Abstract
Competitors are known to be important in governing the outcome of evolutionary diversification during an adaptive radiation, but the precise mechanisms by which they exert their effects remain elusive. Using the model adaptive radiation of Pseudomonas fluorescens, we show experimentally that the effect of competition on diversification of a focal lineage depends on both the strength of competition and the ability of the competitors to diversify. We provide evidence that the extent of diversification in the absence of interspecific competitors depends on the strength of resource competition. We also show that the presence of competitors can actually increase diversity by increasing interspecific resource competition. Competitors that themselves are able to diversify prevent diversification of the focal lineage by removing otherwise available ecological opportunities. These results suggest that the progress of an adaptive radiation depends ultimately on the strength of resource competition, an effect that can be exaggerated or impeded by the presence of competitors.
Keywords: diversification, competitors, resource competition, niche pre-emption, adaptive radiation
1. Introduction
The diversity of life is thought to have arisen, in part, through repeated adaptive radiations, the rapid diversification of a single lineage into an array of ecologically and phenotypically distinct species [1,2]. Competition is commonly cited as an important driver of diversification, especially when individuals share very similar resource requirements or niches and a wide range of under-used resources (ecological opportunities) are available in the environment [3–5]. However, competition can also prevent diversification if competitors use substantially different resources or sets of resources, thereby eliminating ecological opportunity. The effects of competitors on diversification during an adaptive radiation should thus depend on the extent to which their niches overlap: closely overlapping niches generate intense competition promoting diversification, whereas competitors with very different niches can impede diversification.
The idea that competitors can have manifold, and even opposing, effects on diversification has received little attention. The traditional dichotomy is that competition between close relatives (often intraspecific competition) drives divergence via character displacement, whereas competition from other taxa (interspecific competition) inhibits divergence via niche pre-emption [2,4,6]. However, this distinction is somewhat artificial as it fails to capture the idea that the essential differences are in the degree of niche overlap. In addition, the bulk of existing theory focuses on the conditions required to maintain diversity but not its origin. We thus know much about, for example, limiting similarity—the minimum niche differences required for two types to coexist [7,8]—but we know much less about the details of the mechanisms that drove the evolution of those niche differences in the first place. Consequently, understanding the multiple ways in which competition influences adaptation and diversification remains understudied.
Experimental work has shown that intraspecific competition can promote phenotypic divergence [9–11] and expanded resource use [12–14] by a population. Likewise, resource competition is often cited as an important driver of lineage diversification in microbial experiments [15] although this is more often because other potential mechanisms promoting diversification, such as predation or parasites, are excluded by design. However, direct manipulative tests linking resource competition to lineage diversification are lacking. There is stronger experimental evidence that competitors prevent diversification through niche pre-emption. Two microbial experiments, both involving the adaptive radiation of Pseudomonas fluorescens in static microcosms, have shown that niche pre-emption can prevent diversification of the ancestral strain [16,17]. Abundant comparative evidence has implicated interspecific competition in preventing diversification [18–22], and niche expansion [23,24] during a radiation as well.
These results thus suggest that there is a continuum of effects that competitors can have on the diversification of a focal strain. At one end, individuals of the same or very similar genotype will tend to be ecologically similar [7,25] (although very similar genotypes can also display markedly different niche preferences [26]) and so will probably compete directly and intensely for resources [27,28], generating disruptive selection leading to diversification when ecological opportunities are available. The strength of competition under these conditions is determined by the degree of niche overlap, realized as the per capita effect of a competitor on an individual (i.e. competition coefficient [29]), and the number of individuals exerting that effect (i.e. population density). Thus, for a given competition coefficient, species or genotypes with lower population densities experience weaker resource competition, and so are expected to diversify to a lesser extent compared with those with high-density populations. At the other end are genetically and ecologically divergent genotypes, or even different species, whose resource use profiles are so distinct that they effectively do not compete with the focal strain for resources. Rather, they occupy what would (in their absence) be available niche space and so prevent diversification of the focal lineage. In between these two extremes, it is at least conceivable that a competitor can impact diversification through both mechanisms. Indeed, results from a recent experiment show that evolutionary diversification of P. fluorescens occurs more rapidly in the presence of an interspecific competitor—Pseudomonas putida, and the authors suggest that this probably occurs because of increased competition for resources [30].
