaves of C. asiaticum were air dried in the shade and powdered. The extraction was carried out by mixing the powdered (1000 g) leaves with 1:2 (w/v) in 70% ethanol (v/v) for 2 days. The resultant extract was filtered (Chopra et al., 1981) and concentrated by rotary evaporator under reduced pressure and low temperature. The yield of the extract was 12% (w/w) in terms of dried starting material.
2.3. Chemicals
The following reagents were obtained from sigma aldrich Bangalore lycorine, 2-thiobarbituric acid (TBA), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), glutathione (GSH), additionally phosphoric acid (H3PO4), AAPH and trichloroacetic acid (Merck, India). Ammonium persulfate, bisacrylamide (30%), Coomassie Brilliant Blue R-250, sodium dodecyl sulfate (SDS), N,N,N,N-tetramethylethylenediamine (TEMED) and dye reagent were obtained from Genie Bangalore.
2.4. In vitro antioxidant analysis
The measurement of lipid peroxidation by FTC method (Iqbal and Bhanger, 2006). Superoxide anion scavenging activity (Liu et al., 1997) and (Shirwaikar et al., 2006). Scavenging of hydrogen peroxide and free radical scavenging activity (Ruch et al., 1989) of ethanolic extract of C. asiaticum (L) and lycorine were estimated by Techcomp UV spectrophotometer 2310. The concentration of ethanolic extract of C. asiaticum (L) and lycorine was used in this analysis ranging from 0.5 to 2.5 mg/ml and 0.010 mg to 0.050 mg/ml, respectively.
2.5. Ex vivo analysis
2.5.1. Erythrocyte preparation
Blood (5–15 ml) was obtained from normal volunteers via vena puncture. Human erythrocytes from citrated blood were isolated by centrifugation at 3000g for 10 min and washed three times with PBS and then resuspended using the same buffer to the desired hematocrit level (Yang et al., 2006). Cells stored at 4 °C were used within 6 h of sample preparation. In order to induce free radical chain oxidation in erythrocytes by aqueous proxy radicals were generated from thermal decomposition of AAPH (an azo compound) in oxygen. An erythrocyte suspension at 5% hematocrit was incubated with PBS (control) and pre incubated with the ethanolic extract of C. asiaticum (L) (0.5–2.5 mg/ml and lycorine (010 mg–0.050 mg/ml) at 37 °C for 30 min followed by incubation with and without 25 mM AAPH in PBS at pH 7.4. This reaction mixture was shaken gently while being incubated for a fixed interval at 37 °C.
2.5.2. Hemolysis assay
The reaction mixture (200 μl) was removed and centrifuged at 3000g for 2 min with absorbance of the supernatant determined at 540 nm. Reference values were determined using the same volume of erythrocytes in a hypotonic buffer (5 mM phosphate buffer at pH 7.4; 100% hemolysis). The hemolysis percentage was calculated using the formula absorbance of sample supernatant/reference value ×100.
2.5.3. Quantitative estimation of lipid peroxidation of erythrocytes
In vitro peroxidation was assessed using a thiobarbituric acid (TBA) reaction. 1 ml of reaction mixture, 100 μl of H3PO4 (0.44 M) and 250 μl (0.67%) thiobarbituric acid were added and incubated at 95 °C for 1 h, then it was allowed to stand on ice for 10 min before adding 150 μl trichloroaceticacid (20%). After centrifugation at 12,000g for 10 min the peroxide content in the supernatant obtained was assayed using the TBA reaction with the molar extinction coefficient (O.D 523) of malondialdehyde (MDA). Tetraethoxypropane was used as the standard (Yagi, 1984). MDA values were expressed as pmol/g Hb.
