Abstract
The present study was carried out to determine the free radical scavenging potential of culture filtrate of Streptomyces sp. AM-S1. Antioxidant activity of culture filtrate, lyophilized culture filtrate and ethyl acetate extract of Streptomyces sp. AM-S1 was determined by various in vitro assays such as ferric reducing power assay, phosphomolybdenum reduction, DPPH and ABTS radical scavenging activities. The results revealed that the culture filtrate of Streptomyces sp. AM-S1 effectively scavenged DPPH (IC50 90.2 μl/ml) and ABTS (IC50 13.2 μl/ml) radicals in a concentration dependent manner. In all the assays, ethyl acetate extract registered higher antioxidant activity when compared with the lyophilized culture filtrate (LCF). In addition, ethyl acetate extract (1123.4 μmole Fe(II)/mg extract) exhibited higher ferric reducing activity than the standard BHA (814.4 μmole Fe(II)/mg extract). Further works are needed on the isolation and identification of antioxidant molecules from the ethyl acetate extract of Streptomyces sp. AM-S1 culture filtrate.
Keywords: Streptomyces sp. AM-S1, Antioxidant, DPPH, Ferric reducing, ABTS
1. Introduction
Several authors have reported that free radicals induce oxidative damage to biomolecules such as lipids, proteins and DNA. This damage has been implicated in cell disorders and in the development of many diseases including cardiovascular diseases, atherosclerosis, chronic inflammation and other diseases (Valko et al., 2007; Ferreira et al., 2009; Cavar et al., 2012). Antioxidants are the substances that may protect the cells from the oxidative damage caused by free radicals. Natural products have strong antioxidant activity and they have potential beneficial effects on human health. Many plant species and their active principles have been investigated in search of natural antioxidants with pharmacological properties (Ganie et al., 2010; Ak and Gulcin, 2008). Although, microbial secondary metabolites represent a large source of compounds endowed with ingenious structures and potent biological activities. However, the studies on microorganisms with respect to antioxidant and free radical scavenging activities are very limited.
Actinomycetes are one of the important groups of soil microorganisms. They play a significant role in the pharmaceutical and agricultural industries for their capacity to produce biologically active secondary metabolites such as antibiotics, pesticides, anti-parasitic compounds and enzymes like cellulase, xylanase, proteinase and chitinase. Each actinomycete strain has probably genetic potential for producing 10–20 secondary metabolites. It is well known that actinomycetes produce 70–80% of bioactive secondary metabolites, where approximately 60% of antibiotics are isolated from Streptomyces spp. (Ilic et al., 2007). Streptomyces species are widely recognized as industrially important microorganisms because of their ability to produce different kinds of novel secondary metabolites. It has an enormous biosynthetic potential that remains unchallenged, without a potential competitor among other microbial groups (Solanki et al., 2008).
The studies on streptomycetes with respect to free radical scavenging activity are very few and most of the streptomycetes isolated were yet to be screened for bioactive secondary metabolites. The culture filtrate and compounds such as isoflavonoids, diphenazithionin, dihydroherbimycin A, polysaccharide and protocatechualdehyde were isolated from Streptomyces, and have been reported to possess antioxidant and free radical scavenging activity (Chang and Kim, 2007; Zhong et al., 2011a; Saurav and Kannabiran, 2012). However, there is a demand for screening new metabolites that are less expensive and have fewer side effects. Hence a study was carried out to examine the antioxidant activity of Streptomyces sp. AM-S1. Therefore, the present study proposes to investigate antioxidant potential of culture filtrate and its ethyl acetate extract of Streptomyces sp. AM-S1 under various in vitro assays.
2. Materials and methods
2.1. Streptomyces isolate used in this study
Streptomyces sp. AM-S1 strain has been isolated from forest humus soil in Gyeongsan, South Korea by using starch casein agar medium. Based on our previous study, this isolate exhibited higher antagonistic activity against various plant and human pathogens. The 16S rDNA gene sequence of AM-S1 had 99% sequence identity with the 16S rDNA gene sequences from several Streptomyces sp. (Sowndhararajan and Kang, 2012). The sequence of Streptomyces sp. AM-S1 has been submitted to GenBank with the accession number JX444563 (Fig. 1). The present study was carried out to evaluate the free radical potential of Streptomyces sp. AM-S1.