Here, we examine the effects of competitors on diversification using the model adaptive radiation of P. fluorescens SBW25 (hereafter, SBW25) cultured in spatially structured static microcosms. Previous work has shown that frequency-dependent selection is operating in this system [15] and this observation is highly suggestive that intraspecific competition for resources drives the resulting diversification. Our study is aimed at explicitly testing this mechanism and exploring in detail the potentially opposing effects of competition on adaptive diversification. Our strategy is to follow the rate and extent of diversification of SBW25 when it is co-cultured alongside competitors that vary in the strength of competition and in their ability to diversify. This approach allows us to disentangle experimentally the diversification-promoting effects of resource competition from the diversification-preventing effects of niche pre-emption, the two most common modes by which intra- and interspecific competitors, respectively, are thought to exert their effects on a radiating lineage.
We first ask how variation in the strength of intraspecific resource competition impacts diversification. In the absence of interspecific competitors, diversification is expected to occur only when resource competition is sufficiently strong to generate disruptive selection in the presence of abundant ecological opportunity. We test this prediction, manipulating the strength of resource competition experienced by a radiating lineage, by manipulating population density. While the effect of changes in population density driven by changes in the environment on the evolution of diversity in this system has been previously documented (e.g. nutrient concentrations [31], predation [32]), here we take a complementary approach holding environment constant and manipulating population density via genetic differences.
We then ask how competing genotypes (analogous to interspecific competitors), which differ both in their competitive fitness and in their ability to diversify, impact diversification in SBW25. While strong interspecific competitors are expected to out-compete a radiating lineage before the latter has diversified, weaker competitors may either have no effect or promote diversification by increasing the strength of resource competition experienced by the radiating lineage. However, if those competitors also exclude SBW25 from potential niches (i.e. niche pre-emption), their presence is expected to suppress the evolution of diversity in SBW25. Our use of diversifying and non-diversifying competitors allows us to test this idea directly.
2. Material and methods
(a). The Pseudomonas fluorescens radiation
On its own, SBW25 diversifies rapidly and repeatably into characteristic niche specialist morphotypes—smooth (SM), wrinkly spreader (WS) and fuzzy spreader (FS) [15]—resulting from competition for nutrients and oxygen [33,34]. These morphotypes are genetically distinct [15,35] and are easily distinguishable on agar plates and use distinct ecological strategies for acquiring oxygen in static microcosms: motile SM morphotypes are aerotactic and swim towards the air–broth interface; WS morphotypes construct a cellulose-based biofilm at the air–broth interface [36] and FS morphotypes form thin rafts at the air–broth interface that quickly collapse under their own weight and collect in the anoxic zone at the bottom of the microcosm [37]. Diversity among the major morphs is stably maintained by negative frequency-dependent selection [15]; and within each morphotype class, there are often additional phenotypically distinct colony morphs. Note that P. fluorescens is strictly asexual under our experimental conditions, meaning that all genotypes—whether arising de novo through mutation or introduced by design as part of the experiment—are evolutionarily independent lineages that are formally equivalent to species in a sexual system [15].
(b). Fitness measures
We measured the relative fitness of any given strain compared with SBW25 with head-to-head competition experiments. Strains were inoculated as pure cultures from frozen for 24 h in 6 ml shaken King's B (KB) medium (28°C, 150 r.p.m.). Then, 6 ml KB static microcosms were inoculated with 30 μl each of the focal strain and SBW25 and their frequencies estimated at 0 and 24 h by plating on KB agar. We calculated the relative fitness of each strain using the following equation:
| 2.1 |
where Finitial and Ffinal are the initial and final frequencies of the strain of interest, finitial and ffinal are the initial and final frequencies of SBW25 and d is the number of generations or doublings.
(c). Diversification dynamics
We tracked diversification over 7 days after inoculating replicate, static microcosms with a total 60 μl of culture containing 6 ml of KB at 28°C (as in [15]). We destructively sampled three replicate microcosms every 12 h for 5 days, then once a day for another 2 days. Evolved diversity was estimated by plating on KB agar and noting the morphology of between 50 and 200 colonies. Diversity was measured as morphotype richness, the number of morphologically distinct types. When diversity is estimated using Simpson's index, which takes into account relative abundances in addition to richness, similar patterns over time and across treatments were observed; we report only richness estimates here. To prevent potential biases arising from differences in the number of colonies counted per sample, we exclude any morphotypes making up less than 2 per cent (or one in 50 colonies) of the population from our diversity estimates.
The diversification dynamics of the strain of interest in each experiment were summarized by fitting the following modified logistic equation to diversity over time:
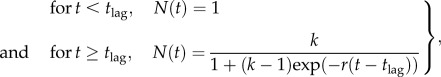 |
2.2 |
where N(t) is diversity at time t, tlag is the time until diversification begins to occur, r is the rate of diversification, k is the extent of evolved diversity. Using these parameter estimates, we took the value of t when N(t) = 0.99k as an estimate of time until maximum diversity is reached, and k as an estimate of the extent of diversification. All model fitting and statistical analyses were performed using R v. 2.13.1 [38].