2.5.4. Determination of glutathione content in erythrocytes
Intracellular glutathione (GSH) was determined by titration with DTNB as described by Van den Berg (1992). After centrifugation of the reaction mixtures (2 ml), 0.6 ml water was added to the erythrocyte pellets for cell lysis. Then 0.5 ml of the lysate was precipitated by the addition of 0.5 ml metaphosphoric acid solution [1.67 g metaphosphoric acid, 0.2 g EDTA (disodium salt) and 30 g NaCl in 100 ml water]. After 5 min the protein precipitate was separated from the remaining solution by centrifugation at 18,000g for 10 min. Then combined 0.45 ml of the solution with 0.45 ml of 300 mM Na2HPO4 and the absorbance at 412 nm was read against a blank consisting of 0.45 ml solution and 0.45 ml water. Then 100 μl DTNB solutions (20 mg DTNB in 100 ml of 1% citrate) were added to the blank and the sample. The absorbance of the sample was again read against the blank at 412 nm. GSH values were expressed as μmol/g Hb.
2.5.5. Preparation of erythrocyte ghosts and analysis of erythrocyte membrane proteins by SDS–PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) of erythrocyte ghosts was prepared from the reaction mixture using hypotonic solution in 30 volumes of 5 mM NaH2PO4 (pH 7.4) as previously described (Dodge et al., 1962). Hemolysate preparations were washed five times with phosphate buffer then centrifuged at 12,000g for 30 min. Protein concentrations of the erythrocyte ghost pellets were determined (Lowry et al., 1951) using BSA fraction V as the standard. SDS–PAGE was carried out on 1.5 mm-thick slab gel with 10% gels for condensation, separations, and stained with Coomassie brilliant blue (Arduini and Strn, 1985).
2.5.6. Statistical analysis
Data were evaluated with SPSS/10 software, hypothesis testing methods included one way analysis of variance (ANOVA) followed by least significant difference (LSD) test P values of less than 0.05 were considered to show statistical significance. All these results were expressed as mean ± SD for six animals in each group.
3. Results and discussion
Membrane lipids are rich in unsaturated fatty acids that are most susceptible to oxidative processes. Especially, linoleic acid and arachidonic acid are targets of lipid per oxidation (Nabavi et al., 2009). The absorbance data of linoleic acid peroxidation determined by the FTC method at 37 °C after the addition of different concentrations of the ethanolic extract of C. asiaticum (L) and lycorine (0.5–2.5 mg/ml and 0.010 mg–0.050 mg/ml). The oxidation of linoleic acid without addition of extracts and lycorine were accompanied by rapid increases of the lipid peroxidation value when compared with ethanolic extract of C. asiaticum (L) and lycorine treated group. The FTC results show maximum inhibition of lipid peroxidation in linoleic acid by ethanolic extract of C. asiaticum (L), lycorine and tocopherol was found to be 26.25 ± 0.25%, 19.30 ± 0.23% and 56 ± 1.81 at concentrations of 2.5 mg/ml, 0.050 mg/ml and 0.125 mg/ml, respectively (Figure 1, Figure 2, Figure 3). However, significant differences in antioxidant activities were found among ethanolic extract of C. asiaticum (L) and lycorine at all the concentration. Similarly, our results concurred with the previous report (Ozsoy et al., 2000); it was found that different concentrations of the extract of Smilax excelsa (0.16–2.5 mg/ml) were added and significant concentration dependent inhibition of lipid peroxidation was observed.
Figure 1.
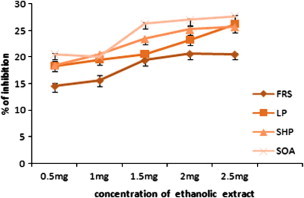
The in vitro antioxidant activity of ethanolic extract of C. asiaticum (L). FRS, free radical scavenging activity, LP, lipid peroxidation; SHP, scavenging of hydrogen peroxide; SOA, superoxide anion scavenging activity. Values are expressed as the mean ± SEM.
Figure 2.
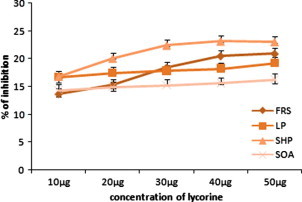
The in vitro antioxidant activity of lycorine. FRS, free radical scavenging activity; LP, lipid peroxidation; SHP, scavenging of hydrogen peroxide; SOA, superoxide anion scavenging activity. Values are expressed as the mean ± SEM.
Figure 3.