Figure 1.

16S rRNA sequences of Streptomyces sp. AM-S1.
2.2. Biomass of actinomycete strain in different liquid media
Growth was monitored by determining the dry cell weight. The liquid culture of Streptomyces sp. AM-S1 strain from the media such as tryptone-yeast extract broth (ISP-1), starch inorganic salt broth (ISP-4), glycerol asparagine broth (ISP-5), glycerol tyrosine broth (ISP-7), starch caesin broth (SCB), maltose yeast extract broth (MYEB), nutrient broth (NB) and potato dextrose broth (PDB), production medium – III (PM-III), and production medium – IV (PM-IV) were taken aseptically on 7th day. The cells were separated from the culture filtrate by centrifugation at 10000 rpm for 10 min and dried at 80 °C for 24 h. Dry cell weight was calculated by the difference in the weights and results were expressed as g/100 ml culture (Thakur et al., 2009).
2.3. Culture filtrate and extract preparation
A loopful of culture of Streptomyces sp. AM-S1 was inoculated into separate 500 mL Erlenmeyer flasks containing 250 ml of maltose yeast extract broth (MYEB) medium (maltose 10 g, yeast extract 2 g, beef extract 1 g and deionized water 1 l) and incubated on a rotary shaker at 150 rpm for 7 days at 30 °C. After incubation, cells were removed by centrifugation (10000 rpm for 10 min at 4 °C) and subsequently passed through a cellulose acetate filter (0.45 μm) to get culture filtrate. One part of the culture filtrate was extracted with ethyl acetate and concentrated by using a rotary evaporator (Eyela N-1000, Tokyo, Japan) to get the crude extract. Another part of culture filtrate was freeze dried under vacuum at −50 °C and to get lyophilized powder (LCF). The culture filtrate was also used directly for the determination of antioxidant activity compared with sterile MYEB medium. The ethyl acetate extract and lyophilized culture filtrate were stored at −20 °C for further use.
2.4. Ferric reducing antioxidant power (FRAP) assay
Antioxidant capacity of culture filtrate, LCF and ethyl acetate extract of Streptomyces sp. AM-S1 was estimated according to the procedure described by Pulido et al. (2000). FRAP reagent (900 μl), prepared freshly and incubated at 37 °C, was mixed with 90 μl of distilled water and 30 μl of test sample or methanol (for the reagent blank). The test samples and reagent blank were incubated at 37 °C for 30 min in a water bath. The final dilution of the test sample in the reaction mixture was 1/34. The FRAP reagent contained 2.5 ml of 20 mmol/l TPTZ solution in 40 mmol/l HCl plus 2.5 ml of 20 mmol/l FeCl3·6H2O and 25 ml of 0.3 mol/l acetate buffer (pH 3.6). At the end of incubation the absorbance readings were taken immediately at 593 nm, using a spectrophotometer. Methanolic solutions of known Fe(II) concentration, ranging from 100 to 2000 μmol/l (FeSO4·7H2O) were used for the preparation of the calibration curve. The values are expressed as μmol Fe(II)/ml culture filtrate or mg extract.
2.5. Phosphomolybdenum reduction assay
The antioxidant activity of samples was evaluated by the green phosphomolybdenum complex according to the method of Prieto et al. (1999). An aliquot of 100 μl of sample solution was combined with 1 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) in a 4 ml vial. The vials were capped and incubated in a water bath at 95 °C for 90 min. After the samples had cooled to room temperature, the absorbance of the mixture was measured at 695 nm against a blank. The results reported are mean values expressed as mg of ascorbic acid equivalents (AAE)/ml culture filtrate or g extract.