(d). Intraspecific competition
To examine the effect of varying the strength of intraspecific resource competition on diversification, we allowed genetically distinct strains of SBW25 carrying costly antibiotic resistance mutations (see the electronic supplementary material, table S1) to diversify in static KB microcosms. The rationale behind this manipulation of intraspecific resource competition comes from the simple logistic model describing density-dependent population growth [29],
| 2.3 |
where N is the population density, t is time, r is the intrinsic growth rate and K is the carrying capacity. The genetically distinct strains used in this experiment do not differ in their carrying capacity (K in equation (2.3)) when grown in static KB microcosms (see final densities in the electronic supplementary material, figure S1) and so do not differ in their per capita interaction strength (1/K). However, the strains do differ in their intrinsic growth rate (r) and so differ in their population densities until carrying capacity is reached after 2–6 days (see the electronic supplementary material, figure S1). Those differences in density generate differences in the overall strength of competition (or the degree to which growth rate is slowed owing to density dependence) experienced by individuals of each strain during the first 2–6 days—the same time period over which adaptive diversification tends to occur in this system [15]. Thus, otherwise isogenic strains that have lower intrinsic growth rates and so lower initial population densities experience weaker resource competition. Strains resistant to the quinolone antibiotic nalidixic acid were originally generated using a conventional fluctuation assay [39] to minimize the number of genetic differences from SBW25. Resistance results from mutations in genes not known to affect SBW25 diversification (gyrases: gyrA, gyrB; topoisomerases: parC, parE; efflux pump regulation: nfxB, mexR). Population density after 24 h and fitness measured in head-to-head competitions (as described earlier) are well correlated in our strains (see the electronic supplementary material, figure S2); we report competitive fitness measures here to facilitate comparison with our other experiments.
An examination of our fitness estimates for these eight strains showed that four strains fell into a ‘low-fitness’ group (mean fitness ± s.e.: 0.694 ± 0.012), and the other four strains fell into a ‘high-fitness’ group (mean fitness ± s.e.: 0.971 ± 0.010). Within these groups, we were unable to detect significant differences in fitness, despite each strain having a unique genotype. For this reason, we test for an effect of fitness on the extent of evolved diversity and time until maximum diversity is reached in a categorical manner, grouping the strains appropriately and using two-sample t-tests.
(e). Interspecific competition
To examine the combined effects of resource competition and niche pre-emption on diversification, we tracked the dynamics of SBW25 diversification in the presence of competitor strains that varied in both their propensity to diversify and their fitness relative to SBW25. We use relative fitness of the competitor strain as a measure of relative strength of interspecific competition against SBW25 as competitor strains with a high relative fitness will grow more quickly, resulting in a higher density and so greater total competitive strength compared with competitor strains with low relative fitness. Six strains were evolved derivatives of SBW25 capable of diversifying into the same range of niche specialists as SBW25 (DIV+: strains A–F), while four were evolved from PBR716 [40], an SBW25 deletion strain that lacks the key operons involved in adaptation to the air–broth interface making it severely compromised in its ability to diversify in static microcosms (DIV−: strains 1–4). DIV− strains thus exert their effects on the focal strain through resource competition while DIV+ strains compete via resource competition and niche pre-emption. All competitor strains are morphologically SM. Fitness relative to SBW25 ranged from 0.71 (weak competitors) to 1.22 (strong competitors). The four low-fitness DIV+ competitors were isolates from P. fluorescens SBW25 : lacZ+ populations evolved for approximately 1000 generations in 2 ml shaken M9 salts media plus either glucose, mannose, xlyose or all three sugars (from [41]). The two high-fitness DIV+ competitors were isolates from P. fluorescens SBW25 : lacZ+populations evolved in 6 ml static KB for 8 days (from [42]). The four DIV− competitors were isolates evolved from P. fluorescens PBR716 in static microcosms containing 6 ml KB, transferred to fresh microcosms every day for 2–6 days. See the electronic supplementary material, table S1 for further details about the strains used. All competitor strains contain a neutral genetic marker enabling us to distinguish them from SBW25 [43].
The experiment was initiated with 30 μl of the focal SBW25 strain and 30 μl of a competitor strain. For logistical reasons, this experiment was run in four blocks (block 1: SBW25 + strain A, SBW25 + strain B; block 2: SBW25 + strain C, SBW25 + strain D; block 3: SBW25 + strain E, SBW25 + strain F; block 4: SBW25 : lacZ + strain 1, SBW25 : lacZ + strain 2, SBW25 : lacZ + strain 3, SBW25 : lacZ + strain 4). Each block included a replicated control treatment—SBW25 evolving in the absence of any competitor strain. As in the intraspecific experiment, we destructively sampled three replicate microcosms every 12 h for 5 days, then once a day for another 2 days. To quantify diversity over time, we again aimed to classify and count at least 50 plated colonies per strain (i.e. both SBW25 and the competitor) per sample; however, this was not always possible for some replicates of the two weakest competitor strains treatments after day 5, as they were essentially out-competed.
