Abstract
Information regarding the simultaneous evaluation of tillage and fertilization on the soil biological traits in canola production is not available. Therefore, field experiments were conducted in 2007–2010 in a split plot based on randomized complete block design with three replications. Main plots consisted of conventional tillage (CT); minimum tillage (MT) and no tillage (NT). Six strategies of fertilization including (N1): farmyard manure (cattle manure); (N2): compost; (N3): chemical fertilizers; (N4): farmyard manure + compost; (N5): farmyard manure + compost + chemical fertilizers and (N6): control, were arranged in sub plots. Results showed that the addition of organic manure increased the soil microbial biomass. No tillage system increased microbial biomass compared to other tillage systems. The activities of all enzymes were generally higher in the N4 treatment. The activity of phosphatase and urease tended to be higher in the no tillage treatment compared to the CT and MT treatments.
Keywords: Enzyme activity, Compost, Farmyard manure, Microbial biomass
1. Introduction
Conventional canola production utilizing tillage, chemical fertilizers, and irrigation can improve the grain yield. However, this intensive production system also can degrade soil quality, enhance runoff by covering the soil with an impervious surface, contribute to surface and impurity pollution and add to production cost (Rice et al., 2001). Alternative systems have been developed that use renewable organic resources and minimize tillage to build soil organic matter and enhance soil quality. Fertilization is one of the soil and crop management practices, which exert a great influence on soil quality (Chander et al., 1998; Mohammadi et al., 2011). Farmyard manure (FYM) and compost have the potential to increase of soil organic matter, in addition to making some nutrient input and stabilizing soil structure. It is well known that organic amendments, such as plant residues, manures and composts have a number of benefits in soil physical and chemical properties. Many reports have also revealed different aspects of biology of soils amended with organic matters, including the number of fungi and bacteria (Nishio and Kusano, 1980), biomass of bacteria and fungi (Lundquist et al., 1999), enzyme activities (Kandeler et al., 1999) and biochemical properties (Lynch, 1983). Microbial communities perform necessary ecosystem services, including nutrient cycling, pathogen suppression, stabilization of soil aggregates, and degradation of xenobiotics. Soil microbial biomass, enzyme activity, and community structure have been shown to respond to agricultural management practices. Alternation to no tillage (NT) or increased cropping intensity increases microbial biomass C (MBC) in response to increase nutrient reserves and improved soil structure and water retention (Biederbeck et al., 2005; Mohammadi, 2011).
Enzyme activities have been indicated as soil properties suitable for use in the evaluation of the degree of alteration of soils in both natural and agro-ecosystems. Soil microbial properties have a strong correlation with soil health. Some researches have already suggested the favorable effects of conservation tillage practices and organic fertilizers on soil enzyme activities (Kandeler et al., 1999). The activity of dehydrogenase is considered an indicator of the oxidative metabolism in soils and thus of the microbiological activity, because it is exclusively intracellular and, theoretically, can function only within viable cells. Urease catalyzes the hydrolysis of urea into CO2 and NH3, which is of specific interest because urea is an important N fertilizer. Urease is released from living and disintegrated microbial cells, and in the soil it can exist as an extracellular enzyme absorbed on clay particles or encapsulated in humic complexes. Phosphatases catalyze the hydrolysis of both organic phosphate (P) esters and anhydrides of phosphoric acid into inorganic P. Phosphatase activity may originate from the plant roots and associated mycorrhiza and other fungi, or from bacteria (Tarafdar and Marschner, 1994).
The objective of this study was to determine the short-term (three years) effects of conservation management practices, such as no-tillage, reduced tillage and organic fertilizers on microbiological soil quality indicators in the canola field.