2.6. Free radical scavenging activity on DPPH
The DPPH radical scavenging activity of culture filtrate, LCF and ethyl acetate extract of Streptomyces sp. AM-S1 was measured according to the slightly modified method of Liyana-Pathirana and Shahidi (2005). Sample extracts at various concentrations were taken and the volume was adjusted to 100 μl with methanol. 900 μl of 0.1 mM methanolic solution of DPPH was added and shaken vigorously. The tubes were allowed to stand for 20 min at 27 °C. The absorbance of the sample was measured at 517 nm. Radical scavenging activity was expressed as the inhibition percentage of free radical by the sample and was calculated using the formula: % DPPH radical scavenging activity = (control OD − sample OD/control OD) × 100.
2.7. Antioxidant activity by the ABTS•+ assay
The total antioxidant activity of the samples was measured by ABTS radical cation decolorization assay according to the method of Re et al. (1999). ABTS•+ was produced by reacting 7 mM ABTS aqueous solution with 2.4 mM potassium persulfate in the dark for 12–16 h at room temperature. Prior to assay, this solution was diluted in ethanol (about 1:89 v/v) and equilibrated at 30 °C to give an absorbance at 734 nm of 0.700 ± 0.02. After the addition of 1 ml of diluted ABTS solution to 10 μl of test sample in ethanol, absorbance was measured at 30 °C exactly 30 min after the initial mixing. The inhibition percentage was calculated for the blank absorbance at 734 nm. Radical scavenging activity was expressed as the inhibition percentage of free radical by the sample and was calculated using the formula: % ABTS radical scavenging activity = (control OD − sample OD/control OD) × 100.
2.8. Statistical analysis
The data were subjected to a one-way analysis of variance (ANOVA) and the significance of the difference between means was determined by Duncan’s multiple range test (P < 0.05) using statistica (Statsoft Inc., Tulsa, OH, USA). Values expressed are means of three replicate determinations ± standard deviations (SD).
3. Results
3.1. Effect of different media on biomass of Streptomyces sp. AM-S1
Growth of Streptomyces sp. in different liquid media was determined by the dry weight of cells. The maximum yield of cell growth of Streptomyces sp. was 2.7 g/100 ml in maltose yeast extract broth medium followed by production medium-IV (1.9 g/100 ml). The poor growth of Streptomyces sp. was observed in the ISP-1, 4, 5 and 7 media (Fig. 2).
Figure 2.
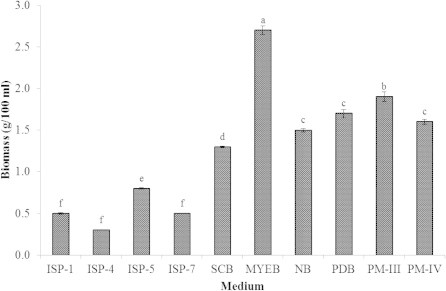
Effect of different media on biomass of Streptomyces sp. on 5th day. ISP-1: Tryptone-yeast extract broth; ISP-4: Starch inorganic salt broth, ISP-5: Glycerol Asparagine broth, ISP-7: Glycerol Tyrosine broth, SCB: Starch Caesin broth, MYEB: Maltose Yeast Extract broth, NB: Nutrient broth, PDB: Potato Dextrose broth, PM-III: Production Medium – III, PM-IV: Production Medium – IV. Values are means of three replicate determinations (n = 3) ± standard deviation. Bars having different letters are significantly different (P < 0.05).
3.2. Ferric reducing antioxidant power assay
The ferric reducing capacity of Streptomyces sp. AM-S1 culture filtrate, LCF and ethyl acetate extract was estimated from their ability to reduce TPTZ-Fe(III) complex to TPTZ-Fe(II) complex. The results are expressed as concentration of substance having ferric-TPTZ reducing ability equivalent to that of 1 μmol concentration of Fe(II) is presented in Table 1. The ferric reducing activity of culture filtrate was 523.5 μmol Fe(II)/ml. Ethyl acetate extract of culture filtrate (1123.4 μmol Fe(II)/mg extract) exhibited higher ferric reducing activity when compared with LCF (452.4 μmol Fe(II)/mg extract) and the standard butylated hydroxyanisole (814.4 μmol Fe(II)/mg extract).