Diversification dynamics were summarized for both the focal strain and the competitor strain in each treatment by fitting equation (2.2). Parameter estimates for the focal strain were then adjusted by estimates from the appropriate competitor-absent control in order to remove any potential block effects. The adjusted extent of diversity and time until maximum diversity is reached were then compared across competitor fitness and competitor diversification potential (DIV−/DIV+) using ANOVAs.
(f). Density–diversity relationships
To investigate the role played by resource competition stemming from interspecific competition on diversification, we regressed SBW25 density on diversity in the presence and absence of DIV+ and DIV− competitors. To control for block effects and to account for temporal autocorrelation in the data, we first subtracted the density and diversity, respectively, of SBW25 diversifying on its own from the comparable value when it diversified in the presence of a competitor. The resulting data thus represent the marginal increase (or decrease) in density and diversity caused by the presence of a competitor at a given time point. The SBW25 density–diversity relationship was tested using an ANOVA with adjusted diversity as the dependent variable and adjusted density, competitor treatment (no competitors/DIV−/DIV+), and their interaction as independent variables, nesting strain within competitor treatment. Adjusted density data were log-transformed before analysis to meet model assumptions.
(g). Carbon-niche similarity
Although the strains used in this study differ in their competitive fitness, they are all morphologically and ecologically identical to the ancestral SBW25, that is, they have SM colony morphologies and grow in the broth phase of the static microcosms. We attempted to further characterize the niche of each strain by measuring its carbon metabolism profile using BIOLOG GN2 plates. BIOLOG GN2 plates are 96-well microwell plates containing 95 different carbon sources plus a carbon-absent control well. Each strain was grown-up overnight from frozen in vials containing 6 ml KB media (28°C, shaken at 150 r.p.m.), starved for 2 h (20 μl of each culture in 20 ml M9 minimal salts at 28°C, 150 r.p.m.) and then 150 μl was transferred into each well of the BIOLOG plates. Optical density (OD) (at 660 nm) was measured at the time of inoculation and after 24 h of growth, and growth on each carbon substrate was calculated as r = ln(ODinitial)−ln(ODfinal). These growth rates then were adjusted by subtracting the maximum growth rate estimate obtained from all the control wells. Growth rate data were then converted into binary ‘growth’/‘no-growth’ data by setting all positive growth estimates to 1 and all zero or negative growth estimates to 0. Niche similarity with SBW25 was calculated as the percentage of a focal strain's ‘growth’/‘no-growth’ niche profile that is identical to that of SBW25. Niche similarity was then compared across strain fitness and between DIV+ and DIV− groups to look for any potentially confounding effects. Data from this study were deposited in the Dyrad repository [44].
3. Results
(a). Intraspecific competition
We found a clear effect of population density, and so strength of resource competition, on diversification (figure 1a,b) with low-fitness strains (light grey) diversifying less than high-fitness strains (dark grey). Specifically, the extent of diversity (estimated using a modified logistic model) increases significantly with fitness (two-sample t-test, t6 = 5.31, p = 0.002; figure 1b) as expected. Time to maximum diversity showed no relationship with fitness (two-sample t-test, t6 = 0.0613, p = 0.953; see the electronic supplementary material, table S2 for parameter estimates).
Figure 1.
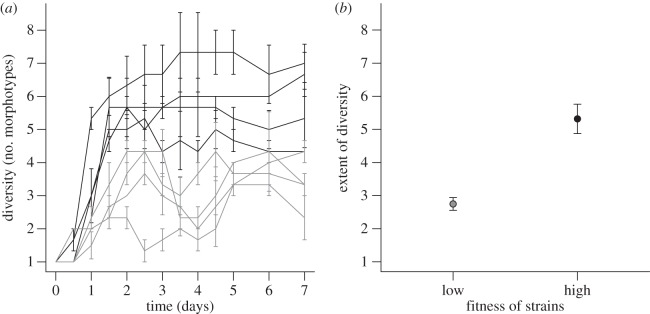
(a) Diversity of low-fitness (grey) and high-fitness (black) naladixic acid-resistant strains over time. Diversity is the number of unique colony morphotypes making up more than 2% of the population (mean±1 s.e.m., n = 3). (b) Extent of diversity of low-fitness and high-fitness naladixic acid-resistant strains. Points represent the mean extent of diversity of four strains±1 s.e.m. Extent of diversity was estimated for each strain using a three-parameter logistic model.