2. Materials and methods
2.1. Site description and experimental design
The experiments were conducted from 2007 to 2010 at the Agricultural Research Center of Sanandaj (ARCS), Kurdistan province, the northwest region of Iran (35°16 lat. N; 47°1 long. E, 1405 m above sea level). The dominant soil type is Inceptisol. The annual temperature averages 12 °C and the annual rainfall averages 512 mm. Experiments were arranged in the split plot based on randomized complete block design with three replications. Main plots consisted of conventional tillage (CT) (moldboard plowing with average depth of 30 cm + two shallow disks followed by secondary tillage with a soil grubber and harrow for seedbed preparation); minimum tillage (MT) (disk harrowing with average depth of 15 cm + one shallow disk harrowing) and no tillage (NT). In NT, crop residues cut by the combine were chopped and spread evenly with a combine-attached chopper. NT plots were seeded with a NT seed drill. Sub-plots were six strategies of supplying the basal fertilizer requirements of canola, including (N1): 30 ton farmyard manure ha−1 (cattle manure); (N2): 15 ton compost ha−1; (N3): 100 kg triple super phosphate ha−1 + 150 kg Urea ha−1; (N4): 15 ton farmyard manure ha−1 + 7.5 ton compost ha−1; (N5): 10 ton farmyard manure ha−1 + 5 ton compost ha−1 + 50 kg triple super phosphate ha−1 + 75 kg Urea ha−1 and (N6) Control (without fertilizer). The amounts of chemical and organic fertilizers were determined according to soil test analysis. Soil texture was clay loam (28% sand, 42% clay and 30% silt) with 0.8% organic matter and a pH of 7.6. The farmyard manure and compost were also analyzed according to Peters et al. (2003) method for chemical and nutrients properties (Table 1). Farmyard manure, compost and chemical fertilizers were added to plots before sowing canola. For CT and MT chemical fertilizer or organic fertilizers were applied and then incorporated with tillage, while for NT treatments, fertilizers were surface applied on the plots. Urea fertilizer was applied equally two times before sowing canola and flowering.
Table 1.
Chemical characteristics of farmyard manure and compost applied to the soil.
| Characteristic | pH | N (%) | P (%) | K (%) | Ca (mg kg−1) | Mg (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| Farmyard manure | 7.45 | 0.47 | 0.49 | 0.31 | 2745 | 1100 | 12 | 25 |
| Compost | 7.21 | 0.78 | 1.15 | 0.51 | 1950 | 1890 | 53 | 295 |
Canola seeds were planted on September 22, 2007, September 12, 2008 and September 18, 2009. Main plot size was of 15 × 20 m and spaces between main plots were three meters. The field was irrigated twice with a 7–9 day interval for the better germination of seeds. The field was also irrigated at stemming and flowering along with fertilization, and at podding and grain filling. Weeds removed by hand in all plots.
2.2. Soil sampling
For soil physical and chemical analyses, soil pH was measured in suspensions with a soil to water (w/w) ratio of 1:2.5. Organic carbon was measured by a colorimetric method with an external heating procedure (Anderson and Ingram, 1993) and total nitrogen in soil was determined using the Kjeldahl method. Soil for microbiological analysis was sampled in canola plots. Soil samples were collected in crop rhizosphere at flowering stage of canola growth. Plants were excavated from four random 0.5-m lengths of a row from each plot. Loose soil was shaken off the roots, and the soil that adhered strongly to the roots was carefully brushed from the roots and kept as rhizosphere soil. The four rhizosphere samples from each plot were combined, passed through a 2-mm sieve and stored at 4 °C until required for analysis.
2.3. Microbial biomass
Microbial biomass carbon (MBC) and biomass N (MBN) contents were estimated by chloroform fumigation extraction (Vance et al., 1987). One 20-g portion (in dry weight) rewetted to 60% was fumigated for 24 h at 25 °C with ethanol-free CHC13. Following fumigant removal, the soil was extracted with 60 mL 0.5 mol L−1 K2SO4 by 30 min horizontal shaking at 200 r min−1 and filtered. The non-fumigated portion was extracted similarly at the time fumigation commenced. Organic C in extracts was determined by a dichromate digestion method and unused dichromate titrated against ferrous ammonium sulfate. Microbial biomass C was calculated as follows: Microbial biomass C = EC/kEC, where EC = organic C extracted from fumigated soils − organic C extracted from non-fumigated soils and kEC = 0.38 (Vance et al., 1987). The Kjeldahl digestion–distillation–titration method was used to determine the total N in the extracts. Microbial biomass N was calculated as follows: Microbial biomass N = EN/kEN, where EN = total N extracted from fumigated soils − total N extracted from non-fumigated soils and kEN = 0.45 (Brookes et al., 1985). Each sample had duplicate analyses and results are expressed on a moisture free basis.
