Abstract
Pleurotus eryngii is a popular mushroom due to its excellent consistency of cap and stem, culinary qualities and longer shelf life. In Bangladesh, where Pleurotus mushrooms are very popular, P. eryngii may take position among the consumers, but currently this mushroom is not cultivated in large scale there. In this study, 3 strains of P. eryngii such as Pe-1 (native to Bangladesh), Pe-2 (germplasm collected from China) and Pe-3 (germplasm collected from Japan) were cultivated on saw dust and rice straw and their growth and yield parameters were investigated. Pe-1 on saw dust showed the highest biological yield and efficiency (73.5%) than other strains. Also, the mycelium run rate and number of fruiting bodies were higher in Pe-1 than other two strains. The quality of mushroom strains was near about similar. On saw dust, the yield and efficiency were better than those cultivated on rice straw, however, on straw; the mushroom fruiting bodies were larger in size. This study shows the prospects of P. eryngii cultivation in Bangladesh and suggests further study in controlled environment for higher yield and production.
Keywords: Pleurotus eryngii, Saw dust, Rice straw, Biological yield
1. Introduction
The oyster mushrooms (Pleurotus spp.) are in the third place after the white button and shiitake among the world mushroom production (Gyorfi and Hajdu, 2007). King oyster mushroom (Pleurotus eryngii) belongs to the family of oyster mushrooms, which is edible, basidiomycetic and saprophytic (Lewinsohn et al., 2002). It is considered as the best one of all Pleurotus species due to its excellent consistency of cap and stem, culinary qualities and longest shelf life than any other oyster mushroom (Yildiz et al., 2002). In the recent year, P. eryngii has been commercially cultivated in China, Japan and Taiwan because its excellent texture and flavor attract consumers (Eguchi et al., 1999, Peng, 1996, Peng, 1998, Royse, 1999).
Many strains of P. eryngii are available in the world, which are extensively cultivated. Different strains of king oyster mushroom response differently to different substrates, supplements, supplementation amount and environmental factors in the aspects of mycelium run, average yield and quality (Visscher, 1989). It can easily and successfully be cultivated on wheat and rice straw, cotton waste and sawdust (Jiskani, 1999). In Bangladesh, three strains of P. eryngii like Pe-1 (native), Pe-2 (germplasm collected from China) and Pe-3 (germplasm collected from Japan) have been cultivated on a small scale since last two years. The temperature range required for cultivation of these strains is 12–17 °C for fruiting body development. Although P. ostreatus, P. florida and P. sajor-caju are widely cultivated all over the year, but widely cultivation of P. eryngii is so difficult without controlled condition, because the average temperature of Bangladesh is higher and even in winter season, it is about 18 °C.
In Bangladesh, sawdust and rice straw are widely used as the main substrate for mushroom cultivation. But still no work has been done to find out the suitability of these locally available lignocellulosic wastes for the cultivation of P. eryngii and also to find out the most cost compatible strains in this environmental condition. If the growing technology will be developed and temperature may be control, that can make this strain most demanded out of all Pleurotus spp. due to its excellent texture and shelf life (Szili and Vessey, 1980). So, to identify the best strain of king oyster mushroom that can be most suitable for culture conditions in Bangladesh in case of sawdust and rice straw substrate, was the main aim of this investigation.
2. Methods and materials
2.1. Strain of king oyster mushroom
Three strains of P. eryngii such as Pe-1 (native to Bangladesh), Pe-2 (collected strain from China) and Pe-3 (collected strain from Japan) were used in this investigation.
2.2. Culture preparation
Pure cultures of different strains were prepared on malt extract agar (MEA) medium. The inoculated Petri dishes were incubated in the growth chamber at 25 ± 2 °C in the dark for about ten days. This culture was used for inoculation of mother culture after completion of the mycelium. Medium of mother culture was prepared by mixing sawdust and wheat bran at the ratio of 2:1 and 0.2% calcium carbonate. The moisture level of the mixture was maintained at 65%. Polypropylene bags of 25 × 17 cm size were filled with 250 g of the mixture and packed tightly. The neck was plugged with cotton and covered with brown paper and tied with a rubber band. The packets were sterilized in an autoclave for 1 h at 121 °C under 1 kg/cm2 pressure. The P. eryngii inoculated packets were placed on a rack in the laboratory at 25 ± 2 °C temperature for incubation. The substrate of the mother culture was colonized by the growth of mycelium within 15–20 days after inoculation. The fully colonized packets were used for spawning.