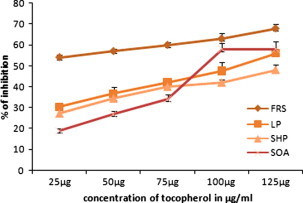
The in vitro antioxidant activity of tocopherol. FRS, free radical scavenging activity; LP, lipid peroxidation; SHP, scavenging of hydrogen peroxide; SOA, superoxide anion scavenging activity. Values are expressed as the mean ± SEM.
The model method of scavenging the stable DPPH radical is widely used to evaluate the free radical scavenging ability of various samples (Koleva et al., 2001). DPPH is a stable nitrogen centered free radical the color of which changes from violet to yellow upon reduction by either the process of hydrogen or electron donation. Substances which are able to perform this reaction can be considered as antioxidants and, therefore, radical scavengers (Dehpour et al., 2009). Figure 1, Figure 2 show the dose response curves of DPPH radical scavenging activities of the ethanolic extract of C. asiaticum (L) and lycorine. The free radical scavenging effect of ethanolic extract of C. asiaticum (L) and lycorine on DPPH radicals increased from 0.5 mg to 2.5 mg/ml and 0.010 mg to 0.050 mg/ml, respectively and was 20.5 ± .023% and 20.92% at concentrations of 2.5 mg/ml and 0.050 mg/ml, respectively, the results show that the ethanol extract of C. asiaticum (L) and lycorine showed significant DPPH radical scavenging activity when compared with tocopherol.
Superoxide radical is known to be very harmful to cellular components as a precursor of the more reactive oxygen species contributing to tissue damage and various diseases (Halliwell and Gutteridge, 1999). The scavenging activities of the extracts on superoxide radicals are shown in Figure 1, Figure 2. It was found that superoxide scavenging activities of the ethanol extract of C. asiaticum and lycorine increases with an increase of their concentrations. The ethanolic extract of C. asiaticum (L) (27.69 ± 0.16%), lycorine (16.29 ± 0.74%) and tocopherol (58 ± 35%) exhibited maximum percentage of superoxide radical scavenging activity at 2.5 mg/ml, 0.050 mg/ml and 0.125 mg/ml, respectively. However, other concentrations also exhibited significant superoxide radical scavenging properties. The increase of superoxide radical scavenging activity with antioxidant thus indicates the consumption superoxide radical by ethanol extract of C. asiaticum (L) and lycorine in the reaction mixture.
Hydrogen peroxide itself is not very reactive but it can sometimes be toxic to cells, since it may give rise to hydroxyl radicals inside the cell (Halliwell, 1991). Hydrogen peroxide is unique in that it can be converted to the highly damaging hydroxyl radical or be catalyzed and excreted harmlessly as water. The ethanolic extract of C. asiaticum (L) and lycorine were capable of scavenging hydrogen peroxide in a concentration dependent manner (Figure 1, Figure 2). The ethanolic extract of C. asiaticum (L) showed more potent scavenging hydrogen peroxide than the lycorine. Moreover, compared with positive control, less activity was observed by 25.67 ± 0.17%, 23.07 ± 0.31% and 48 ± 27% at concentrations of 2.5 mg/ml, 0.050 mg/ml and 0.125 mg/ml, respectively. Scavenging of H2O2 by ethanolic extract of C. asiaticum (L) and lycorine may be attributed to donate the electrons to H2O2 and thus neutralizing it to water.
AAPH is a water soluble azo compound which is used extensively as a free radical generator often in the study of lipid peroxidation and the characterization of antioxidants (Noguchi et al., 1998, Rice Evans and Miller, 1994). The erythrocyte membrane contains abundant polyunsaturated fatty acids which are very susceptible to free radical induced peroxidation. Since the generation rate of free radical from the decomposition of AAPH at physiological temperature can be easily controlled, this water soluble azo compound can be used as free radical resource to attack the erythrocyte membrane to induce hemolysis. Hence, the AAPH induced hemolysis provides a good approach to research the free radical induced membrane damage (Zou et al., 2001).