Table 1.
FRAP, Phosphomolybdenum reduction activities of Streptomyces sp. AM-S1 culture filtrate, lyophilized culture filtrate and ethyl acetate extract.
| Sample | FRAP (μmole Fe(II)/ml) | Phosphomolybdenum (mg AAE/ml) |
|---|---|---|
| Maltose yeast extract medium | 48.7 ± 2.4 | 11.4 ± 0.5 |
| Culture filtrate | 523.5 ± 18.5 | 95.9 ± 3.4 |
| (μmole Fe(II)/mg extract) | (mg AAE/g extract) | |
| Lyophilized culture filtrate | 452.4 ± 14.5 | 83.7 ± 4.5 |
| Ethyl acetate extract | 1123.4 ± 54.8 | 145.6 ± 11.8 |
| Butylated hydroxyanisole (BHA) | 814.4 ± 24.9 | 478.4 ± 24.9 |
Values are mean of three replicate determinations (n = 3) ± standard deviation.
FRAP – Ferric reducing antioxidant power; AAE – Ascorbic acid equivalent.
3.3. Phosphomolybdenum reduction assay
The reduction of molybdenum complex from Mo(V) to Mo(IV) by the culture filtrate, LCF and ethyl acetate extract is depicted in Table 1. Increase of the absorbance indicated the increase of the total antioxidant capacity and the values are expressed as ascorbic acid equivalent. The culture filtrate of Streptomyces sp. AM-S1 significantly reduced phosphomolybdenum complex (95.9 mg AAE/ml). Moreover, the phosphomolybdenum reduction activity of ethyl acetate extract (145.6 mg AAE/mg extract) was higher than the LCF (83.7 mg AAE/mg extract).
3.4. DPPH• radical scavenging assay
The results of DPPH radical scavenging activity of the culture filtrate and LCF and ethyl acetate extract are presented in Figs. 3 and 4. In this study, all the tested samples significantly scavenged the DPPH with increasing concentrations. The scavenging activity of DPPH radical by the culture filtrate was ranged between 9.3% and 57.4% with the IC50 of 90.2 μl/ml (Fig. 3). Whereas, the IC50 of LCF and ethyl acetate extract was 119.3 and 68.4 μg/ml, respectively.
Figure 3.
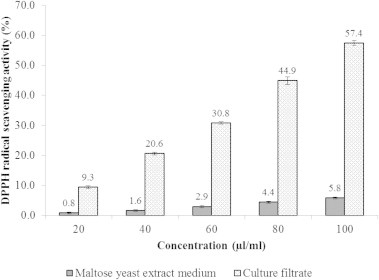
DPPH radical scavenging activity of maltose yeast extract medium and culture filtrate of Streptomyces sp. AM-S1. Values are means of three replicate determinations (n = 3) ± standard deviation.
Figure 4.
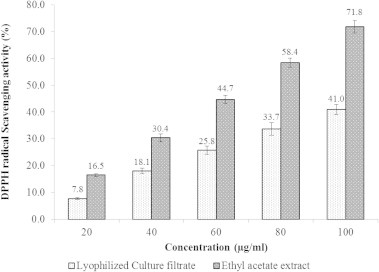
DPPH radical scavenging activity of lyophilized culture filtrate and ethyl acetate extract of Streptomyces sp. AM-S1. Values are means of three replicate determinations (n = 3) ± standard deviation.
3.5. ABTS•+ radical scavenging activity
ABTS radical scavenging activity of culture filtrate and MYEB medium is shown in Fig. 5. The radical scavenging activity of culture filtrate is in a concentration dependent manner and the radical scavenging activity was 92.7% at 25 μl/ml. Meanwhile, MYEB medium showed 20.5% of radical scavenging activity at 25 μl/ml. At the concentration of 25 μg/ml, the radical scavenging activity of LCF and ethyl acetate extract was 78.4% and 94.2% with the IC50 of 16.1 and 12.0 μg/ml, respectively (Fig. 6).