2.3. Spawn preparation
Two different substrates namely, sawdust (SD) and rice straw (RS) were used as culture media. In case of SD, sun dried SD, wheat bran and rice husk were mixed together at 176 g, 88 g and 11 g, respectively for each 550 g substrate. Water was added to adjust moisture content at 65% and CaCO3 was mixed at the rate of 0.2% of the mixture. Substrate mixture was filled into autoclavable polypropylene plastic bottles (900 ml) and a hole of about 2/3 deep of the volume of the bottle was made for space to put the inoculums. The bottles were sterilized at 121 °C for 1 h under 1 kg/cm2 pressure. After cooling down to room temperature the sterilized bottles were inoculated with the mother culture of the selected strains to be tested separately. In case of RS substrate, dried RS was chopped into 2–4 cm length and placed in hot water. After half an hour the burner was stopped and this straw was kept to cool. After cooling, the straw was spread on the polypropylene sheet for removal of excess water. Then the polypropylene bags were filled with substrate of 500 g. During bagging the packets were inoculated separately with the mother culture of selected strains to be tasted. These inoculated bottles and bags were incubated in a dark room at 25 ± 2 °C temperature for mycelium growth.
2.4. Cropping and harvesting
After completion of mycelial growth, the bottles of sawdust were uncapped and soaked in water for 3–5 min. But the spawn bags of rice straw were opened by square shaped (1″ × 1″) cut on the different place in a culture house. The temperature, relative humidity and light were maintained at 13–22 °C, 70–85% and about 180–250 lux, respectively. Carbon dioxide concentration was not monitored and controlled instrumentally. Mushroom were harvested when the mushroom cap surface were flat to slightly up-rolled at the cap margins. One flush of mushroom in each bottle or bag was harvested. The yield of mushrooms and their different quality parameters were recorded regularly.
2.5. Statistical analysis
The experiment was done completely randomized design with 10 replications (n = 10). Data was analyzed and graph was constructed by statistical program, SPSS-12.0 and Microsoft Excel.
3. Results
The growth and yield pattern of the P. eryngii strains cultivated on saw dust (SD) and rice straw (RS) is shown in Table 1. When P. eryngii strains were cultivated on SD, the highest mycelium run rate (MRR) was observed for Pe-1 (0.57 cm/day) which was significantly different (P ⩽ 0.05) from MRR of Pe-2 (0.32 cm/day) and Pe-3 (0.36 cm/day). Similarly, on RS, the MRR of Pe-1 (0.50 cm/day) was significantly different (P ⩽ 0.05) from MRR of Pe-2 (0.30 cm/day) and Pe-3 (0.31 cm/day). MRR for each strain was slightly lower on RS than SD, but this was not significant.
Table 1.
The growth and yield pattern of three strains of Pleurotus eryngii cultivated on saw dust (SD) and rice straw (RS).
| Substrate: SD |
Substrate: RS |
|||||
|---|---|---|---|---|---|---|
| Pe-1 | Pe-2 | Pe-3 | Pe-1 | Pe-2 | Pe-3 | |
| MRR | 0.57 ± 0.02a | 0.32 ± 0.01b | 0.36 ± 0.01b | 0.50 ± 0.04p | 0.30 ± 0.02q | 0.31 ± 0.02q |
| NPI | 4.25 ± 1.31 | 4.5 ± 0.65 | 3.75 ± 0.63 | 3.79 ± 0.52 | 4.0 ± 0.04 | 3.7 ± 1.0 |
| DFPI | 17.0 ± 0.5⁎ | 15.75 ± 1.0⁎ | 16.8 ± 0.7 | 12.75 ± 1.7p,q | 11.25 ± 1.1p⁎ | 15.8 ± 1.4q |
| DFH | 26.5 ± 0.96 | 26.5 ± 0.5 | 30.0 ± 2.48 | 27.1 ± 1.2 | 29.4 ± 0.9 | 27.6 ± 2.0 |
| NFB | 3.25 ± 0.75 | 2.75 ± 0.25 | 2.25 ± 0.25 | 2.3 ± 0.2p⁎ | 1.2 ± 0.05q⁎ | 1.1 ± 0.02q⁎ |
Results are mean ± SEM (n = 10). Values with different superscript in a same row for each substrate are significantly different at P ⩽ 0.05 (a, b, c for SD and p, q, r for RS).