Figure 4, Figure 5 represents the human erythrocyte hemolysis induced by the AAPH with the addition of ethanolic extract of C. asiaticum (L) and lycorine. The hemolysis is lagged indicating that endogenous antioxidants in the erythrocytes can trap radicals to protect them against free radical induced hemolysis as described previously (Zou et al., 2001). Both the ethanolic extract of C. asiaticum (L) and lycorine (at 0.5–2.5 mg/ ml and 0.010 mg–0.050 mg/ml, respectively) reduced the AAPH induced hemolysis in a concentration and time dependent manner. The dose dependent association can be demonstrated between different interval time and ethanolic extract of C. asiaticum (L) and lycorine concentration. This may explain its protective effect against free radicals and also the stabilizing effect on the red blood cell membrane.
Figure 4.
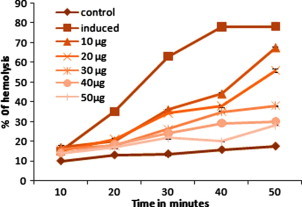
Effect of ethanolic extract of C. asiaticum (L) on AAPH induced the hemolysis GSH amendment in the human erythrocytes. Erythrocyte suspension was incubated with PBS (control) and preincubated with ethanolic extracts of C. asiaticum (L) at different concentrations for 30 min. The product was then incubated with 25 mM AAPH for 10, 20, 30, 40, and 50 min at 37 °C. Values are expressed as the mean ± SEM of three experiments. ∗ Indicates a significant difference from AAPH induced group (P < 0.05 level).
Figure 5.
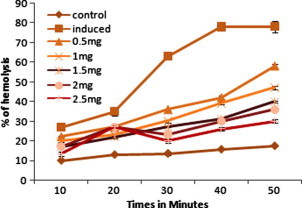
Effect lycorine on AAPH induced the hemolysis GSH amendment in the human erythrocytes. Erythrocyte suspension was incubated with PBS (control) and preincubated with lycorine at different concentrations for 30 min. The product was then incubated with25 mM AAPH for 10, 20, 30, 40, and 50 min at 37 °C. Values are expressed as the mean ± SEM of three experiments. ∗ Indicates a significant difference from AAPH induced group (P < 0.05 level).
Many studies have focused on the free radical initiated peroxidation of membrane lipid associated with a variety of pathological events. All natural and synthetic antioxidants have been used to trap peroxyl radical and other radicals to protect the membrane lipids against free radical chain reactions (Adom and Liu, 2005). Similarly, our present result shows the ethanolic extract C. asiaticum (L) and lycorine would prevent the erythrocyte damage by AAPH induced lipid peroxidation. The AAPH induced lipid peroxidation of erythrocytes is reflected in the MDA generation that occurs with the addition of ethanolic extract of C. asiaticum (L) and lycorine. MDA levels for the normal-group erythrocytes were 5.3 ± 0.12, 6.2 ± 0.42 and 6.8 ± 0.47 pmol/g Hb, respectively at 20, 40, and 60 min and it was increased to 24.42 ± 0.6, 43.4 ± 0.45 and 57.8 ± 0.16 pmol/g Hb after incubation with 25 mM AAPH. The addition of AAPH caused time dependent lipid peroxidation of erythrocytes. The ethanolic extract of C. asiaticum (L) and lycorine significantly inhibited the AAPH induced MDA formation. The results were presented in Table 1, Table 2.
Table 1.
Effect of lycorine on erythrocyte MDA content (pmol/g Hb).
| Time of incubation (min) | Normal | Induced | 10 μg | 20 μg | 30 μg | 40 μg | 50 μg |
|---|---|---|---|---|---|---|---|
| 20 | 5.3 ± 0.12 | 24.42 ± 0.6 | 20.31 ± 0.88⁎ | 18.69 ± 0.1⁎ | 15.49 ± 0.13⁎ | 14.17 ± 0.1⁎ | 7.1 ± 0.86⁎ |
| 40 | 6.2 ± 0.42 | 43.4 ± 0.45 | 32.2 ± 0.58⁎ | 27.44 ± 0.32⁎ | 25.3 ± 0.21⁎ | 25.17 ± 0.56⁎ | 24.9 ± 0.24⁎ |
| 60 | 6.8 ± 0.47 | 57.8 ± 0.16 | 36.12 ± 0.21⁎ | 30.76 ± 0.71⁎ | 27.47 ± 0.27⁎ | 27.7 ± 0.12⁎ | 23.54 ± 0.15⁎ |
Effect of lycorine on AAPH induced MDA alteration in the human erythrocytes. Erythrocyte suspension was incubated with PBS (control) or pre incubated with lycorine at different concentrations for 30 min. The product was then incubated with 25 mM AAPH for 20, 40, and 60 min at 37 °C. Values are expressed as the mean ± ± SEM three experiments.
