Abstract
Protease inhibitors are well known to have several applications in medicine and biotechnology. Several plant sources are known to return potential protease inhibitors. In this study plants belonging to different families of Leguminosae, Malvaceae, Rutaceae, Graminae and Moringaceae were screened for the protease inhibitor. Among them Moringa oleifera, belonging to the family Moringaceae, recorded high level of protease inhibitor activity after ammonium sulfate fractionation. M. oleifera, which grows throughout most of the tropics and having several industrial and medicinal uses, was selected as a source of protease inhibitor since so far no reports were made on isolation of the protease inhibitor. Among the different parts of M. oleifera tested, the crude extract isolated from the mature leaves and seeds showed the highest level of inhibition against trypsin. Among the various extraction media evaluated, the crude extract prepared in phosphate buffer showed maximum recovery of the protease inhibitor. The protease inhibitor recorded high inhibitory activity toward the serine proteases thrombin, elastase, chymotrypsin and the cysteine proteases cathepsin B and papain which have more importance in pharmaceutical industry. The protease inhibitor also showed complete inhibition of activities of the commercially available proteases of Bacillus licheniformis and Aspergillus oryzae. However, inhibitory activities toward subtilisin, esperase, pronase E and proteinase K were negligible. Further, it was found that the protease inhibitor could prevent proteolysis in a commercially valuable shrimp Penaeus monodon during storage indicating the scope for its application as a seafood preservative. This is the first report on isolation of a protease inhibitor from M. oleifera.
Keywords: Moringa oleifera, Protease inhibitor, Seafood preservative, Therapeutic proteases
1. Introduction
Enzyme inhibitors have received increasing attention as useful tools not only for the study of enzyme structures and reaction mechanisms but also for potential utilization in pharmacology (Cyran, 2002; Imada, 2005; Robert, 2005) and agriculture (Ahn et al., 2004). Specific and selective protease inhibitors are potentially powerful tools for inactivating target proteases in the pathogenic process of human diseases such as emphysema, arthritis, pancreatitis, thrombosis, high blood pressure, muscular dystrophy, cancer and AIDS (Johnson and Pellecchia, 2006). Protease inhibitors are one of the prime candidates with highly proven inhibitory activity against insect pests and are also known to improve the nutritional quality of food. Insects that feed on plant material possess alkaline guts and depend predominantly on serine proteases for digestion of food materials and, therefore, protease inhibitors by virtue of their anti-nutritional interaction can be employed effectively as defense tools (Ryan, 1990). Microbial food spoilage is an area of global concern as it has been estimated that as much as 25% of all food produced is lost post-harvest owing to microbial activity (Baird-Parker, 2003). The use of an adequate amount of natural protease inhibitors is an effective way to extend the shelf life of many types of seafood such as salted fish products. This is due to the fact that the inhibitors can retard several deteriorative processes like protein degradation caused by the action of endogenous and exogenous proteases, during the food processing and preservation (Reppond and Babbitt, 1993). Hence, protease inhibitors continue to attract the attention of researchers due to their increasing use in medicine and biotechnology (Dunaevsky et al., 1998).
A large number of protease inhibitors have been isolated and identified from several plants (Tamir et al., 1996). Most of the naturally occurring protease inhibitors were found in plants and are well characterized. They belong to the group of serine protease inhibitors which include trypsin (Richardson, 1991). However, protease inhibitor has not been so far reported from Moringa oleifera, a panotropical multipurpose tree with a high biomass yield and capable of tolerating unfavorable environmental conditions (Foidl et al., 2001). M. oleifera grows well throughout the tropics and almost every part of the plant is of value for food. The flowers, leaves, and roots are used in folk remedies for treatment of tumors and the seeds for abdominal tumors. Bark regarded as antiscorbic and exudes a reddish gum with properties of tragacanth is sometimes used for diarrhea. Roots are bitter and act as a tonic to the body and lungs. They are used as expectorant, mild diuretic, as stimulant in paralytic afflictions, in epilepsy, and in hysteria (Hartwell, 1971). In addition, several low molecular weight bioactive compounds from Moringa seeds with bactericidal, fungicidal and immunosuppressive activities (Mahajan and Mehta, 2010) and some anti inflammatory agents (Caceres et al., 1991; Cheenpracha et al., 2010) were also reported. Moreover bioactive nitrile glycosides niaziridin and niazirin in the leaves, pods and bark (Khanuja et al., 2005; Shanker et al., 2007) and coagulant lectin as bio insecticide (Oliveira et al., 2010) were also reported from this plant. In this communication, we report the inhibitory activity of protease inhibitor isolated from M. oleifera against therapeutically important and commercially available proteases. Further their potential for use as seafood preservative against proteolysis in Penaeus monodon on storage was also evaluated.
2. Materials and methods
2.1. Screening of plants for protease inhibitor
Plants, which are available from nearby areas around Cochin University of Science and Technology campus, India, and belonging to the families of Leguminosae, Malvaceae, Graminae, Rutaceae and Guttiferae were used as source materials for screening protease inhibitors. Different plant parts including seeds, leaves, flowers and bark were used for the study.
2.2. Extraction and recovery of protease inhibitor
Ideal solution that enables maximal extraction of the protease inhibitor from the plant material was optimized by preparing crude extract of leaves with different solutions. Fresh leaves (25 g) from the mature plant was blended with 100 mL each of sodium chloride 15% (w/v) (Wu and Whitaker, 1990), sodium hydroxide 0.2% (w/v), hydrochloric acid 0.05 M (Tawde, 1961), phosphate buffer 0.1 M (pH 7) (Wu and Whitaker, 1990) and distilled water.