Notification indicates significant difference (P ⩽ 0.05) between parameters of a single strain cultivated on different substrate. MRR – mycelium run rate (cm/day); NPI – number of primordial initiation, DFPI – days to first primordia initiation; DFH – days required to first harvest, NFB – number of fruiting bodies.
Number of primordia varied from 3.7 to 4.5 among the strains on two substrates but the variation was not significant at P ⩽ 0.05. On SD, the highest number of primordial initiation (NPI) was observed for Pe-2 (4.5), which was followed by Pe-1 (4.25) and Pe-3 (3.75). On RS, similar trend was observed: 4.0 for Pe-2, 3.79 for Pe-1 and 3.7 for Pe-3.
On SD, fewest days (15.75) were required for primordial initiation (DFPI) in case of Pe-2, which was non-significantly different from Pe-1 (17.0) and Pe-3 (16.0). On RS, fewest days were required again for Pe-2 (11.3), which was followed by Pe-1 (12.75) and Pe-3 (15.75). DFPI of Pe-2 and Pe-3 were significantly different (P ⩽ 0.05). DFPI of Pe-1 and Pe-2 on RS were also significantly different (P ⩽ 0.05) from those on SD.
Days required to first harvest the mushroom fruiting bodies (DFH) did not vary significantly, which ranged from 26.5 (Pe-1 and Pe-2 on SD) to 30.0 (Pe-3 on SD).
The number of fruiting bodies of Pe-1 (3.25), Pe-2 (2.75) and Pe-3 (2.25) on SD were significantly higher (P ⩽ 0.05) than corresponding strains on RS (2.3, 1.2 and 1.1 for Pe-1, Pe-2 and Pe-3, respectively).
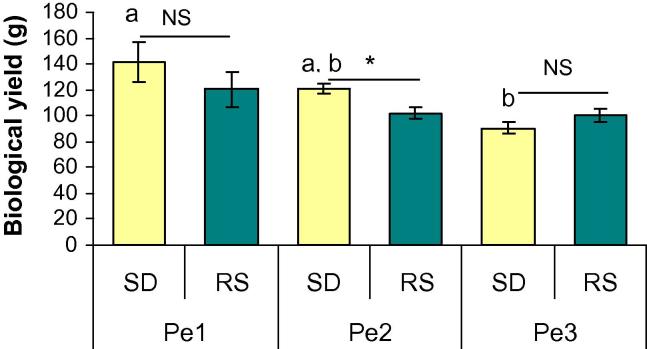
Fig. 1 represents the biological yield of P. eryngii strains on SD and RS. On SD, the highest yield was observed in case of Pe-1 (141 g) which was non-significantly followed by Pe-2 (120.5 g). The yield of Pe-3 (90.5 g) was significantly lower (P ⩽ 0.05) than that of Pe-1. The highest biological yield on RS was found for Pe-1 (120.25 g) which was followed by Pe-2 (102 g) and Pe-3 (100 g). In this study, for every strain, yield was lower on RS, among which difference between Pe-2 on SD and RS was significantly different at P ⩽ 0.05.
Figure 1.
The biological yield of Pleurotus eryngii mushroom strains cultivated on saw dust (SD) and rice straw (RS). Results are mean ± SEM (n = 10). Bars with different letter for each substrate are significantly different at P ⩽ 0.05 (a, b, c for SD and p, q, r for RS). ∗Indicates significant difference (P ⩽ 0.05) between parameters of a single strain cultivated on different substrate and ‘NS’ indicates non-significant.