(b). Interspecific competition
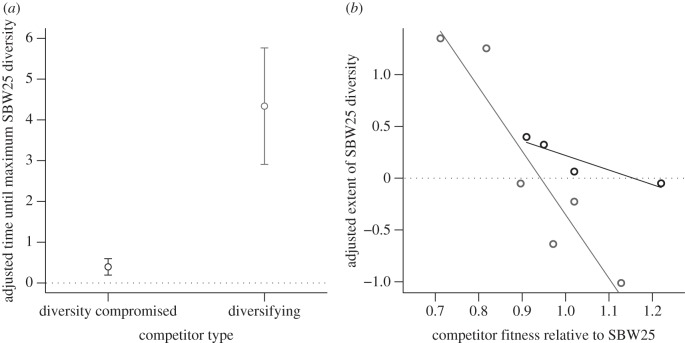
We found a striking difference in the effect of DIV+ and DIV− competitors on the rate and extent of SBW25 diversification. Inspection of figure 2a,b reveals that strong DIV+ competitors (dark grey lines) almost completely prevent SBW25 diversification, as expected, but strong DIV− competitors do not. More quantitatively, DIV+ strains slow the rate of SBW25 diversification (t-test, T5 = 3.7214, p = 0.01369) but DIV− strains do not (t-test, T3 = 1.6102, p = 0.2057) independently of competitor fitness (linear regression, p = 0.2914; figure 3a). There is a negative relationship between competitor fitness and the extent of SBW25 diversity for both DIV+ and DIV− strains (figure 3b; ANOVA: main effect of competitor fitness, F1,6 = 31.08, p = 0.0014), although this relationship is stronger for the DIV+ competitors than for the DIV− competitors (ANOVA: competitor fitness × competitor type, F1,6 = 7.63, p = 0.0327; DIV+ slope: −6.15, DIV− slope: −1.41). Note that the sign of the effect depends on the fitness of the competitors: weak competitors cause diversity to be higher, whereas strong competitors cause it to be lower, than would be the case in the absence of competitors. Proximately, this extra diversity is not owing to the occurrence of unusual or rare morphotypes but rather is associated with more types within either the WS or SM niche classes. Note also that a regression of the realized extent of competitor diversity on SBW25 diversity shows a significantly negative relationship for DIV+ competitors, but for DIV− competitors, the slope of this relationship did not differ significantly from zero (figure 4a; DIV+: p = 0.0032, DIV−: p = 0.819), and the DIV+ and DIV− slopes are not significantly different from each other (ANCOVA, interaction of competitor type and competitor diversity: F1,6 = 1.2488, p = 0.3065). These results are consistent with the idea that niche pre-emption is the mechanism by which DIV+ competitors prevent diversification.
Figure 2.
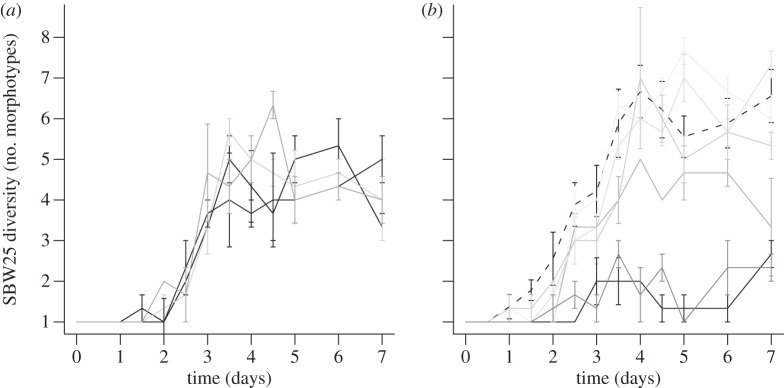
Diversity of SBW25 in the presence of (a) DIV− competitors and (b) DIV+ competitors over time, where light-to-dark grey corresponds to low-to-high competitor fitness. Each panel includes SBW25 in the absence of competitors (dashed black line) for that experiment. SBW25 diversity is the number of unique colony morphotypes making up more than 2% of the population (mean±1 s.e.m., n = 3).
Figure 3.
(a) Adjusted time until maximum SBW25 diversity is reached in the presence of DIV− and DIV+ competitors. (b) Adjusted extent of SBW25 diversity in DIV− (black circles) and DIV+ (grey circles) competitors, plotted against fitness of the competitor relative to SBW25. Statistically significant regression lines are shown. Time until maximum diversity and extent of diversity were estimated using a three-parameter logistic model (see §2) and adjusted by parameter estimates for SBW25 in the absence of competitors.