Significant difference from AAPH group (P < 0.05) level.
Table 2.
Effect of Crinumasiaticum on erythrocyte MDA content (pmol/g Hb/g Hb).
| Time of incubation (min) | Normal | Induced | 0.5 mg | 1.0 mg | 1.5 mg | 2 mg | 2.5 mg |
|---|---|---|---|---|---|---|---|
| 20 | 5.3 ± 0.12 | 24.4 ± 0.6 | 18.4 ± 0.22⁎ | 16.4 ± 0.27⁎ | 14.3 ± 0.35⁎ | 12.3 ± 0.18⁎ | 8.34 ± 0.11⁎ |
| 40 | 6.2 ± 0.42 | 43.4 ± 0.45 | 32.5 ± 0.27⁎ | 28.3 ± 0.37⁎ | 26.6 ± 0.25⁎ | 23.5 ± 0.28⁎ | 20.9 ± 0.31⁎ |
| 60 | 8.6 ± 0.47 | 57.8 ± 0.16 | 28.02 ± 0.34⁎ | 26.2 ± 0.23⁎ | 25.5 ± 0.76⁎ | 23.5 ± 0.09⁎ | 19.2 ± 0.78⁎ |
Effect of Crinumasiaticum on AAPH induced MDA alteration in the human erythrocytes. Erythrocyte suspension was incubated with PBS (control) or pre incubated with Crinumasiaticum at different concentrations for 30 min. The product was then incubated with 25 mM AAPH for 20, 40, and 60 min at 37 °C. Values are expressed as the mean ± SEM three experiments.
Significant difference from AAPH group (P < 0.05 level).
GSH is the major non-enzymatic antioxidant, regulator of intracellular redox homeostasis and is ubiquitously present in all cell types (Meister and Anderson, 1983). In the present study, normal human erythrocytes incubate with 25 mM APPH for 1 h resulted in loss of GSH. However, erythrocytes treated with ethanolic extract of C. asiaticum (L) and lycorine were reverted to the reduced level of GSH when compared with APPH induced erythrocytes. Several workers have suggested that GSH provides the first line of defense during oxidative insult (Vissers and Winterbourne, 1995). In the presence of free radicals, mainly H2O2, GSH acts as an electron donor and is oxidized into GSSG by glutathione peroxidase. The GSSG generated is later converted back to GSH by glutathione reductase in which reduced nicotinamide adenine dinucleotide phosphate is the hydrogen donor. When cellular GSSG levels increase during oxidative stress, GSSG is exported into extracellular fluid to avoid thiol toxicity. In the AAPH-induced erythrocytes hemolysis shows the rapid depletion of GSH that might be caused by oxidation processes taking place in erythrocyte membranes. The resultant lipid peroxides might also react with GSH and that led to a decrease in GSH, it means increase in GSSG. If GSSG accumulates within the cell, it can form protein glutathione adducts via reversible thiol exchange reactions referred to as S-glutathionylation of protein. Protein S-glutathionylation has been implicated in the buffering of oxidative stress, stabilization of extracellular proteins, protection of proteins against irreversible oxidation of critical cysteine residues, and regulation of enzyme activity. Glutathionylated proteins are more stable than GSSG and less prone to enzymatic reduction by glutathione reductase (Table 3, Table 4).
Table 3.