Figure 5.
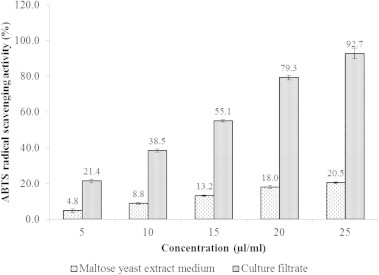
ABTS radical scavenging activity of maltose yeast extract medium and culture filtrate of Streptomyces sp. AM-S1. Values are means of three replicate determinations (n = 3) ± standard deviation.
Figure 6.
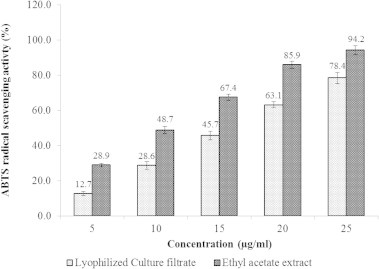
ABTS radical scavenging activity of lyophilized culture filtrate and ethyl acetate extract of Streptomyces sp. AM-S1. Values are means of three replicate determinations (n = 3) ± standard deviation.
4. Discussion
Actinomycetes are Gram-positive filamentous bacteria. The majority of this group is saprophytic and found widely distributed in the soil. They are growing extensively in soils rich in organic matters. Actinomycetes form the source of three-fourth of all the known products, of which, Streptomyces spp. are very important candidates (Saisivam and Kishan, 2006). Different kinds of pathways are associated with secondary metabolites produced by streptomycetes, including antimicrobial, antitumor and enzyme inhibitors (Wu et al., 2007).
In the present study, the strain Streptomyces sp. AM-S1 has been isolated from forest humus soils and registered higher antagonistic activity against Rhizoctonia solani with the inhibition zone of 41 mm. Hence, the isolate Streptomyces sp. AM-S1 was used in this study to determine the free radical potential. The results of different media on biomass of Streptomyces sp. AM-S1 revealed that the growth of Streptomyces sp. in different media is useful for the optimization of growth and production of biologically active metabolites. Nutritional requirements of Streptomyces play an important role during metabolite synthesis process. Amongst various nutritional requirements, carbon and nitrogen resources are the most important impact factors. Based on the results, the MYEB medium has been selected because of the maximum growth of Streptomyces sp. AM-S1 in this medium.
Many reports evidenced the use of antioxidant substances in reducing the level of oxidative stress and in slowing or preventing the development of free radical mediated diseases. Many synthetic antioxidants have shown toxic and/or mutagenic effects. Therefore consideration has been given to naturally occurring antioxidants (Zhuang et al., 2012). In the present study, various methods of in vitro assays were performed to determine the antioxidant activity of culture filtrate of Streptomyces sp. AM-S1.
The FRAP assay is commonly used in routine analysis for evaluation of antioxidant capacity. The reducing capacity of a compound might serve as a significant indicator of its potential antioxidant capacity. FRAP assay measures the reducing capability of tested sample by increasing sample absorbance based on the ferrous ions released (Prior et al., 2005). Similarly, in another total antioxidant assay is based on the reduction of Mo(V)–Mo(IV) by the antioxidant substance in the sample and subsequent formation of a green phosphate/Mo(V) complex at acidic pH with an absorbance maximum at 695 nm (Sowndhararajan et al., 2010). Increase of the absorbance indicated the increase of the total antioxidant capacity. The culture filtrate and its extracts showed significant ferric and phosphomolybdenum reducing potential. However, ethyl acetate extract of Streptomyces sp. AM-S1 culture filtrate exhibited maximum reducing capacity in both of the assays.