Figure 4.
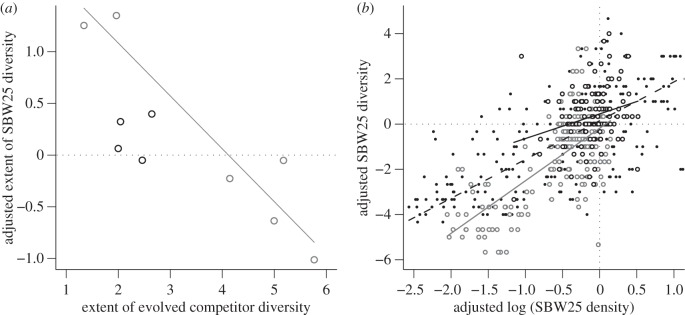
(a) Adjusted extent of SBW25 diversity in the presence of DIV+ (grey circles) and DIV− (black circles) competitors, plotted against extent of evolved competitor diversity. Statistically significant regression lines are shown. (b) SBW25 diversity in the presence of DIV+ (grey open circles, grey solid line), the DIV− (black open circles, black dashed line) competitors and the absence of competitor strains (black points, black solid line) plotted against log-transformed SBW25 density. Diversity and density measures were adjusted by SBW25 density and diversity measures in the absence of competitor strains at the same time point. Lines show regression fits for each group.
(c). Density–diversity relationship
Figure 4b shows a significant positive relationship between SBW25 density and SBW25 diversity across all treatments (nested-ANOVA; adjusted density: F1,12 = 262.452, p < 0.0001). The marginal effect of an increase in SBW25 density on diversity is larger in the presence of non-diversifying (DIV−) competitors and smaller in the presence of diversifying (DIV+) competitors than when competitors are absent altogether, a result confirmed both by a marginally significant effect of competitor type (F2,12 = 3.691, p = 0.05631) and a significant interaction between adjusted density and competitor type (F2,12 = 7.631, p = 0.00727). Over the range of densities where SBW25 experiences antagonistic effects (adjusted log(density) < 0), the effect of adding competitors is to increase SBW25 diversity when the competitors are unable to diversify (DIV− competitors) and to lower it when they can (DIV+ competitors). This result provides direct support for the idea that weak, non-diversifying competitors increase the effective amount of resource competition experienced by the radiating lineage. Weak diversifying competitors, on the other hand, act to impede diversification of SBW25 by radiating to occupy what would otherwise be available niche space.
(d). Carbon-niche similarity
There is no evidence of any relationship between competitor strain fitness and carbon-niche similarity with SBW25 (see the electronic supplementary material, figure S3; linear regression: p = 0.702). In addition, there is no relationship between competitor carbon-niche overlap with SBW25 and evolved diversity in SBW25 (see the electronic supplementary material, figure S4; linear regression: p = 0.9779).
4. Discussion
We have investigated the effects of different kinds of competition on the rate and extent of diversification in a model adaptive radiation. Briefly, our main results are: (i) the extent of diversification is directly, positively related to the strength of intraspecific resource competition; (ii) interspecific competitors can prevent diversification through niche pre-emption or, if they are particularly strong competitors, through resource competition; (iii) weak interspecific competitors can, counterintuitively, promote diversification by increasing resource competition. We discuss each of these results in turn later.
(a). Intraspecific competition
We found that the extent, but not the rate, of diversification increases significantly with the strength of intraspecific resource competition. More specifically, a cost of adaptation caused by the presence of antibiotic resistance mutations kept population densities low enough to prevent substantial diversification. Evidently, there is some threshold level of population density that must be reached for the pronounced and rapid diversification characteristic of other experiments with the P. fluorescens system to proceed. To the extent that this model system represents a good guide to adaptive radiations in the ‘real’ world, it suggests that simply gaining access to novel ecological opportunities is not by itself sufficient to drive diversification. Population densities must also be high enough to generate strong resource competition capable of generating disruptive selection.
This result is somewhat different from that observed in the previous experiments with this system where the presence of predators that reduce population densities, and so the strength of resource competition, slowed the emergence of diversity but did not change the extent of diversification [32]. Predation has been shown to promote the emergence of WS morphs that gain protection from predation by virtue of being part of the biofilm [32,45], suggesting that the high levels of diversity observed under predation are owing to a fortuitous alignment of selective forces where both predation-resistance and resource competition favour biofilm formation. Without the additional selective advantage of predation-resistance driving diversification, in our experiment diversity differences between the strong and weak competition treatments emerge early on and are then maintained throughout the course of the experiment.