Effect of ethanolic extract of Crinumasiaticum (L) on erythrocyte GSH content (μmol/g Hb).
| Time of incubation (min) | Normal | Induced | 0.5 mg | 1.0 mg | 1.5 mg | 2 mg | 2.5 mg |
|---|---|---|---|---|---|---|---|
| 20 | 2.94 ± 0.28 | 0.98 ± 0.52 | 1.41 ± 0.36⁎ | 1.85 ± 0.17⁎ | 1.87 ± 0.12⁎ | 1.92 ± 0.125⁎ | 2.3 ± 0.24⁎ |
| 40 | 2.93 ± 0.22 | 0.68 ± 0.42 | 1.21 ± 0.35⁎ | 1.73 ± 0.17⁎ | 1.80 ± 0.11⁎ | 1.87 ± 0.135⁎ | 2.0 ± 0.14⁎ |
| 60 | 2.9 ± 0.13 | 0.38 ± 0.46 | 0.94 ± 0.31⁎ | 1.65 ± 0.28⁎ | 1.77 ± 0.23⁎ | 1.82 ± 0.38⁎ | 1.93 ± 0.56⁎ |
Effect Crinumasiaticum (L) on AAPH-induced GSH amendment in the human erythrocytes. Erythrocyte suspension was incubated with PBS (control) or preincubated with ethanolic extracts of C. asiaticum at different concentrations for 30 min. The product was then incubated with 25 mM AAPH for 20, 40, and 60 min at 37 °C. Values are expressed as the mean ± SEM of three experiments.
Significant difference from AAPH group (P < 0.05 level).
Table 4.
Effect of lycorine on erythrocyte GSH content (μmol/g Hb).
| Time of incubation (min) | Normal | Induced | 10 μg | 20 μg | 30 μg | 40 μg | 50 μg |
|---|---|---|---|---|---|---|---|
| 20 | 2.94 ± 0.28 | 0.98 ± 0.52 | 1.32 ± 0.16⁎ | 1.38 ± 0.28⁎ | 1.60 ± 0.88⁎ | 1.68 ± 0.1⁎ | 1.62 ± 0.17⁎ |
| 40 | 2.93 ± 0.22 | 0.68 ± 0.42 | 1.18 ± 0.16⁎ | 1.18 ± 0.23⁎ | 1.32 ± 0.34⁎ | 1.54 ± 0.3⁎ | 1.6 ± 0.20⁎ |
| 60 | 2.9 ± 0.13 | 0.38 ± 0.46 | 0.84 ± 0.32⁎ | 0.95 ± 0.12⁎ | 1.07 ± 0.23⁎ | 1.21 ± 0.2⁎ | 1.50 ± 13⁎ |
Effect of lycorine on AAPH-induced GSH amendment in the human erythrocytes. Erythrocyte suspension was incubated with PBS (control) or preincubated with lycorine at different concentrations for 30 min. The product was then incubated with 25 mM AAPH for 20, 40, and 60 min at 37 °C. Values are expressed as the mean ± SEM of three experiments.
Significant difference from AAPH group (P < 0.05 level).
The membrane proteins of erythrocytes are basically composed of band A, B, C, D, E and other accessory proteins. However, expression of these bands vary from APPH induced and drug treated. It is pointed out that some of the higher and lower molecular weight proteins were over expressed in APPH induced erythrocytes when compared to normal and drug treated. Oxidants produce alterations in erythrocyte membranes as manifested by a decreased cytoskeleton protein content (LMW proteins) and production of HMW proteins (Snyder et al., 1985, Flynn et al., 1983). Similarly, the present results showed over expression of some proteins above the proteins band A when human erythrocytes incubate with AAPH for 3 h. These results assign that both ethanolic extract of C. asiaticum (L) (2.5 mg/ml) and lycorine (0.050 mg/ml) prevent the AAPH induced changes in the erythrocyte membrane proteins. The results were showed in Fig. 6.
Figure 6.