The DPPH free radical scavenging assay has been widely used to evaluate antioxidant capacities. Antioxidants react with DPPH, reducing a number of DPPH molecules equal to the number of available hydroxyl groups (Matthaus, 2002). The degree of discoloration indicates that the samples to scavenge DPPH radical due to its ability to donate hydrogen proton. Similar to DPPH, the decolorization of ABTS•+ radical reflects the capacity of an antioxidant species to donate electrons or hydrogen atoms to inactivate this radical species. The ABTS•+ radical cation is generated from the reaction of ABTS with potassium persulfate overnight in water (Re et al., 1999). The study demonstrated that the culture filtrate, LCF and ethyl acetate extract of Streptomyces sp. AM-S1 have hydrogen donating ability and could serve as primary antioxidants. The MYEB medium also registered some level of antioxidant and free radical scavenging activity that might be due to the presence of chemical components in the medium.
Saurav and Kannabiran (2012) demonstrated that the DPPH radical scavenging and phosphomolybdenum reduction activities of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one (DMBPO) extracted from marine Streptomyces VITSVK5 exhibited strong activity (44.13% and 50.10% at 5 μg/ml, respectively). In addition, the compounds isolated from Streptomyces, 2-allyoxyphenol and streptopyyrolidine have been reported to possess antioxidant activity (Arumugam et al., 2010; Shin et al., 2008).
Prashith Kekuda et al. (2010) studied that the butanol extract of culture filtrate of two Streptomyces sp. effectively scavenged the DPPH radical. The radical scavenging activity of Streptomyces sp. isolates 1 and 2 was found to be 58.71% and 59.97% at 0.5 mg/ml, respectively. In the present study, ethyl acetate extract of Streptomyces sp. AM-S1 culture filtrate registered very strong DPPH (IC50 of 68.4 μg/ml) and ABTS (IC50 of 12 μg/ml) radical scavenging activity than the strain Streptomyces Eri12 with the IC50 of 842.18 and 172.43 μg/ml, respectively (Zhong et al., 2011b). Moreover, Huang and Chang (2012) reported that the DPPH and ABTS radical scavenging activities of Bifidobacterium adolescentis culture filtrate were 88% and 81.5%, respectively at the final concentration of 7.5% (v/v). The culture filtrate of present isolate also showed higher scavenging activity in both of the DPPH (57.4% at 100 μl) and ABTS (92.7% at 25 μl) assays. Its higher scavenging activities indicated that the mechanism of antioxidant action was as a hydrogen donor and it could terminate the oxidation process by converting free radicals into the stable forms.
The results indicated that the ethyl acetate extract of Streptomyces sp. AM-S1 culture filtrate provides significant free radical potential under various in vitro assays. Hence, the present data suggest that the ethyl acetate extract of culture filtrate could be a potential source of natural antioxidant for the treatment of radical related diseases. Further studies are needed to isolate and identify the antioxidant molecules from the ethyl acetate extract of Streptomyces sp. AM-S1 culture filtrate.