An alternative explanation for our results is that the resistance mutations themselves have unknown pleiotropic effects that in some way compromise diversification independently of their effects on resource competition. This explanation seems unlikely because, as we noted earlier, none of the mutations conferring resistance occur in genes known to be involved in diversification into the broad niche classes in this system (SM, WS and FS). Two of the naladixic acid-resistant strains we used do have large multi-gene deletions that might be expected to have wide reaching effects; however, removal of those two strains from the analysis did not qualitatively change our results. Furthermore, all resistant strains diversified into the three available broad niche specialist classes; differences in diversity arise from differences in the number of types that evolve within the broad niche classes. We were also unable to detect any significant differences between these strains in their carbon-niche profiles (see the electronic supplementary material, figure S5). Thus, there appears to be no reason to expect potential pleiotropic effects to vary systematically across fitness and/or diversification potential in a way that would confound our results.
(b). Interspecific competition
Our co-culture experiments show clearly that the dynamics of diversification can be modulated by the strength of interspecific competition. Strong competitors can prevent diversification through a combination of niche pre-emption and competitive exclusion. Weak competitors, surprisingly, can actually promote diversification, presumably because competitor density is sufficiently high in the initial phases of the radiation to increase the strength of resource competition experienced by the radiating lineage but not so high that they themselves diversify.
Direct evidence for both niche pre-emption and resource competition as significant mechanisms modulating the outcome of competition during the radiation comes from a regression of the extent of competitor diversity on SBW25 diversity. The slope of this regression is negative for DIV+ competitors but not for DIV− competitors, consistent with the idea that competitor diversification leads to niche pre-emption and prevents SBW25 diversification (figure 4a). This result is also consistent with that seen in other experiments with this system, where niche pre-emption has been directly manipulated [16,17] and cannot be explained by pre-existing differences in metabolic niche space, as the competitors and SBW25 do not differ in carbon-use profiles (t-test, p = 0.1604). Our results thus lend further support to the idea that interspecific competitors can prevent diversification of a focal lineage by removing or reducing the extent of ecological opportunity through niche pre-emption. Note also that strong, non-diversifying competitors also prevented diversification but, because they were unable to radiate themselves, they necessarily did so through direct resource competition that resulted in the competitive exclusion of SBW25. Taken together, these results provide direct evidence that interspecific competitors can prevent diversification through niche pre-emption, and at its extreme this leads to competitive exclusion.
In general, it is competition for resources that drives the adaptive radiation of SBW25 in this system, and the positive relationship seen between density and diversity in figure 4b supports this. While the presence of interspecific competitors modifies the details of this relationship, it is always the case that a higher density SBW25 population results in increased SBW25 diversity. However, the marginal effect of an increase in SBW25 density on diversity is larger in the presence of non-diversifying (DIV−) competitors, and smaller in the presence of diversifying (DIV+) competitors, than when competitors are absent altogether. This result suggests that non-diversifying competitors tend to increase the effective amount of resource competition experienced by the radiating lineage, whereas diversifying competitors tend to impede the radiation through niche pre-emption. Interestingly, the additional diversity that arises in the presence of weak interspecific competitors is not due to the occurrence of unusual or rare morphotypes but rather is associated with more types within the SM and WS broad niche classes. As mentioned earlier, these results cannot be explained by pre-existing differences in carbon use, as there is no relationship between competitors strain carbon use and competitor strain fitness, and no relationship between competitor strain carbon use and evolved SBW25 diversity.
(c). Summary
We have provided direct evidence that the extent of diversification under intraspecific competition is determined by the strength of resource competition. Interspecific competitors, by contrast, can either increase the strength of resource competition experienced by a radiating lineage, promoting diversification or remove ecological opportunities available to it, and so prevent diversification. Which of these two outcomes will be realized in any ‘real’ radiation will thus depend on both the strength of the competitor itself as well as its propensity to diversify. In our study, ‘propensity to diversify’ was a constructed genetic property particular to our experimental design; however in nature, we expect differences in species' underlying genetic architecture to cause populations to vary in their propensity to diversify in any given environment [40].
An incipiently radiating lineage that gains access to novel ecological opportunities in the presence of a weak competitor, or a competitor that is incapable of diversifying rapidly, will find little obstacle to diversification and may even diversify more extensively than it would in the absence of the competitor. It is possible that additional diversity driven by the presence of weak competitors contributes to the ‘overshooting’ pattern observed in some extant radiations [46], with diversity peaking and then dropping again as those competitors are eventually lost. The extra diversity that comes through the presence of a weak competitor also constitutes an example of how the presence of one type can cause diversification in another, an example of ‘diversity begetting diversity’ [47,48]. By contrast, the presence of a strong competitor that is itself capable of diversifying, or one that has already diversified [16,17], will prevent diversification in the focal lineage. These results also bear on the issue of the evolutionary fate of invasive species outside their native range: an exotic species that colonizes a novel environment which lacks the usual suite of competitors, or is itself a stronger competitor against existing native species, should be expected to diversify. As a whole, our study underlines the complexities of competition's important role in the evolution of diversity and helps to focus our understanding of the ways in which competition may be at work in other systems.