Samples were washed thoroughly in distilled water and air-dried. An extract was prepared in a 500 mL conical flask by homogenizing 25 g of plant materials in 100 mL of extractant in an electrical blender. The homogenate was further mixed thoroughly by incubating the contents at room temperature (RT, 28 ± 2 °C) in a rotary shaker for 30 min at 150 rpm. The slurry was then filtered through cheesecloth and the filtrate was centrifuged (10,000 rpm, 15 min, 4 °C) for removing any cell debris that remains in the preparation (Pichare and Kachole, 1996). The clear supernatant obtained represented the crude extract, and was assayed for protease inhibitor activity and protein content.
2.3. Purification of protease inhibitor
Protease inhibitor isolated from leaves of mature M. oleifera (extracted with phosphate buffer, 0.1 M, pH 7) and purified by conventional protein purification techniques in combination with ion-exchange chromatography, gel filtration by Sephadex G75, and preparative PAGE (Bijina, 2006) was used.
2.4. Protease inhibitor assay
Protease inhibitor activity was assayed according to the method of Kunitz (1947) with slight modifications. One mL aliquot of trypsin [EC 3.4.21.4, SRL, India(1000 units/mg)] (0.5 mg/mL prepared in 0.1 M phosphate buffer pH 7) was pre-incubated with 1 mL of a suitable dilution of the protease inhibitor at 37° C for 15 min. To the above mixture 2 mL of 1% Hammerstein casein (SRL, India) (prepared in 0.1 M phosphate buffer) was added and incubated at 37° C for 30 min. The reaction was terminated by the addition of 2.5 mL of 0.44 M trichloroacetic acid (TCA) solution. The reaction mixture was transferred to a centrifuge tube and the precipitated protein was removed by centrifugation at 10,000 rpm for 15 min (Sigma, Germany). The absorbance of the clear supernatant was measured at 280 nm in a UV–Visible spectrophotometer (Shimadzu, Japan) against appropriate blanks. The TCA soluble peptide fractions of casein formed by the action of trypsin in the presence and absence of the inhibitor were quantified by comparing with tyrosine as standard. One unit of trypsin activity was defined as the amount of enzyme that liberated 1 μg of tyrosine per milliliter of the reaction mixture per minute under the assay conditions. One unit of inhibitor activity was defined as the decrease by one unit of absorbance of TCA soluble casein hydrolysis product liberated by trypsin action measured at 280 nm per minute under the assay conditions. The protease inhibitor activity was expressed in terms of percent inhibition. Appropriate blanks for the enzyme, inhibitor, and the substrate were also included in the assay along with the test.
2.5. Protein estimation
Protein content was determined according to the method of Lowry et al. (1951) using Bovine Serum Albumin (BSA) (SRL, India) as the standard and the concentration was expressed in milligram per milliliter (mg/mL).
2.6. Effect of protease inhibitor on proteases with therapeutic importance
Effect of purified protease inhibitor on chymotrypsin (EC.3.4.21.1, Product No. C3142, Sigma–Aldrich), thrombin (EC.3.4.21.5, Product No. T7513, Sigma–Aldrich), Elastase (EC.3.4.21.37, Product No. E8140, Sigma–Aldrich), cathepsin-B (EC.3.4.22.1, Product No. C6286, Sigma–Aldrich), papain (EC.3.4.22.2, Sisco Research Laboratories Pvt. Ltd., India), and collagenase (EC.3.4.24.3, Product No. C2674, Sigma–Aldrich) activities were determined as described below.
Enzymes were assayed for their activities as per the protocols detailed below and their inhibitions in the presence of M. oleifera protease inhibitor were also assayed in the same manner with the addition of the inhibitor (0.27 mg/mL) to the respective reaction mixture and pre-incubation for 10 min followed by assay of residual enzyme activity. Protease inhibitor unit is defined as the amount of protease inhibitor that inhibited one unit of respective enzyme activity. Protease inhibitor activity of the respective enzyme is finally expressed in terms of percent inhibition for comparative purposes.
(a) Cathespin-B activity was assayed using 1.25-2.5 units/mL of cathepsin B (Sigma–Aldrich) in cold deionised water as described by Barrett (1981), using 20 mM N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide in dimethyl sulphoxide as substrate at 30 °C for 5 min. One unit of enzyme will release one micromole of p-nitro aniline per min from N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide at pH 7 at 30° C. (b) Thrombin activity of protease inhibitor was evaluated using 1 mg/mL of thrombin (Sigma–Aldrich) in 0.1 M Tris–HCl buffer pH 7.5 and 1% Hammerstein casein as substrate at 37° C for 30 min adopting Kunitz caseinolytic method (Kunitz, 1947) (c) Elastase activity was tested using 10 μg/mL of elastase (Sigma–Aldrich) solution according to Kunitz caseinolytic method (Ian, 2001; Kunitz, 1947). (d) Collagenase activity was assayed using 1 mg/mL of collagenase (Sigma–Aldrich) and 1% gelatin as substrate (Ian, 2001) at 37° C for 30 min. (e) Papain activity was evaluated by Caseinolytic method (Murachi, 1970) using papain (6 mg/mL) and 1% Hammerstein casein as substrate at 37° C for 30 min. The reaction was interrupted by adding 1.5 mL of TCA (5% w/v). (f) Chymotrypsin activity was assayed according to the modified method of Fritz et al. (1966). Chymotrypsin from Bovine pancreas (Sigma–Aldrich) was prepared by dissolving freeze dried pancreas in 0.001 M HCl at a concentration of 1 mg/mL. Standard assay mixture contained 0.05 M Tris–HCl buffer, pH 7.6, 20 mM peptide substrate, N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide, 0.27 mg/mL of inhibitor and chymotrypsin (10 μg/mL). One unit of enzyme is defined as the amount of enzyme that resulted in the conversion of 1 μmol substrate per minute.