2.4. Soil enzyme activities
Protease (EC 3.4.21–24) activity was determined according to Kandeler (1996). One g field-moist soil was incubated in a rotating water bath for 2 h in 5 ml casein solution (2%, w/v) and 5 ml 0.05 M Tris buffer (pH 8.1) at 50 °C. The reaction was stopped with 5 ml 0.92 MTCA. Folin-Ciocalteu’s reagent was added to form a colored complex with the aromatic amino acids formed during the incubation, and the absorbance was determined at 700 nm (Perkin Elmer Lambda 25 UV/VIS). To measure alkaline (EC 3.1.3.1) and acid phosphatase (EC 3.1.3.2) enzymes (Mandal et al., 2007) p-nitrophenyl phosphate disodium (0.115 M) was used as the substrate. Soil samples (1 g) were treated with 2 ml of 0.5 M sodium acetate buffer with a pH of 5.5 (using acetic acid) (Naseby and Lynch, 1997) and 0.5 ml of substrate and were incubated at 37 °C for 90 min. Cooling at 2 °C for 15 min inhibited the reaction. The treated samples were then mixed with 2 ml of 0.5 M NaOH and 0.5 ml of 0.5 M CaCl2 (to inhibit the enzyme reaction) and centrifuged at 4000 rpm for 5 min. Using spectrometry at 398 nm the produced p-nitrophenol was measured (Tabatabai and Bremner, 1969). Urease (EC 3.5.1.5) activity was measured using 0.5 M urea as a substrate in 0.1 M phosphate buffer at pH 7.1 (Nannipieri et al., 1974). The produced by urease activity was determined using a flow injection analyser (FIAStar, Tecator, S). To account for the fixation by soils, solutions with concentrations in the range of those released by urease activity were incubated with these soils. Dehydrogenase activity was determined by the reduction of triphenyl tetrazolium chloride (TTC) to triphenyl formazan (TPF) as described by Serra-Wittling et al. (1995) with modifications. Briefly, moist soil (2 g) was treated with 2.5 ml of 1% TTC–Tris buffer (pH 7.6), and then incubated at 37 °C in darkness for 24 h. All enzyme activity values were calculated based on of oven-dry (105 °C) weight of soil.
2.5. Statistical analysis
Using SAS (SAS Institute, 2003) data were subjected to analysis of variance, including combined analysis. Analysis of variance (ANOVA) was used to detect the treatments effect on measured variables, and the least significant difference (LSD) was used to compare means of measured enzyme activities and microbial biomass carbon (P < 0.05). In addition correlation coefficients among soil enzymes and microbial biomass were also determined.
3. Results and discussion
3.1. Microbial biomass
The results indicated statistically significant (p < 0.05) differences in the level of MBC in the soil between various methods of tillage and fertilization. There were no significant differences between interaction effect of tillage and fertilization on MBC. The pattern of variation of MBC in the soil during the three years of study was similar. The addition of compost or FYM, significantly (p < 0.05) increased the soil MBC in comparison to the chemical fertilizer and the control. Higher levels of MBC in N1 and N2 treatments could be due to greater amounts of biogenic materials like mineralizable nitrogen, carbohydrates and water soluble carbon. Integrated use of chemical fertilizers and organic matter (N5) brings in more MBC in soil compared to their single application (Table 2). Similar observations were recorded by Leita et al. (1999). Fertilizers may meet up the demand of mineral nutrition required by the microbes but not that of carbon, which is a major component of microbial cells. Integrated application of organic and inorganic materials provides a balanced supply of mineral nutrients as well as carbon.
Table 2.