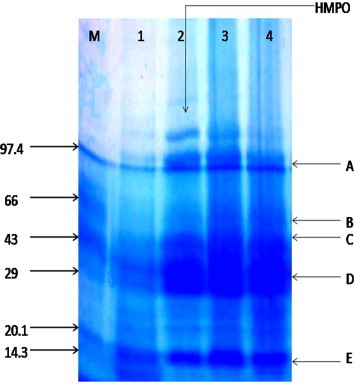
Effects of ethanolic extract of C. asiaticum (L) and lycorine on AAPH induced alteration in erythrocyte membrane proteins analyzed using SDS–PAGE. Erythrocyte suspension at 5% hematocrit was incubated with PBS (control) and preincubated with ethanolic extract of C. asiaticum (L) and lycorine for 30 min. The product was incubated with 25 mM AAPH for 6 h at 37 °C. Lane M: Marker protein Lane a: intact erythrocyte membrane proteins. Lane b: erythrocytes oxidized with 25 mM AAPH. Lane’s c and d: erythrocytes preincubated with ethanolic extract of C. asiaticum (L) and lycorine at 2.5 mg/ml and 0.050 μg/ml. The amount of layered protein was 40 μg in each well. This experiment was repeated two times with similar results were achieved. HMPO: represents high molecular weight protein over expression.
4. Conclusion
It was concluded that ethanolic extract of C. asiaticum (L) and lycorine can reduce the AAPH induced erythrocyte hemolysis, lipid/protein per oxidation and cell damage. The ethanolic extract of this plant may be used as a source of natural antioxidants as their crude extracts and lycorine exhibit significant free radical scavenging activity when compared with tocopherol. The results indicate that the different concentration of ethanolic extract of C. asiaticum (L) and lycorine exhibited significant antioxidant activities. The maximum percentage of free radicals activity were observed by C. asiaticum when compared with lycorine, however, tocopherol showed higher free radical scavenging activity than the C. asiaticum and lycorine. Therefore, purpose of the present finding confirmed that ethanolic extract of C. asiaticum (L) and lycorine possibly will have defensive antioxidant and have better application in food and drug products.
Acknowledgement
The authors are grateful to the PRIST University, Thanjavur, Tamilnadu, India for their financial and excellent technical support.
References
- Adom K.K., Liu R.H. Rapid peroxyl radical scavenging capacity (PSC) assay for assessing both hydrophilic and lipophilic antioxidants. J. Agric. Food Chem. 2005;53(17):6572–6580. doi: 10.1021/jf048318o. [DOI] [PubMed] [Google Scholar]
- Arduini A., Strn A. Spectrin degradation in intact red blood cells by phenylhydrazine. Biochem. Pharmacol. 1985;34:4283–4289. doi: 10.1016/0006-2952(85)90286-2. [DOI] [PubMed] [Google Scholar]
- Braughler J.M., Hall E.D. Central nervous system trauma and stroke biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radical Biol. Med. 1989;6:289–301. doi: 10.1016/0891-5849(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Chopra R.N., Nair S.L., Chopra J.C. CSIR Publication; New Delhi: 1981. Glossary of Indian Medicinal Plants. p. 50. [Google Scholar]
- Dehpour A.A., Ebrahimzadeh M.A., Nabavi S.F., Nabavi S.M. Antioxidant activity of methanol extract of Ferulaassafoetida and its essential oil composition. Grasas Aceites. 2009;60(4):405–412. [Google Scholar]
- Dodge J.T., Mitchell C., Hanahan D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1962;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Elgorashi E.E., Stafford G.I., Van Staden J. Acetyl cholin esterase enzyme inhibitory effects of amaryllidaceae alkaloids. Planta Med. 2004;70:260. doi: 10.1055/s-2004-818919. [DOI] [PubMed] [Google Scholar]
- Evans P., Halliwell B. Free radicals and bearing. Ann. N.Y. Acad. Sci. 1999;884:19. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Flynn T.P., Allen D.W., Johnson G.J., White J.G. Oxidant damage of the lipids and proteins of the erythrocyte membranes in unstable hemoglobin disease and evidence for the role of lipid peroxidation. J. Clin. Invest. 1983;71:1215–1223. doi: 10.1172/JCI110870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frlich I., Riederer P. Free radical mechanisms in dementia of Alzheimer type and the potential for antioxidative treatment. Drug Res. 1995;45:443–449. [PubMed] [Google Scholar]