2.7. Effect of protease inhibitor on commercially available proteases
Commercially available proteases-subtilisin (EC.3.4.21.14, Product No. P5380, Sigma–Aldrich), esperase (EC.3.4.21.62, Novozyme), pronase E (EC.3.4.24.31, Product No. P6911, Sigma–Aldrich), proteinase K (EC.3.4.21.64, Product No. P2308, Sigma–Aldrich), and that obtained from Bacillus sp. (Product No. P3111, Sigma–Aldrich), Bacillus licheniformis (Product No. P4860, Sigma–Aldrich), B. amyloliquefaciens (Product No. P1236, Sigma–Aldrich) and Aspergillus oryzae (Product No. P6110, Sigma–Aldrich) were evaluated for the effect of the protease inhibitor on their activities. In addition, alkaline protease isolated from Engyodontium album (BTMFS10) and available in our laboratory was also evaluated. Protease assays were carried out by adopting Kunitz caseinolytic method (Kunitz, 1947) using Hammerstein casein as substrate. Respective protease inhibitor activities were assayed in the same manner with the addition of the inhibitor (0.27 mg/mL) to the respective reaction mixture and pre incubation for 10 min followed by assay of residual enzyme activity. Protease inhibitor unit is defined as the amount of protease inhibitor that inhibited one unit of respective enzyme activity.
2.8. Effect of protease inhibitor on proteolysis in shrimp P. monodon during storage
P. monodon, known for its delicacy across the globe and with commercial significance, was selected for the study. The “peeled and undeveined” style of shrimp, which is the most common type that is processed and exported, was used for the experiments. Fresh shrimps obtained from fish landing site was collected in sterile containers under iced condition, transported to the laboratory immediately, and processed. The shrimps were peeled and the head removed aseptically to obtain “peeled and undeveined” condition. The sample (10 g) was taken in a sterile polyethylene bag, sealed, and kept at each storage condition as the control. For the experiments, three different sets of samples of the same weight were taken, under the same conditions as that of the control, in sterile polyethylene bags. They were added with 10 mL aliquots of purified protease inhibitor at different concentrations (0.05, 0.1 and 0.2 mg/mL) and mixed well. The test samples thus prepared were then incubated at RT (28 ± 2 °C), 4 °C and −20 °C for 8, 24 and 168 h, respectively.
After incubation for specified periods, samples were drawn and analyzed for microbial load (total viable counts) and protein content in the flesh. Preparation of flesh extract and estimation of microbial load were performed using Flesh agar plates (Chandrasekaran et al., 1985) incorporated with 1% casein. Total protein of the samples was extracted using 5% NaCl in 0.02 M sodium bicarbonate (Chandrasekaran, 1985) and estimated as mentioned above.
3. Results and discussion
3.1. Screening of plants for protease inhibitor
Most of the natural protease inhibitors are proteinaceous in nature and are located mainly in seeds, leaves and tubers, which act as specific defense and regulatory proteins. Many reports are available on the isolation, purification and characterization of protease inhibitor from seeds of legume plants (Ryan, 1990). Hence, few plants belonging to different families of Leguminosae, Malvaceae, Rutaceae, Graminae and Moringaceae were screened for protease inhibitor. Results presented in Table 1 shows that the plants belonging to Leguminosae family have maximum percent of inhibition toward trypsin. Maximum inhibitory activity was shown by Cicer arietinum (98.21%) followed by Momordica charantia (86.77%), M. oleifera (76.7%) and Adathoda vasica (76.12%).
Table 1.
Screening of plants for protease inhibitor.
| Serial No. | Name of plants | Inhibitory activity (%) | Specific inhibitory activity (units/mg protein) |
|---|---|---|---|
| 1 | Adathoda vasica | 76.12 | 115.33 |
| 2 | Allium sepa | 0.00 | 0.00 |
| 3 | Azadirachta indica | 6.50 | 0.77 |
| 4 | Amaranthus viridis | 51.21 | 98.48 |
| 5 | Arachis hypogea | 39.90 | 62.34 |
| 6 | Beta vulgaris | 0.00 | 0.00 |
| 7 | Carica papaya | 17.84 | 20.74 |
| 8 | Cassia fistula | 30.00 | 40.54 |
| 9 | Catharanthus roseus | 46.64 | 466.40 |
| 10 | Cicer arietinum | 98.21 | 10.68 |
| 11 | Cucurbita pepo | 22.40 | 27.32 |
| 12 | Dolichos biflorus | 60.74 | 33.74 |
| 13 | Hibiscus esculantus | 17.49 | 97.17 |
| 14 | Momordica charantia | 86.77 | 74.16 |
| 15 | Moringa oleifera | 76.70 | 109.57 |
| 16 | Ocimum sanctum | 50.60 | 56.22 |
| 17 | Oryza sativa | 15.33 | 4.26 |
| 18 | Phaseolus mungo | 56.00 | 80.00 |
| 19 | Pisum sativum | 55.98 | 75.65 |
| 20 | Phaseolus aureus | 18.64 | 32.70 |
| 21 | Solanum tuberosum | 73.78 | 163.96 |
| 22 | Triticum vulgare | 42.34 | 49.23 |
3.2. Selection of potential source and isolation of protease inhibitor from M. oleifera
In the present study, potential source for protease inhibitor was selected based on the activity of the protease inhibitor. The plants with more than 60% protease inhibitor activity were further screened after partial purification of the molecules using ammonium sulfate precipitation in order to select the potential proteinaceous protease inhibitor. Among the plants evaluated, although M. oleifera showed maximal percent of protease inhibition (92%) compared to others (Table 2), maximum specific activities of protease inhibitors were recorded with M. charantia (1144) and Solanum tuberosum (192.36) compared to M. oleifera (76.66). It was noted that among the five plants selected, extracts of M. oleifera, after ammonium sulfate fractionation, recorded high level of protease inhibitor activity. Hence, M. oleifera was selected for further studies. M. charantia and S. tuberosum were not considered for further studies since protease inhibitor from these plants were already well characterized.