Effects of fertilization method on microbial biomass and soil enzyme activities.
| Treatments | MBC (mg C kg−1 soil) | MBN (mg N kg−1 soil) | Protease (μg tyrosine g−1 h−1) | Acid phosphatase (μg PNP g−1 h−1) | Alkaline phosphatase (μg PNP g−1 h−1) | Urease (μg urea hydrolyzed g−1 h−1) | Dehydrogenase (μg triphenylformazan g−1 24 h−1) |
|---|---|---|---|---|---|---|---|
| Basal fertilizers | |||||||
| FYM (N1) | 278.4 c | 98.6 b | 86.5 c | 167.4 b | 2987.3 b | 49.6 a | 60.1 b |
| Compost (N2) | 312.6 c | 92.6 b | 94.6 bc | 169.2 b | 3001.4 b | 44.4 b | 62.9 ab |
| Chemical fertilizer (N3) | 196.3 d | 75.2 c | 87.1 c | 158.1 c | 2678.6 c | 28.8 c | 21.2 d |
| FYM + compost (N4) | 409.5 b | 141.2 a | 110.3 a | 226.6 a | 3314.4 a | 49.8 a | 63.8 a |
| FYM + compost + chemical (N5) | 691.2 a | 145.8 a | 96.2 b | 169.2 b | 2879.1 bc | 29.4 c | 53.7 c |
| Control (N6) | 89.3 e | 66.3 d | 73.1 d | 41.8 d | 2658.7 c | 27.9 c | 20.8 d |
Mean values in each column with the same letter(s) are not significantly different using LSD tests at 5% of probability.
NT system increased MBC compared to other tillage systems (Fig. 1).
Figure 1.
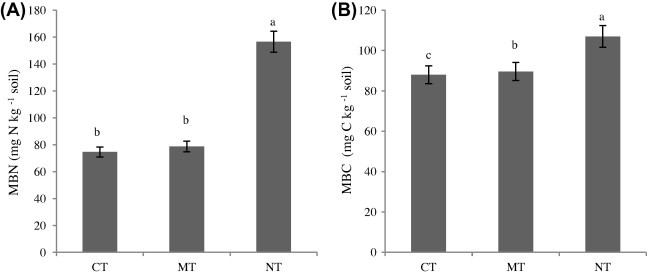
Effect of tillage practices on MBN (A) and MBC (B) in soil. (CT: conventional tillage, MT: minimum tillage; NT: no tillage).
Conventional tillage decreases soil organic matter and soil structure, and it is due to decrease soil microbial communities. Madejon et al. (2007) observed that conservation tillage increased MBC and microbial activities. Along with microbial biomass changes, one might also expect shifts in microbial community structure to occur due to the temporal increase in microbial niche, water retention or reduced physical disturbance with no-tillage.
Analysis of variance showed that tillage, fertilization and interaction of them had significant effects on MBN. The evaluation interaction of tillage and fertilization showed that the MBN content was from 59.11 to 167.96 mg N kg−1 soil (Fig. 2). Nitrogen microbial biomass contents were the highest in the NTN5 treatment.
Figure 2.
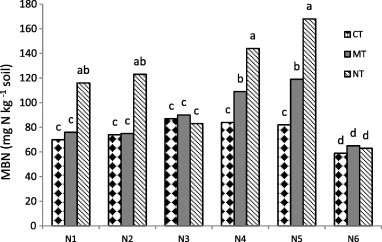
Interactive effect of fertilizers and tillage on microbial biomass of nitrogen. (CT: conventional tillage, MT: minimum tillage; NT: no tillage & N1: FYM; N2: compost; N3: chemical fertilizers; N4: FYM + compost N5: FYM + compost + chemical fertilizers; N6: control).