- Githens T.S. University of Pennsylvania Press; Philadelphia, PA: 1949. Drug Plants of Africa. [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med. 1991;91:14–22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutterridge J.M.C. second ed. Clarendon Press; Oxford: 1989. Free Radicals in Biology and Medicine. [Google Scholar]
- Halliwell B., Gutteridge J.M.C. In: Free Radicals in Biology and Medicine. third ed. Halliwell B., Gutteridge J.M.C., editors. Oxford University Press; 1999. Free radicals, other reactive species and disease; pp. 639–645. [Google Scholar]
- Hohmann J., Forgo P., Molnar J., Wolfard K., Molnár A., Thalhamme T. Ntiproliferative amaryllidaceae alkaloids isolated from the bulbs of Sprekeliaformosissima and Hymenocallisfestalis. Planta Med. 2002;68:454. doi: 10.1055/s-2002-32068. [DOI] [PubMed] [Google Scholar]
- Iqbal S., Bhanger M.I. Effect of season and production location on antioxidant activity of Moringaoleifera leaves grown in Pakistan. J. Food Compos. Anal. 2006;19:544–551. [Google Scholar]
- Jimenez A., Santos A., Alonso G., Vazquez D. Inhibitors of protein synthesis in eukaryotic cells. Comparative effects of some amaryllidaceae alkaloids. Biochim. Biophys. Acta. 1976;425:342. doi: 10.1016/0005-2787(76)90261-6. [DOI] [PubMed] [Google Scholar]
- Koleva I., van Beek T.A., Linssen J.P.H., De Groot A., Evstatieva L.N. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal. 2001;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H. Identification of natural compounds with antiviral activities against SARS associated coronavirus. Antiviral Res. 2005;7:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Hu W.X., He L.F., Ye M., Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004;578:245–250. doi: 10.1016/j.febslet.2004.10.095. [DOI] [PubMed] [Google Scholar]
- Liu F., Ooi V.E.C., Chang S.T. Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci. 1997;60:763–771. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Nabavi S.M., Ebrahimzadeh M.A., Nabavi S.F., Bahramian F. Invitro antioxidant activity of Phytolaccaamericana berries. Pharmacologyonline. 2009;1:81–88. [Google Scholar]
- Noguchi N., Takahashi M., Tsuchiya J. Action of 21 aminosteroid U74006F as an antioxidant against lipid peroxidation. Biochem. Pharmacol. 1998;55:785–791. doi: 10.1016/s0006-2952(97)00533-9. [DOI] [PubMed] [Google Scholar]
- Ozsoy N., Can A., Yanardag R., Akev N. Smilaxexcelsa L. leaf extracts. Food Chem. 2000;110:571–583. [Google Scholar]
- Rice Evans C., Miller N. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234(24):279–293. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Shirwakar A., Rajendran K., Punitha I.R.S. In-vitro antioxidant studies on the benzyltetraisoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006;29(9):1906–1910. doi: 10.1248/bpb.29.1906. [DOI] [PubMed] [Google Scholar]
- Snyder L.M., Fortier N.L., Trainor J., Jacobs Leb.L., Lubin B., Chiu D., Shohet S., Mohandas N. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin hemoglobin crosslinking. J. Clin. Invest. 1985;76:1971–1977. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg J.J., Opden Kamp J.A., Lubin B.H., Roelofsen B., Kuypers F.A. Kinetics and site specificity of hydroperoxide induced oxidative damage in red blood cells. Free Radical Biol. Med. 1992;12:487–498. doi: 10.1016/0891-5849(92)90102-m. [DOI] [PubMed] [Google Scholar]
- Vissers M.C.M., Winterbourne C.C. Oxidation of intracellular glutathione after exposure of human red blood cells to hypochlorous acid. Biochem. J. 1995;37:57–62. doi: 10.1042/bj3070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–331. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- Yang H.L., Chen S.-C., Chang N.-W., Chang J.-M., Lee M.-L., Tsai P.-C., Fu H.-H., Kao W.-W., Chiang H.-C., Wang H.-H., Hseu Y.C. Protection from oxidative damage using Bidenspilosa extracts in normal human erythrocytes. Food Chem. Toxicol. 2006;44:1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Zou C.G., Agar N.S., Jones G.L. Oxidative insult to human red blood cells induced by free radical initiator AAPH and its inhibition by a commercial antioxidant mixture. Life Sci. 2001;69(1):75–86. doi: 10.1016/s0024-3205(01)01112-2. [DOI] [PubMed] [Google Scholar]