Table 2.
Protease inhibitory activity of ammonium sulfate precipitated fractions of different plant extracts.
| Serial No. | Name of plants | Saturation of (NH4)2SO4 (%) | Protease inhibition (%) | Specific activity (units/mg protein) |
|---|---|---|---|---|
| 1 | Adathoda vasica | 30–60 | 31.16 | 103.86 |
| 2 | Cicer areitinum | 0–30 | 2.60 | 13.00 |
| 3 | Momordica charantia | 30–60 | 57.20 | 1144.00 |
| 4 | Moringa oleifera | 30–60 | 92.00 | 76.66 |
| 5 | Solanum tuberosum | 30–60 | 48.09 | 192.36 |
In spite of the fact that M. oleifera is known for containing several low molecular weight bioactive constituents with pharmaceutical and industrial applications (Kalogo et al., 2000) protein inhibitors from the Moringaceae family was not yet reported.
3.3. Distribution of protease inhibitor in different parts of M. oleifera
From the data presented in Fig. 1, it is evident that among the different parts of M. oleifera tested, the crude extract isolated from the mature leaves and seeds showed highest level of inhibition against trypsin. The crude extract prepared from leaves showed maximum percent of inhibition (77%) followed by the seed extract (63%). On evaluation of the distribution of protease inhibitor in different plant tissues of the mature M. oleifera plant, it was observed that the mature leaves had maximum percent of protease inhibition with maximum specific inhibitor activity followed by the seeds when compared to flowers, roots and bark. Since leaves are the major tissues attacked by pest and pathogens, the accumulation of this protease inhibitor is maximum in leaves, compared to other parts, indicating a tissue specific expression of these proteins. In winged bean plant, western blot analysis of the expression of the protease inhibitor in different tissues suggested that the expression of the protease inhibitor is tissue specific and species specific (Datta et al., 2001). Hence, it may be presumed that leaves and seeds of M. oleifera are rich sources of the protease inhibitor, which is mostly directed toward serine proteases such as trypsin and chymotrypsin.
Figure 1.
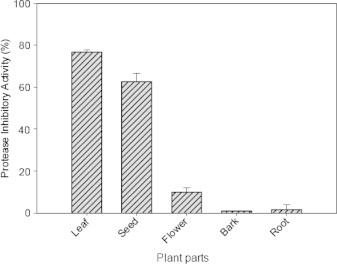
Distribution of protease inhibitor in different parts of Moringa oleifera.
3.4. Extraction of protease inhibitor from M. oleifera using different solvents
Among the various extraction media evaluated for recovering protease inhibitor from plant sources, the crude extract prepared in phosphate buffer showed maximum protease inhibitor activity (79%) followed by that prepared in distilled water (68%) (Fig. 2). The inhibition of trypsin by the extract prepared in sodium chloride and sodium hydroxide was very less compared to that prepared in distilled water and phosphate buffer. Whereas, in terms of protein content in the crude extract, phosphate buffer enabled maximal protein content compared to other media, while distilled water extract contained very less protein although it showed high protease inhibitor activity. The specific protease inhibitor activity obtained for each extract is presented in Table 3.
Figure 2.
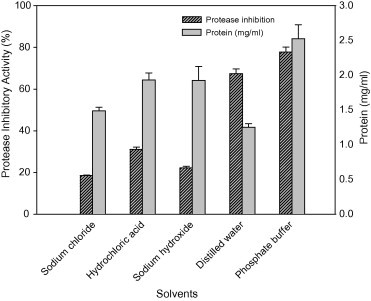
Extraction of protease inhibitor using different solvents.
Table 3.
Specific activity of protease inhibitor extracted with different extractants.
| Serial No. | Extraction medium | Specific protease inhibitor activity (units/mg protein) |
|---|---|---|
| 1 | Sodium chloride (15%) | 12.20 ± 0.05 |
| 2 | Hydrochloric acid (0.05 M) | 16.60 ± 0.04 |
| 3 | Sodium hydroxide (0.2%) | 11.60 ± 0.04 |
| 4 | Distilled water | 56.60 ± 0.23 |
| 5 | Phosphate buffer (0.1 M) | 30.20 ± 0.10 |
The extraction medium has a major role in the complete extraction of the protein from any desired source. Hence different solvents were used for extracting proteinaceous protease inhibitors from the leaves. The protein concentration and the protease inhibitor activity were maximal in the extract prepared with phosphate buffer, which facilitated the complete release of proteins from the leaves into the solvent with maximum inhibitor activity. The protease inhibitor activities in the extracts prepared with sodium hydroxide and sodium chloride were very less compared to that prepared in phosphate buffer, and distilled water. Even though extraction with distilled water yielded maximum specific activity, phosphate buffer was selected as the potent extraction medium for maximal extraction of protease inhibitor from the leaves since loss in inhibitor activity was negligible during further purification. In fact 0.1 M phosphate buffer (pH 7.6) was reported to be a good extractant for the maximal extraction of proteins from Cajanus cajan seeds with high amount of trypsin inhibitor activity and protein concentration (Pichare and Kachole, 1996).
3.5. Effect of protease inhibitor on proteases with therapeutic importance
From the results presented in Fig. 3 it was noted that the protease inhibitor obtained from M. oleifera could have high affinity toward the serine proteases thrombin, elastase followed by chymotrypsin which is known for their pathological and therapeutic importance. Cysteine proteases cathepsin B and papain also recorded appreciable level of inhibition while there was no significant inhibition toward collagenase. Serine protease inhibitors modulates protease activities and controls a variety of the critical protease mediated processes like coagulation, fibrinolysis and tissue remodeling (Laskowski and Kato, 1980), and also in the neurobiology of aging (Higgins et al., 1990) and the development of cancer (Koivunen et al., 1991). It is reported that a Kunitz type trypsin inhibitor from Enterolobium contortisiliquum seeds strongly inhibited Bovine trypsin and chymotrypsin and also some serine proteases involved in the blood clotting cascade and the fibrinogen proteolysis: human plasma kallikrein, Factor XIIa and plasmin (Isabel et al., 1996). The high affinity of the protease inhibitor toward thrombin indicates scope for its use as an anticoagulant agent.