Higher levels of microbial biomass in organic manure treated entire soil could be due to greater amounts of biogenic materials like mineralizable nitrogen, water soluble carbon and carbohydrates. Fertilizers may meet up the demand of mineral nutrition required by the microbes but not that of carbon, which is a major component of microbial cells. Integrated application of organic and inorganic materials provides a balanced supply of mineral nutrients as well as carbon. MBN is considered to be more sensitive than total organic nitrogen to indicate soil changes because it is related to soil microorganisms that are sensitive to soil variations (Liu et al., 2003). The soil microbial biomass nitrogen is the active component of the soil organic pool. Ren and Stefano (2000) suggested that the MBC and MBN can provide an effectively early warning of the improvement or deterioration of soil quality as a result of different management practices (Powlson, 1994). Thus, the changes in biomass measured over relatively short periods can indicate the trends in total organic matter content long before these can be detected by chemical analysis. The soil microbial biomass carbon is the early indicator of soil organic carbon, soil microbial biomass nitrogen involved in soil nitrogen mineralization.
3.2. Soil enzyme activities
The activities of all enzymes varied significantly in different fertilization methods. Only, urease activity was significantly affected by the two-way interactions of fertilizers × tillage. The pattern of variation of enzyme activity in the soil during the three years of study was similar; however, urease activity was higher in the first year. The activities of all enzymes were generally higher in the N4 treatment than in the unfertilized and chemical fertilizer treatments (Table 2). There were no differences in phosphatase activity between the compost treatment and the FYM treatments. The dehydrogenase, phosphatase and urease activities in the N3 treatment were significantly lower than in the FYM and compost treatments. As shown in Table 2, alkaline and acid phosphatase generally increased with compost application. Increased phosphatase activity could be responsible for the hydrolysis of organically bound phosphate into free ions, which were taken up by plants. Tarafdar and Marschner (1994) reported that plants can utilize organic P fractions from the soil by phosphatase activity enriched in the soil–root interface. The observed increases in enzyme activities due to organic manure are in accordance with previous studies. Martens et al. (1992) reported that addition of the organic matter maintained high levels of phosphatase activity in soil during a long term study. Giusquiani et al. (1994) reported that phosphatase activities increased when compost was added at rates of up to 90 ton ha−1 and the phosphatases continued to show a linear increase with compost rates of up to 270 ton ha−1 in a field experiment. Application of nitrogen fertilizers significantly decreased urease activity while addition of organic manure increased its activity. The authors concluded that because the chemical fertilizers used in the experiments contained NH4+ and that the reaction products of urease being NH4+, microbial induction of urease activity had been inhibited. The effect of organic manures on enzyme activities is probably a combined effect of a higher degree of stabilization of enzymes to humic substances and an increase in microbial biomass with increased soil carbon concentration (Martens et al., 1992). This is also indicated by the strong correlation of protease, acid phosphatase and urease with microbial soil C concentrations. Only alkaline phosphatase activity showed statistically non-significant, correlations with MBC (Table 3). Compost application increased dehydrogenase activity (Table 2). Stronger dehydrogenase activity in compost applied plots may be due to higher organic matter content (Wlodarczyk et al., 2002). Marinari et al. (2000) reported that a higher level of dehydrogenase activity was observed in soil treated with compost and farmyard manure compared to soil treated with mineral fertilizer.
Table 3.
Correlation coefficients between enzyme activity and microbial biomass.
| MBN | MBC | Protease | Acid phosphatase | Alkaline phosphatase | Urease | Dehydrogenase | |
|---|---|---|---|---|---|---|---|
| MBN | 1 | ||||||
| MBC | −0.123 ns | 1 | |||||
| Protease | 0.715 ⁎⁎ | 0.873 ⁎⁎ | 1 | ||||
| Acid phosphatase | 0.134 ns | 0.712 ⁎⁎ | 0.665 ⁎⁎ | 1 | |||
| Alkaline phosphatase | 0.098 ns | 0.389 ns | 0.632 ⁎⁎ | 0.733 ⁎⁎ | 1 | ||
| Urease | 0.789 ⁎⁎ | 0.812 ⁎⁎ | 0.332 ns | 0.523 ⁎⁎ | 0.249 ns | 1 | |
| Dehydrogenase | 0.456⁎ | 0.671 ⁎⁎ | 0.703 ⁎⁎ | 0.783 ⁎⁎ | 0.674 ⁎⁎ | 0.512 ⁎⁎ | 1 |
Significance levels of correlations:
P < 0.05;
P < 0.01; ns P > 0.05.