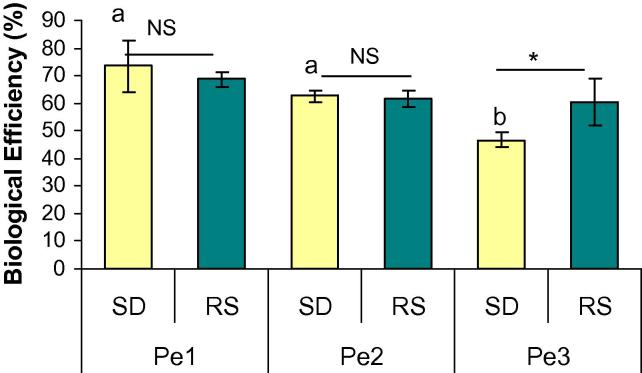
Fig. 2 represents the biological efficiency of P. eryngii strains on SD and RS. Biological efficiency (BE) was determined by the following formula:
Highest efficiency was found in case of Pe-1 (73.5%) which was followed by Pe-2 (62.6%) on SD. Biological efficiency of Pe-3 (46.75%) was significantly lower than those of Pe-1 and Pe-2. Similar trend was observed on RS (68.7% for Pe-1, 61.5% for Pe-2 and 60.4% for Pe-3), but these were not significantly different. Like biological yield, the biological efficiency (BE) of the strains was also lower when RS was used as substrate, among which difference between Pe-3 on SD and RS was significantly different at P ⩽ 0.05.
Figure 2.
The biological efficiency of Pleurotus eryngii mushroom strains cultivated on saw dust (SD) and rice straw (RS). Results are mean ± SEM (n = 10). Bars with different letter for each substrate are significantly different at P ⩽ 0.05 (a, b, c for SD and p, q, r for RS). ∗Indicates significant difference (P ⩽ 0.05) between parameters of a single strain cultivated on different substrate and ‘NS’ indicates non-significant.
Table 2 represents the quality of P. eryngii strains produced on SD and RS. Length of stalk (LS) of Pe-1, Pe-2 and Pe-3 were 8.0 cm, 8.0 cm and 6.63 cm respectively on SD. On RS, LS were 7.4, 7.6 and 8.3 cm of Pe-1, Pe-2 and Pe-3, respectively. Interestingly LS of Pe-3 was lowest among the strains on SD but highest on RS. LS of Pe-3 on SD and RS were significantly different (P ⩽ 0.05). Diameter of stalk (DS) of the strains on two substrates did not vary significantly, ranging from 2.7 cm (Pe-2 on SD) to 3.2 cm (Pe-2 and Pe-3 on RS). Similarly, thickness of pileus (TP) of the strains on two substrates did not vary significantly, ranging from 1.43 cm (Pe-2 on SD) to 1.83 cm (Pe-1 on SD). On SD, highest diameter of pileus (DP) was found in case of Pe-2 (7.13 cm) which differed with Pe-1 (6.83 cm) non-significantly and with Pe-3 (5.25 cm) significantly. But on RS, the variation of DP among the strains was not significant (6.9 cm, 7.5 cm and 8.2 cm of Pe-1, Pe-2 and Pe-3, respectively). Like LS, the DP of Pe-3 was lowest among the strains on SD but highest on RS and DP of Pe-3 on SD and RS were significantly different (P ⩽ 0.05).
Table 2.
The quality of Pleurotus eryngii mushroom strains produced on saw dust (SD) and rice straw (RS).
| Substrate: SD |
Substrate: RS |
|||||
|---|---|---|---|---|---|---|
| Pe-1 | Pe-2 | Pe-3 | Pe-1 | Pe-2 | Pe-3 | |
| LS (cm) | 8.0 ± 0.61 | 8.0 ± 0.20 | 6.63 ± 0.55 | 7.4 ± 0.6 | 7.6 ± 0.9 | 8.3 ± 0.3⁎ |
| DS (cm) | 3.05 ± 0.17 | 2.7 ± 0.18 | 3.13 ± 0.24 | 3.1 ± 0.09 | 3.2 ± 0.35 | 3.2 ± 0.25 |
| TP (cm) | 1.83 ± 0.12 | 1.43 ± 0.22 | 1.60 ± 0.07 | 1.7 ± 0.55 | 1.66 ± 0.07 | 1.75 ± 0.4 |
| DP (cm) | 6.83 ± 0.4a,b | 7.13 ± 0.35a | 5.25 ± 0.12b | 6.9 ± 0.7 | 7.5 ± 0.6 | 8.2 ± 0.5⁎ |
Results are mean ± SEM (n = 10). Values with different superscript in a same row for each substrate are significantly different at P ⩽ 0.05 (a, b, c for SD and p, q, r for RS).