Figure 3.
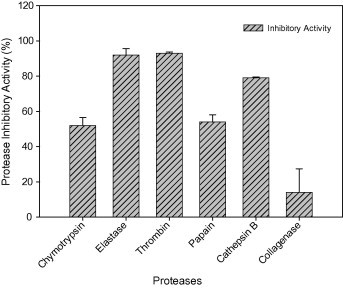
Inhibitory activity of Moringa oleifera protease inhibitor toward pharmaceutically important proteases.
It has been suggested that a cathepsin B-like protease, a secretory form of lysosomal cathepsin B, present in some cancer exudates, is involved in the invasive process of cancer pathology. Cysteine protease inhibitor present in the same fluid strongly inhibited the action of cathepsin B in the cancer cells. Thus the inhibitor could play a protective role in the tumor invasion (Keppler et al., 1985). Cathepsin B is known as the major virulent proteases present in many protozoan diseases like Leishmaniasis. An attractive target for new chemotherapy for Leishmaniasis is two cysteine proteases: cathepsin B-like and cathepsin L-like, which are required for parasitic growth and virulence (Mottram et al., 1998). It is reported that peptide based cysteine protease inhibitors alter the Golgi complex ultra structure and function in Trypanosoma cruzi (Juan et al., 1998). In this context, the protease inhibitor from M. oleifera has promise as a possible drug against cathepsin B. It was reported that bitter gourd, M. charantia contains both trypsin and elastase inhibitory protein (Hamato et al., 1995). Plants are the richest source of antifungal proteins. The potent antifungal protein isolated from broad bean is a trypsin chymotrypsin inhibitor (Banks et al., 2002; Giudici et al., 2000). From the results it is inferred that there is scope for the use of this protease inhibitor from M. oleifera as a drug in pharmaceutical industries against elastase, thrombin, chymotrypsin and cathepsin B.
Based on the ability to inhibit proteases of insect digestive tracts, protease inhibitors have been shown to have potential usefulness as antifeedent agents. The majority of the protease inhibitors exhibiting anti-feedent properties reported so far is active against the neutral serine proteases such as trypsin and chymotrypsin (Ryan, 1990). Studying plant defense responses and devising newer and ecofriendly strategies for plant protection against pests and pathogens is today one of the most dynamic areas of research in plant science. The insecticidal effect of protease inhibitors, especially serine and cysteine protease inhibitors, have been studied by direct incorporation assays or by in vitro inhibition studies. They induced delayed growth and development, reduced fecundity and sometimes increased mortality (Annadana et al., 2002; Azzouz et al., 2005; Oppert et al., 1993, 2003). It may be possible to develop the M. oleifera protease inhibitor, by virtue of its activity against trypsin and chymotrypsin, for the direct application as a biocontrol agent for the protection of plants against phytopathogenic fungi and to insects by encapsulation for surface application or can be sprayed directly, once required feasibility studies are conducted.
3.6. Effect of protease inhibitor on commercially available proteases
The affinity of M. oleifera protease inhibitor with different industrially important proteases is also evaluated. From the results presented in Fig. 4, it was inferred that the protease inhibitor could completely inhibit the commercially available proteases obtained from Bacillus sp., B. licheniformis, and A. oryzae compared to reduced level of inhibition of protease of B. amyloliquefaciens. Further 76% inhibition of activity in the protease isolated from E. album was also noticed. In contrast the commercial proteases subtilisin, esperase, pronase E, and proteinase K were not markedly inhibited by M. oleifera protease inhibitor. From the results it was noted that M. oleifera protease inhibitor has high inhibitory activity against bacterial proteases and protease of A. oryzae and E. album.
Figure 4.
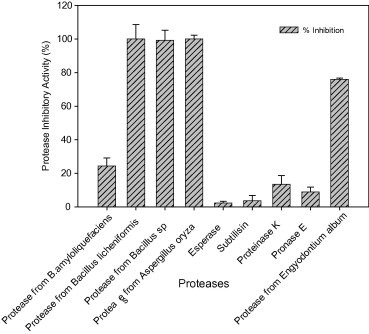
Inhibitory activity of Moringa oleifera protease inhibitor toward different commercially important proteases.
Currently, a large proportion of commercially available proteases are derived from Bacillus strains (Abdel-Naby et al., 1998; Mabrouk et al., 1999), although potential use of fungal proteases is being increasingly realized (Joo et al., 2001). These proteases have wide range of applications as additives in various industries such as detergents, leather processing, and pharmaceuticals. Hence in the present study these commercial enzymes were evaluated along with E. album, a strain which was recognized to produce protease that has potential for applications in detergent industry (Chellappan et al., 2006). The results obtained for the inhibition studies against these commercial enzymes indicate probable application for the M. oleifera protease inhibitor in regulating their activity.
Protease inhibitor (API-1) isolated from actinomycete was reported to improve the thermal stability of protease of fungus Conidiobolus macrosporous that has potential biotechnological applications in both detergent and leather industries (Pandhare et al., 2002). The alkaline protease was stable only up to 40 °C and lost its activity on increasing the temperature. It was found that the binding of API-1 enhanced the stability of enzyme at 50 °C up to 1 h (Pandhare et al., 2002). Thus thermal inactivation of detergent proteases can be prevented by binding of protease inhibitor. In the present study it was noted that the protease inhibitor did not show any appreciable inhibitory activity against detergent enzymes. This observation points out to the probability that the protease inhibitor could be developed toward use as stabilizer for imparting thermal stability of detergent enzymes.