The enzyme activity in organic amendment soil increased by an average two to fourfold compared with the un-amended soil. Application of compost caused a significant increase in dehydrogenase activity (Martens et al., 1992). These results were similar to our finding that dehydrogenase in rhizosphere soil of N2 treatment was average three times higher than that of mineral fertilizer (N3) treatments.
In addition, the higher organic matter in the compost treatment may supply more favorable conditions for the accumulation of enzymes in the soil matrix, since soil organic constituents are thought to be important in forming stable complexes with free enzymes. Soil factors, including redox potential (Eh) and pH can affect the rate of enzyme mediated reactions by influencing the redox status and ionization respectively, as well as solubility of enzymes, substrates and cofactors. In addition, some enzymes may predominate at specific pH levels. Application of compost and FYM caused a faster and higher reduction of soil, and at the same time increased the soil pH. Application of chemical fertilizers decreased soil pH and compost amended plots increased soil pH in a tropical Aeric Endoaquept planted to rice under flooded condition (Nayak et al., 2007). Soil dehydrogenase activity showed a negative correlation with Eh and a positive relationship with Fe2+ content, suggesting aeration status is the main factor determining the activity (Wlodarczyk et al., 2002).
Results showed significant differences (p < 0.05) in the enzyme activities in the soil between various methods of tillage. The activity of acid, alkaline phosphatase and protease tended to be higher in the NT treatment compared to the MT and CT treatments. However, the activity of urease and dehydrogenase was similar in NT and MT treatments (Fig. 3). Finding of Jin et al. (2009) has already suggested the positive effects of conservation tillage practices on soil enzyme activities. The generally higher enzyme activities in NT mainly resulted from the larger water availability in the plots rather than the better soil fertilities. Urease activity under NTN4 treatment in the three years of our study was the highest of all treatments. In this treatment co-application of compost and farmyard manure in no tillage system assembles good condition for urease activity. The higher bulk density could account for this difference. Enzyme activities were shown to be linearly related to soil bulk density (Li et al., 2002).
Figure 3.
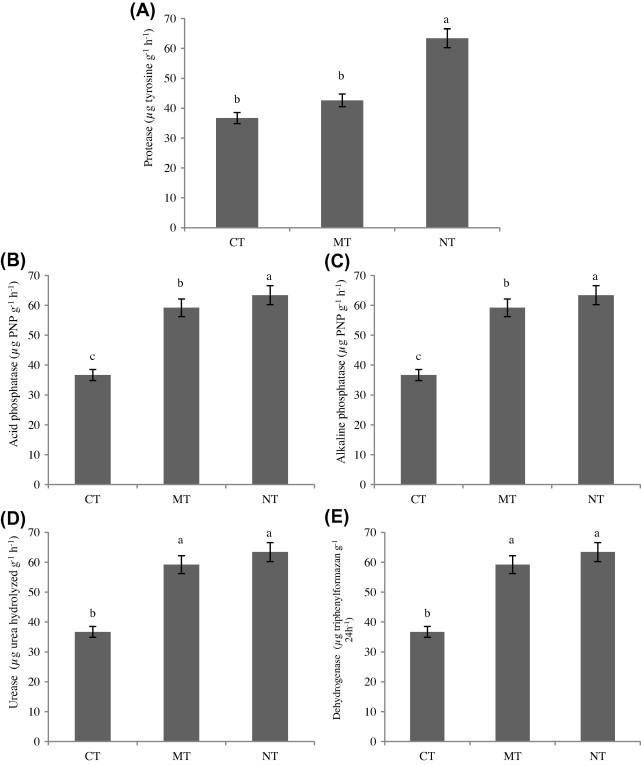
Effect of tillage practices on protease (A), acid phosphatase (B), alkaline phosphatase (C), urease (D), and dehydrogenase (E) activity in soil. (CT: conventional tillage, MT: minimum tillage; NT: no tillage).