Acknowledgements
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ008157), Rural Development Administration, Republic of Korea.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ak T., Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Arumugam M., Mitra A., Jaisankar P., Dasgupta S. Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl. Microbiol. Biotechnol. 2010;86:109–117. doi: 10.1007/s00253-009-2311-2. [DOI] [PubMed] [Google Scholar]
- Cavar S., Kovac F., Maksimovic M. Evaluation of the antioxidant activity of a series of 4-methylcoumarins using different testing methods. Food Chem. 2012;133:930–937. [Google Scholar]
- Chang H.B., Kim J.H. Antioxidant properties of dihydroherbimycin A from a newly isolated Streptomyces sp. Biotechnol. Lett. 2007;29:599–603. doi: 10.1007/s10529-006-9288-z. [DOI] [PubMed] [Google Scholar]
- Ferreira I.C.F.R., Barros L., Abreu R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009;16:1543–1560. doi: 10.2174/092986709787909587. [DOI] [PubMed] [Google Scholar]
- Ganie S.A., Haq E., Masood A., Zargar M.A. Antioxidant activities of methanolic rhizome extract of Podophyllum hexandrum against CCl4 induced kidney and lung injury in rats. J. Pharmacol. Toxicol. 2010;5:334–342. [Google Scholar]
- Huang H.C., Chang T.M. Antioxidative properties and inhibitory effect of Bifidobacterium adolescentis on melanogenesis. World J. Microbiol. Biotechnol. 2012;28:2903–2912. doi: 10.1007/s11274-012-1096-0. [DOI] [PubMed] [Google Scholar]
- Ilic S.B., Konstantinovic S.S., Todorovic Z.B., Lazic M.L. Characterization and antimicrobial activity of the bioactive metabolites in streptomycete isolates. Microbiology. 2007;76:421–428. [PubMed] [Google Scholar]
- Liyana-Pathirana C.M., Shahidi F. Antioxidant activity of soft commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005;53:2433–2440. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- Matthaus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002;50:3444–3452. doi: 10.1021/jf011440s. [DOI] [PubMed] [Google Scholar]
- Prashith Kekuda T.R., Shobha K.S., Onkarappa R. Studies on antioxidant and anthelmintic activity of two Streptomyces species isolated from Western Ghat soils of Agumbe Karnataka. J. Pharm. Res. 2010;3:26–29. [Google Scholar]
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitative of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Wu X.L., Schaich K. Standardized method for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Pulido R., Bravo L., Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Saisivam S., Kishan V. Taxonomy, fermentation and biological activities of a new strain of Streptomyces luridus from Indian soil. Ind. J. Microbiol. 2006;46:153–160. [Google Scholar]
- Saurav K., Kannabiran K. Cytotoxicity and antioxidant activity of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi J. Biol. Sci. 2012;19:81–86. doi: 10.1016/j.sjbs.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.J., Kim T.S., Lee H.S., Park J.Y. Streptopyrrolidine, an angiogenesis inhibitor from a marine-derived Streptomyces spp. KORDI-3973. Phytochemistry. 2008;69:2363–2366. doi: 10.1016/j.phytochem.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Solanki R., Khanna M., Lal R. Bioactive compounds from marine actinomycetes. Ind. J. Microbiol. 2008;48:410–431. doi: 10.1007/s12088-008-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowndhararajan K., Kang S.C. In vitro antagonistic potential of Streptomyces sp. AM-S1 against plant and human pathogens. J. Agric. Chem. Environ. 2012;1:41–47. [Google Scholar]
- Sowndhararajan K., Siddhuraju P., Manian S. In vitro evaluation of the antioxidant activities in the differentially processed seeds from underutilized legume, Bauhinia vahlii Wight & Arn. Food Sci. Biotechnol. 2010;19:503–509. [Google Scholar]
- Thakur D., Bora T.C., Bordoloi G.N., Mazumdar S. Influence of nutrition and culturing conditions for optimum growth and antimicrobial metabolite production by Streptomyces sp. 201. J. Mycol. Med. 2009;19:161–167. [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wu X.C., Chen W.F., Qian C.D., Li O. Isolation and identification of newly isolated antagonistic Streptomyces sp. strain AP19-2 producing chromomycins. J. Microbiol. 2007;45:499–504. [PubMed] [Google Scholar]
- Zhong K., Gao X.L., Xu Z.J., Gao H. Antioxidant activity of a novel Streptomyces strain Eri12 isolated from the Rhizoma Curcumae Longae. Curr. Res. Bacteriol. 2011;4:63–72. [Google Scholar]
- Zhong K., Gao X.L., Xu Z.J., Li L.H. Isolation and characterization of a novel Streptomyces strain Eri11 exhibiting antioxidant activity from the rhizosphere of Rhizoma Curcumae Longae. Afr. J. Microbiol. Res. 2011;5:1291–1297. [Google Scholar]
- Zhuang Y., Chen L., Sun L., Cao J. Bioactive characteristics and antioxidant activities of nine Peppers. J. Funct. Foods. 2012;4:331–338. [Google Scholar]