3.7. Effect of protease inhibitor on protein degradation in shrimp P. monodon during storage
The possible use of protease inhibitor toward controlling rapid proteolysis and consequent spoilage in the shrimp, P. monodon was evaluated by treating the peeled and undeveined samples with protease inhibitor and incubation at different storage temperatures. It was found that protease inhibitor highly influenced the microbial load and thus only 4 × 102 (cfu/mL) of total viable count (TVC) was recorded in the protease inhibitor treated samples compared to 8 × 1010 of (cfu/mL) of TVC in the control samples (data not shown). From the results presented in Fig. 5 it was noted that, at a dosage of 0.2 mg/mL, there was 41% reduction in protein content of the untreated sample at RT after 8 h of incubation when compared to the respective control. But at reduced temperatures (4 and −20 °C) there was no considerable variation in the protein contents of the untreated samples during storage when compared to the initial values recorded at 0 h. Whereas, in protease inhibitor treated samples there was no reduction in the level of protein content indicating retardation in proteolysis at each storage temperature studied. The impact of protease inhibitor dosage on control of proteolysis was in a linear proportion with the tested concentrations (data not shown) and the highest dosage tested (0.2 mg/mL) alone was presented in Fig. 5. It is concluded that further studies with purified protease inhibitor would facilitate appropriate selection of right dosage for rapid control of proteolysis in sea food.
Figure 5.
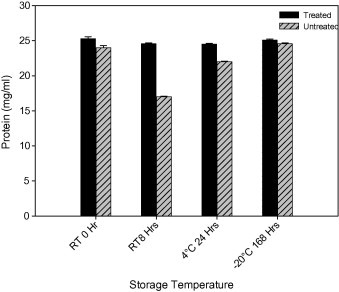
Effect of protease inhibitor on the proteolysis in shrimp Penaeus monodon during storage at different temperatures.
The protein hydrolysis in fish and shrimp muscle is generally an undesirable process and accounts for the loss of commodity during post harvest treatment. The presence of proteases causes the softening of some fish muscle and also causes gel weakening in ‘Surimi’. The protease-producing organisms are responsible for the fish and shrimp muscle degradation during preservation (Chandrasekaran, 1985; Chandrasekaran et al., 1987). At present, chemical preservatives and antibiotics are used, but the use of enzyme inhibitor, which is a part of the edible plant, could be the best alternative and safe preservative. Many legume protease inhibitors have been found to have inhibitory effects on the extracts of fish enzymes (Soottawat et al., 1999). In this context, use of natural protease inhibitor will be not only safe and effective for the control of proteolysis and extension of shelf life but also environmental friendly. The protease inhibitor isolated from M. oleifera could be used as a safe seafood preservative preventing proteolysis and consequent spoilage for proteinaceous sea food.
4. Conclusions
Based on the results obtained for the studies conducted with the M. oleifera protease inhibitor on various proteases, it is concluded that M. oleifera protease inhibitor has immense potential for the development of suitable drugs in pharmaceutical industries against thrombin, elastase, chymotrypsin, trypsin, cathepsin, and papain. Further this protein protease inhibitor could become an ideal candidate for use as a seafood preservative against proteolysis, in various biotechnological applications especially in food industry, and also as a biocontrol defense protein for the protection of plants against pest and pathogen infestations.
References
- Abdel-Naby M.A., Ismail A.S., Ahmed S.A., Abdel-Fattah A.F. Production and immobilization of alkaline protease from Bacillus mycoides. Bioresour. Technol. 1998;64:205–210. [Google Scholar]
- Ahn J.E., Salzman R.A., Braunagel S.C., Koiwa H., Zhu-Salzman K. Functional roles of specific bruchid protease isoforms in adaptation to a soybean protease inhibitor. Insect Mol. Biol. 2004;13:649–657. doi: 10.1111/j.0962-1075.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- Annadana S., Peters J., Gruden K., Schipper A., Outchkourov N.S., Beekwilder M.J., Udayakumar M., Jongsma M.A. Effects of cysteine protease inhibitors on oviposition rate of the western flower thrips, Frankliniella occidentalis. J. Insect Physiol. 2002;48:701–706. doi: 10.1016/s0022-1910(02)00093-8. [DOI] [PubMed] [Google Scholar]
- Azzouz H., Cherqui A., Campan E.D.M., Rahbe Y., Duport G., Jouanin L., Kaiser L., Giordanengo P. Effects of plant protease inhibitors, Oryzacystatin I and soybean Bowman–Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphelinidae) J. Insect Physiol. 2005;51:75–86. doi: 10.1016/j.jinsphys.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Baird-Parker T.C. The production of microbiologically safe and stable foods. In: Lund B.M., Baird-Parker T.C., Gould G.W., Gaithersburg, editors. The Microbiological Safety and Quality of Food. Aspen Publishers Inc.; 2003. pp. 3–18. [Google Scholar]
- Banks W., Niehoff M., Brown R., Chen Z., Cleveland T. Transport of an antifungal trypsin inhibitor isolated from corn across the blood-brain barrier. Antimicrob. Agents Chemother. 2002;46:2633–2635. doi: 10.1128/AAC.46.8.2633-2635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A.J. Alpha 2-macroglobulin. Methods Enzymol. 1981;80:737–754. doi: 10.1016/s0076-6879(81)80056-0. [DOI] [PubMed] [Google Scholar]
- Bijina, B., 2006. Isolation, purification and characterization of protease inhibitor from Moringa oleifera. Ph D thesis, University of Science and Technology, Cochin.