4. Conclusion
The present study provides information on soil microbial biomass dynamics and biocatalytic activities as influenced by organic and inorganic fertilization in canola production conditions. The results demonstrate that microbial biomass and soil enzyme activity are sensitive in discriminating between organic fertilizers and inorganic fertilizer application on a short-term basis. Soil microbial biomass and enzymatic properties were also closely related with the C inputs. Consistent distinctions in enzyme activities were observed between different tillage practices. These differences were most pronounced between no tillage at the one hand and conventional and reduced tillage at the other hand.
Acknowledgments
We would like to thank Dr Farhad Saber Ali (Tarbiat Modares University, Iran) for valuable comments on the manuscript and Professor Ali Mohammad Modarres Sanavi (Tarbiat Modares University, Iran) for help with the analysis of data. The Sanandaj Agriculture Research Center for Farm support and Islamic Azad University, Sanandaj Branch is acknowledged for financial support.
References
- Anderson J.M., Ingram J.S. CAB International; Wallingford, UK: 1993. Tropical Soil Biology and Fertility. A Handbook of Methods. [Google Scholar]
- Biederbeck V.O., Zentner R.P., Campbell C.A. Soil microbial populations and activities as influenced by legume green fallow in a semiarid climate. Soil Biol. Biochem. 2005;37:1775–1784. [Google Scholar]
- Brookes P.C., Landman A., Pruden G., Jenkinson D.S. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method for measuring microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985;17:837–842. [Google Scholar]
- Chander K., Goyal S., Nandal D.P., Kapoor K.K. Soil organic matter, microbial biomass and enzyme activities in a tropical agroforestry system. Biol. Fertility Soils. 1998;27:168–172. [Google Scholar]
- Giusquiani P.L., Gigliotti G., Businelli D. Long-term effects of heavy metals from composted municipal waste on some enzyme activities in a cultivated soil. Biol. Fertility Soils. 1994;17:257–262. [Google Scholar]
- Jin K., Sleutel S., Buchan D., De Neve S., Cai D.X., Gabriels D., Jin J.Y. Changes of soil enzyme activities under different tillage practices in the Chinese Loess Plateau. Soil Till. Res. 2009;104:115–120. [Google Scholar]
- Kandeler E. Protease activity. In: Schinner F., Ohlinger R., Kandeler E., Margesin R., editors. Methods in Soil Biology. Springer; Berlin/Heidelberg/New York: 1996. pp. 165–168. [Google Scholar]
- Kandeler E., Tscherko D., Spiegel H. Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a Chernozem under different tillage management. Biol. Fertility Soils. 1999;28:343–351. [Google Scholar]
- Leita L., De Nobilli M., Mondini C., Muhlbacova G., Marchiol L., Bragato G., Contin M. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biol. Fertility Soils. 1999;4:371–376. [Google Scholar]
- Li C.H., Ma B.L., Zhang T.Q. Soil bulk density effects on soil microbial populations and enzyme activities during the growth of maize (Zea mays L.) planted in large pots under field exposure. Can. J. Microbiol. 2002;82:147–154. [Google Scholar]
- Liu M.Q., Hu F., He Y.Q., Li H.X. Seasonal dynamics of soil microbial and its microbial biomass and its significance to indicate soil quality under different vegetations restored on degraded red soils. Acta Pedo. Sinica. 2003;40:937–944. [Google Scholar]
- Lundquist E.J., Jackson L.E., Scow K.M., Hsu C. Changes in microbial biomass and community composition and soil carbon and nitrogen pools after incorporation of rye into three California agricultural soils. Soil Biol. Biochem. 1999;31:221–236. [Google Scholar]