Notification indicates significant difference (P ⩽ 0.05) between parameters of a single strain cultivated on different substrate. LS – length of stalk, DS – diameter of stalk, DP – diameter of pileus, TP – thickness of pileus.
4. Discussion
Saw dust and rice straw are the most available agricultural wastage in Bangladesh. For this, in this study, these two substrates were used for the production of three different strains of P. eryngii. The mycelium run rate (MRR) was highest for Pe-1 in both substrates but MRR of each strain was slightly lower on RS than SD, however, this was not significant. This result is similar to the findings of Khandakar et al. (2008), who investigated the mycelial growth on different culture media.
Number of primordia varied from 3.7 to 4.5 among the strains on two substrates without any significance. Amin et al. (2007) also did not find any significant variation of primordial initiation number of Pleuratus spp. between SD and RS. The DFPI of different strains of P. eryngii ranged 11.3–17.0 days. These periods were shorter than the data from the study of Kirbag and Akyuz (2008), who reported that the time need for primordial initiation of P. eryngii was 26.2–44.2 days, depending on the type of substrate used and the rate of additive matter. Days required to first harvest of P. eryngii was also found different from other study (Akyuz and Yildiz, 2007).
In this study, the number of fruiting bodies of different strains on SD was significantly higher than corresponding strains on RS. Amin et al. (2007) found the maximum number of fruiting bodies of different oyster mushroom species on SD when compared with RS. Although king oyster gives small number of fruiting body, texture and shelf life is very higher than other Pleurotus spp. Similar result was found in shiitake mushroom (Sarker et al., 2009).
The biological yield of P. eryngii strains on SD and RS was found 90.5–141 g in first flush in this study. This result is similar to the findings of Peng et al. (2001), who found the biological yield of king oyster mushroom to vary between 88 and 146 g in first flush on sawdust. Kirbag and Akyuz (2008) found 14.4 g mushroom from 100 g of substrate on wheat straw and 19 g on wheat straw when added 10% rice bran as a supplement. In this study, for every strain, yield was lower on RS, among which difference between Pe-2 on SD and RS was significantly different at P ⩽ 0.05. Amin et al. (2007) reported sawdust better then RS as the substrate for Pleurotus spp. Kirbag and Akyuz (2008) found 48.05% biological efficiency on wheat straw. In this study, the BE was found higher with difference among strains and substrates. Peng et al. (2000) also reported the different biological efficiency in different strains on sawdust. The differences between the values may arise from the fact that the strain and culture media used were different.
The quality of P. eryngii of this study was much better than other oyster mushroom’s quality reported in other studies. For example, LS, DS, DP and TP were higher in P. eryngii when compared with other Pleurotus spp. (Alam et al., 2007, Sarker et al., 2007, Shelly et al., 2009).
The difference of growth and yield of P. eryngii and their quality may be due to the genotype of mushroom strains and the biological structure of the substrate. The production was highest in Pe-1, which is native to Bangladesh, probably due to environmental suitability of this strain. But the production and biological efficiency were comparatively lower than other Pleurotus species cultivated in Bangladesh. This is probably due to the low temperature. As P. eryngii is a mushroom of cold temperature, the short winter period of Bangladesh does not provide enough support to high yield of this mushroom. By considering the popularity of this mushroom to the consumers, further researches are required in controlled environment to increase production of this mushroom.
References
- Akyuz M., Yildiz A. Cultivation of Pleurotus eryngii (DC ex Fr.) Quel. on agricultural wastages. Philipp. Agric. Sci. 2007;90:344–348. [Google Scholar]
- Alam N., Amin S.M.R., Sarker N.C. Efficacy of five different growth regulators on the yield and yield contributing attributes of Pleurotus ostreatus (Jacquin ex Fr.) Kummer. Bangladesh J. Mushroom. 2007;1:51–55. [Google Scholar]
- Amin S.M., Rahman M.M., Hossain M.M., Haque M.M., Sarker N.C. Effect of different substrates on the growth and yield of five selected oyster mushrooms. Bangladesh J. Mushroom. 2007;1:21–25. [Google Scholar]
- Eguchi, F., Watanabe, Y., Sudo, K., Higaki, M., 1999. Pharmacological effects of Pleurotus eryngii on the hyperlipemia. In: Proceedings of 3rd International Conference on Mushroom Biology and Mushroom Products. University of Western Sydney, Hawkesbury, pp. 333–339.