- Caceres A., Cabrera O., Morales O., Mollinedo P., Mendia P. Pharmacological properties of Moringa oleifera: preliminary screening for antimicrobial activity. J. Ethnopharmacol. 1991;33:213–216. doi: 10.1016/0378-8741(91)90078-r. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran, M. 1985. Studies on microbial spoilage of Penaeus indicus. Ph D thesis, Cochin University of Science and Technology, Cochin.
- Chandrasekaran M., Lakshmanaperumalsamy P., Chandramohan D. Fish flesh agar medium – A suitable experimental medium for the detection of spoilage bacteria. Antonie Van Leeuwenhoek. 1985;51:219–225. doi: 10.1007/BF02310014. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran M., Lakshmanaperumalsamy P., Chandramohan D. Spoilage bacteria of Penaeus indicus. Fish Technol. 1987;24:122–125. [Google Scholar]
- Cheenpracha S., Park E.-J., Yoshida W.Y., Barit C., Wall M., Pezzuto J.M., Chang L.C. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorgan. Med. Chem. 2010;18:6598–6602. doi: 10.1016/j.bmc.2010.03.057. [DOI] [PubMed] [Google Scholar]
- Chellappan S., Jasmin C., Basheer S.M., Elyas K.K., Bhat S.G., Chandrasekaran M. Production, purification and partial characterization of a novel protease from marine Engyodontium album BTMFS10 under solid state fermentation. Process Biochem. 2006;41:956–961. [Google Scholar]
- Cyran R. BCC Research; Norwalk: 2002. C-202R New Developments in Therapeutic Enzyme Inhibitors and Blockers. [Google Scholar]
- Datta K., Usha R., Dutta S.K., Singh M. A comparative study of the winged bean protease inhibitors and their interactions with proteases. Plant Physiol. Biochem. 2001;39:949–959. [Google Scholar]
- Dunaevsky Y.E., Pavlukova E.B., Beliakova G.A., Tsybina T.A., Gruban T.N., Belozersky M.A. Protease inhibitors in buckwheat seeds: comparison of anionic and cationic inhibitors. J. Plant Physiol. 1998;152:696–708. [Google Scholar]
- Foidl N., Makkar H.P.S., Becker K. The potential of Moringa oleifera for agricultural and industrial uses. In: Fuglie L.J., editor. The Miracle Tree: The Netherlands, Technical Centre for Agricultural and Rural Cooperation, Senegal Food Product Safety. State Institute for Quality Control of Agricultural Products (RIKILT); 2001. pp. 45–76. [Google Scholar]
- Fritz H., Hartwich G., Hoppe E.W., Seylers Z. On protease inhibitors. I. Isolation and characterization of trypsin inhibitors from dog pancreas tissue and pancreas secretion. Physiol. Chem. 1966;345:150–165. [PubMed] [Google Scholar]
- Giudici M., Regente C., de la Canal L. A potent antifungal protein from Helianthus annuus flowers is a trypsin inhibitor. Plant Physiol. Biochem. 2000;38:881–888. [Google Scholar]
- Hamato N., Koshiba T., Pham T.-N., Tatsumi Y., Nakamura D., Takano R., Hayashi K., Hong Y.-M., Hara S. Trypsin and elastase inhibitors from bitter gourd (Momordica charantia LINN) seeds: purification, amino acid sequences, and inhibitory activities of four new inhibitors. J. Biochem. 1995;117:432–437. doi: 10.1093/jb/117.2.432. [DOI] [PubMed] [Google Scholar]
- Hartwell J.L. Plants used against cancer: a survey. Lloydia. 1971:30–34. [PubMed] [Google Scholar]
- Higgins G., Oyler G., Neve R., Chen K., Gage F.H. Altered levels of amyloid protein precursor transcripts in the basal forebrain of behaviorally impaired aged rats. Proc. Natl. Acad. Sci. USA. 1990;87:3032–3036. doi: 10.1073/pnas.87.8.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ian M.C. Matrix metallo proteinases protocols. In: John M.W., editor. Methods in Moleculer Biology. Humanapress Inc.; UK: 2001. p. 392. [Google Scholar]
- Imada C. Enzyme inhibitors and other bioactive compounds from marine actinomycetes. Antonie van Leeuwenhoek. 2005;87:59–63. doi: 10.1007/s10482-004-6544-x. [DOI] [PubMed] [Google Scholar]
- Isabel F.C.B., Luiza V.O.M., Mariana S.A., Misako U.S., Michael R., Hanz F., Claudio A.M.S. Primary structure of a Kunitz type trypsin inhibitor from Enterolobium contortisiliquum seeds. Phytochem. 1996;41:1017–1022. doi: 10.1016/0031-9422(95)00710-5. [DOI] [PubMed] [Google Scholar]
- Johnson S., Pellecchia M. Structure and fragment based approaches to protease inhibition. Curr. Top. Med. Chem. 2006;6:317–329. doi: 10.2174/156802606776287072. [DOI] [PubMed] [Google Scholar]
- Joo H.S., Park G.C., Kim K.M., Paik S.R., Chang C.S. Novel alkaline protease from the polychaeta, Periserrula leucophryna: purification and characterization. Process Biochem. 2001;36:893–900. [Google Scholar]
- Juan C.E., Patricia S.D., James P., Ivi H., Dorothy F.B., McKerrowo H. Cysteine protease inhibitor alter Golgi complex ultra structure and function in Trypanosoma cruzi. J. Cell Sci. 1998;111:597–606. doi: 10.1242/jcs.111.5.597. [DOI] [PubMed] [Google Scholar]
- Kalogo Y., Rosillon F., Hammes F., Verstraete W. Effect of a water extract of Moringa oleifera seeds on the hydrolytic microbial species diversity of a UASB reactor treating domestic wastewater. Lett. Appl. Microbiol. 2000;31:259–264. doi: 10.1046/j.1365-2672.2000.00814.x. [DOI] [PubMed] [Google Scholar]
- Keppler D., Pagano M., Dalet-Fumeron V., Engler R. Regulation of neoplasm-specific cathepsin B by cysteine-protease inhibitors present in cancerous exudates. CR Acad. Sci. III. 1985;300:471–474. [PubMed] [Google Scholar]
- Khanuja, S.P.S., Arya, J.S., Tiruppadiripuliyur, R.S.K., Saikia, D., Kaur, H., Singh, M., Gupta, S.C., Shasany, A.K., Darokar, M.P., Srivastava, S.K., Gupta, M.M., Verma, S.C., Pal, A., 2005. Nitrile glycoside useful as a bioenhancer of drugs and nutrients, process of its isolation from Moringa oleifera, United States Patent, India, CSIR, New Delhi.