- Lynch J.M. Blackwell; Oxford: 1983. Soil Biotechnology. p. 191. [Google Scholar]
- Madejon E., Moreno F., Murillo J.M., Pelegrn F. Soil biochemical response to long-term conservation tillage under semi-arid Mediterranean conditions. Soil Till. Res. 2007;94:346–352. [Google Scholar]
- Mandal A., Patra A.K., Singh D., Swarup A., Masto R.E. Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresour. Technol. 2007;98:3585–3592. doi: 10.1016/j.biortech.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Marinari S., Masciandaro G., Ceccanti B., Grego S. Influence of organic and mineral fertilizers on soil biological and physical properties. Bioresour. Technol. 2000;72:9–17. [Google Scholar]
- Martens D.A., Johanson J.B., Frankenberger J.W.T. Production and persistence of soil enzymes with repeated addition of organic residues. Soil Sci. 1992;153:53–61. [Google Scholar]
- Mohammadi K. Lambert Academic Publishing; 2011. Soil, Plant and Microbe Interactions. p. 120. [Google Scholar]
- Mohammadi K., Ghalavand A., Aghaalikhani M., Heidari G.R., Shahmoradi B., Sohrabi Y. Effect of different methods of crop rotation and fertilization on canola traits and soil microbial activity. Aust. J. Crop Sci. 2011;5:1261–1268. [Google Scholar]
- Nannipieri P., Ceccanti B., Cervelli S., Sequi P. Use of 0.1 M pyrophosphate to extract urease from a podzol. Soil Biol. Biochem. 1974;6:359–362. [Google Scholar]
- Naseby D.C., Lynch J.M. Rhizosphere soil enzymes as indicators of perturbation caused by a genetically modified strain of Pseudomonas fluorescens on wheat seed. Soil Biol. Biochem. 1997;29:1353–1362. [Google Scholar]
- Nayak D.R., Babu Y.J., Adhya T.K. Long-term application of compost influences microbial biomass and enzyme activities in a tropical Aeric Endoaquept planted to rice under flooded condition. Soil Biol. Biochem. 2007;39:1897–1906. [Google Scholar]
- Nishio M., Kusano S. Fluctuation patterns of microbial numbers in soil applied with compost. Soil Sci. Plant Nutr. 1980;26:581–593. [Google Scholar]
- Peters J., Combs S., Hosklns B., Jarman J., Kovar J., Watson M., Wolf A., Wolf N. University of Wisconsin-Madison; 2003. Recommended Methods of Manure Analysis. [Google Scholar]
- Powlson D.S. The soil microbial biomass: before, beyond and back. In: Ritz K., editor. Beyond the Biomass. Wiley; Chichester: 1994. pp. 3–20. [Google Scholar]
- Ren T.Z., Stefano G. Soil bio-indicators in sustainable agriculture. Sci. Agric. Sin. 2000;33:68–75. [Google Scholar]
- Rice P.J., McConnell L.L., Heighton L.P., Sadeghi A.M., Isensee A.R., Teasdale J.R., Abdul-Baki A.A., Harman-Fetcho J.A., Hapeman C.J. Runoff loss of pesticides and soil: a comparison between vegetative mulch and plastic mulch in vegetable production systems. J. Environ. Qual. 2001;30:1808–1821. doi: 10.2134/jeq2001.3051808x. [DOI] [PubMed] [Google Scholar]
- SAS Institute, 2003. The SAS system for windows. Release 9.1, SAS Inst., Cary, NC.
- Serra-Wittling C., Houot S., Barriuso E. Soil enzymatic response to addition of municipal solid-waste compost. Biol. Fert. Soils. 1995;20:226–236. [Google Scholar]
- Tabatabai M.A., Bremner J.M. Use of p-nitrophenol phosphate in assay of soil phosphatase activity. Soil Biol. Biochem. 1969;1:301–307. [Google Scholar]
- Tarafdar J.C., Marschner H. Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem. 1994;26:387–395. [Google Scholar]
- Vance E.D., Brookes P.C., Jenkinson D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987;19:703–707. [Google Scholar]
- Wlodarczyk T., Stepniewski W., Brzezinska M. Dehydrogenase activity, redox potential, and emissions of carbon dioxide and nitrous oxide from Cambisols under flooding conditions. Biol. Fertility Soils. 2002;36:200–206. [Google Scholar]