- Gyorfi J., Hajdu C.S. Casing-material experiments with P. eryngii. Int. J. Horticult. Sci. 2007;13:33–36. [Google Scholar]
- Jiskani, M.M., 1999. A Brief Outline “The Fungi” (Cultivation of Mushrooms). Izhar Pub. Tandojam, Pakistan, p. 94.
- Khandakar J., Yesmin S., Sarker N.C., Amin S.M.R. Effect of media on mycelial growth of edible mushrooms. Bangladesh J. Mushroom. 2008;2:53–56. [Google Scholar]
- Kirbag S., Akyuz M. Effect of various agro-residues on growing periods, yield and biological efficiency of Pleurotus eryngii. J. Food Agric. Environ. 2008;6:402–405. [Google Scholar]
- Lewinsohn D., Wasser S.P., Reshetnikov S.V., Hadar Y., Nevo E. The Pleurotus eryngii species complex in Israel: distribution and morphological description of a new takson. Mycotaxon. 2002;81:51–67. [Google Scholar]
- Peng, J.T., 1996. Research on the Automatic Production of Pleurotus eryngii (DC.:Fr.) Quel (in Chinese). In: A Report on Agricultural Research in the Republic of China on Taiwan (Crops) 1992–1996, Council of Agriculture, Executive Yuan, ROC, pp. 89–91.
- Peng, J.T., 1998. Research on the Automatic Production of King Oyster Mushroom (in Chinese). In: Proceedings of 1998 Agricultural Science and Technology Exhibition. Council of Agriculture, Executive Yuan, ROC, pp. 44–46.
- Peng J.T., Lee C.M., Tsai Y.F. Effect of rice bran on the production of different king oyster mushroom strains during bottle cultivation. J. Agric. Res. China. 2000;49:60–67. [Google Scholar]
- Peng J.T., Dai M.C., Tsai Y.F., Chen M.H., Chen J.T. Selection and breeding of king oyster mushroom. J. Agric. Res. China. 2001;50:43–58. [Google Scholar]
- Royse D.J. Yield stimulation of king oyster mushroom, Pleurotus eryngii, by brewer’s grain and spawn mate IISE supplementation of cottonseed hull and wood chip substrates. Mushroom News. 1999;47:4–8. [Google Scholar]
- Sarker N.C., Hoossain M.M., Sultana N., Karim A.J.M.S., Amin S.M.R. Performance of different substrates on growth and yield of Pleurotus ostreatus (Jacquin ex Fr.) Kummer. Bangladesh J. Mushroom. 2007;1:9–20. [Google Scholar]
- Sarker N.C., Ahmed S., Hossain K., Jahan A., Quddus N.M.M. Performance of different strains of shitake mushroom (Lentinus edodes) on saw dust. Bangladesh J. Mushroom. 2009;3:1–7. [Google Scholar]
- Shelly N.J., Amin S.M.R., Nuruddin M.M., Ahmed K.U., Khandakar J. Comparative study on the yield and yield related attributes of some newly introduced strains of Pleurotus cystidiosus with Pleurotus ostreatus. Bangladesh J. Mushroom. 2009;3:67–72. [Google Scholar]
- Szili, I., Vessey, E., 1980. A csiperke es mas gombak haztaji termesztese. Mezogazdasagi Kiado, Buddapest, pp. 152–153.
- Visscher H.R. Supplementation of the substrate for Pleurotus species at filling. Mushroom Sci. 1989;12:229–240. [Google Scholar]
- Yildiz S., Yildiz U.C., Geze E.D., Temiz A. Some lignocellulosic wastes used as raw material in cultivation of the Pleurotus ostreatus culture mushroom. Process Biochem. 2002;38:301–306. [Google Scholar]