- Koivunen E., Ristimaki A., Itkonen O., Vuento M., Stenman U. Tumor associated trypsin participates in cancer cell-mediated degradation of extracellular matrix. Cancer Res. 1991;51:2107–2112. [PubMed] [Google Scholar]
- Kunitz M. Crystalline soyabean trypsin inhibitor II. General properties. J. Gen. Physiol. 1947;30:291–310. doi: 10.1085/jgp.30.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M.J., Kato I. Protein inhibitors of protienase. Ann. Rev. Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosenbrough N.J., Farr A.L., Randal R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- Mabrouk S.S., Hashem A.M., El-Shayeb N.M.A., Ismail A.S., Abdel-Fattah A.F. Optimization of alkaline protease productivity by Bacillus licheniformis ATCC 21415. Bioresour. Technol. 1999;69:155–159. [Google Scholar]
- Mahajan S.G., Mehta A.A. Immunosuppressive activity of ethanolic extract of seeds of Moringa oleifera Lam. in experimental immune inflammation. J. Ethnopharmacol. 2010;130:183–186. doi: 10.1016/j.jep.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Mottram J.C., Brooks D.R., Coomps G.H. Roles of cysteine proteinases of Trypanosomes and Leishmania in host-parasite interactions. Curr. Opin. Microbiol. 1998;1:455–460. doi: 10.1016/s1369-5274(98)80065-9. [DOI] [PubMed] [Google Scholar]
- Murachi T. Bromelain enzymes. Methods Enzymol. 1970;19:274–283. doi: 10.1016/s0076-6879(76)45042-5. [DOI] [PubMed] [Google Scholar]
- Oliveira C., Luz L., Paiva P., Coelho L., Marangoni S., Macedo M. Evaluation of seed coagulant Moringa oleifera lectin (cMoL) as a bioinsecticidal tool with potential for the control of insects. Process Biochem. 2010;46:498–504. [Google Scholar]
- Oppert B., Morgan T.D., Culbertson C., Kramer K.J. Dietary mixtures of cysteine and serine proteinase inhibitors exhibit synergistic toxicity toward the red flour beetle, Tribolium castaneum. Comp. Biochem. Physiol. 1993;105:379–385. [Google Scholar]
- Oppert B., Morgan T.D., Hartzer K., Lenarcic B., Galesa K., Brzin J., Turk V., Yoza K., Ohtsubo K., Kramer K.J. Effects of proteinase inhibitors on digestive proteinases and growth of the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) Comp. Biochem. Physiol. Part C. 2003;134:481–490. doi: 10.1016/s1532-0456(03)00042-5. [DOI] [PubMed] [Google Scholar]
- Pandhare J., Zog K., Deshpande V. Differential stabilities of alkaline protease inhibitors from actinomycetes: effect of various additives on thermostability. Bioresour. Technol. 2002;84:165–169. doi: 10.1016/s0960-8524(02)00025-1. [DOI] [PubMed] [Google Scholar]
- Pichare M.M., Kachole M.S. Protease inhibitors of Pigeon pea (Cajanus cajan) and its wild derivatives. Physiol. Plantarum. 1996;98:845–851. [Google Scholar]
- Reppond K.D., Babbitt J.K. Protease inhibitors affect physical properties of arrowtooth flounder and walleye pollack surimi. J. Food Sci. 1993;58:96–98. [Google Scholar]
- Richardson M. Seed storage proteins: the enzyme inhibitors. Methods Plant Biochem. 1991;5:295–305. [Google Scholar]
- Robert A.C. Wiley; Germany: 2005. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists. [PubMed] [Google Scholar]
- Ryan C.A. Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Ann. Rev. Phytopathol. 1990;28:425–449. [Google Scholar]
- Shanker K., Gupta M.M., Srivastava S.K., Bawankule D.U., Pal A., Khanuja S.P.S. Determination of bioactive nitrileglycoside(s) in drumstick (Moringa oleifera) by reverse phase HPLC. Food Chem. 2007;105:376–382. [Google Scholar]
- Soottawat B., Somkid K., Angkana S. Inhibitory effects of legume seed extracts on fish proteinases. J. Sci. Food Agric. 1999;79:1875–1881. [Google Scholar]
- Tamir S., Bell J., Finlay T.H., Sakal E., Smirnoff S., Gaur S., Birk Y. Isolation, characterization and properties of a trypsin–chymotrypsin inhibitor from Amaranth seeds. J. Prot. Chem. 1996;15:219–229. doi: 10.1007/BF01887402. [DOI] [PubMed] [Google Scholar]
- Tawde S. Isolation and partial characterisation of red gram (Cajanus cajan) trypsin inhibitor. Ann. Biochem. Exp. Med. 1961;21:359–366. [PubMed] [Google Scholar]
- Wu C., Whitaker J.R. Purification and partial characterization of four trypsin/chymotrypsin inhibitors from red kidney beans (Phaseolus vulgaris var. Linden) J. Agric. Food Chem. 1990;38:1523–1529. [Google Scholar]