Acknowledgements
S.F.B. thanks the members of the Rainey laboratory for stimulating discussions during her visit. L. Harmon provided comments on an earlier version of the manuscript.
Funding statement
This work was supported by an NSERC (Canada) Postgraduate Graduate Scholarship to S.F.B. and an NSERC Discovery Grant to R.K.
References
- 1.Benton MJ. 1995. Diversification and extinction in the history of life. Science 268, 52–58 (doi:10.1126/science.7701342) [DOI] [PubMed] [Google Scholar]
- 2.Simpson GG. 1953. The major features of evolution. New York, NY: Columbia University Press [Google Scholar]
- 3.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596 (doi:10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 4.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press [Google Scholar]
- 5.Dieckmann U, Doebeli M. 1999. On the origin of species by sympatric speciation. Nature 400, 354–357 (doi:10.1038/22521) [DOI] [PubMed] [Google Scholar]
- 6.Lack D. 1947. Darwin's finches. Cambridge, UK: Cambridge University Press [Google Scholar]
- 7.Kassen R, Bell G. 2000. The ecology and genetics of fitness in Chlamydomonas. X. The relationship between genetic correlation and genetic distance. Evolution 54, 425–432 (doi:10.1554/0014-3820(2000)054[0425:TEAGOF]2.0) [DOI] [PubMed] [Google Scholar]
- 8.Abrams P. 1983. The theory of limiting similarity. Annu. Rev. Ecol. Syst. 14, 359–376 (doi:10.1146/annurev.es.14.110183.002043) [Google Scholar]
- 9.Tyerman JG, Bertrand M, Spencer CC, Doebeli M. 2008. Experimental demonstration of ecological character displacement. BMC Evol. Biol. 8, 34 (doi:10.1186/1471-2148-8-34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolnick D. 2004. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution 58, 608–618 (doi:10.1554/03-326) [PubMed] [Google Scholar]
- 11.Schluter D. 1996. Ecological causes of adaptive radiation. Am. Nat. 148, S40–S64 (doi:10.1086/285901) [Google Scholar]
- 12.Bolnick DI, Ingram T, Stutz WE, Snowberg LK, Lau OL, Paull JS. 2010. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B 277, 1789–1797 (doi:10.1098/rspb.2010.0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persson A, Hansson A. 1999. Diet shift in fish following competitive release. Can. J. Fish. Aq. Sci. 56, 70–78 (doi:10.1139/cjfas-56-1-70) [Google Scholar]
- 14.Pacala SW, Roughgarden J. 1985. Population experiments with the Anolis lizards of St Maarten and St Eustatius. Ecology 66, 129–141 (doi:10.2307/1941313) [Google Scholar]
- 15.Rainey PB, Travisano M. 1998. Adaptive radiation in a heterogeneous environment. Nature 394, 69–72 (doi:10.1038/27900) [DOI] [PubMed] [Google Scholar]
- 16.Brockhurst MA, Colegrave N, Hodgson DJ, Buckling A. 2007. Niche occupation limits adaptive radiation in experimental microcosms. PloS ONE 2, e193 (doi:10.1371/journal.pone.0000193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukami T, Beaumont HJE, Zhang X-X, Rainey PB. 2007. Immigration history controls diversification in experimental adaptive radiation. Nature 446, 436–439 (doi:10.1038/nature05629) [DOI] [PubMed] [Google Scholar]
- 18.Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457, 830–836 (doi:10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 19.Svänback R, Eklov P, Fransson R, Holmgren KO. 2008. Intra-specific competition drives multiple species trophic polymorphism in fish communities. Oikos 117, 114–124 (doi:10.1111/j.2007.0030-1299.16267.x) [Google Scholar]
- 20.Jonsson B, Jonsson N. 2001. Polymorphism and speciation in Arctic charr. J. Fish Biol. 58, 605–638 (doi:10.1006/jfbi.2000.1515) [Google Scholar]
- 21.Losos JB, de Queiroz K. 1997. Evolutionary consequences of ecological release in Caribbean Anolis lizards. Biol. J. Linn. Soc. 61, 459–483 (doi:10.1111/j.1095-8312.1997.tb01802.x) [Google Scholar]
- 22.Robinson BW, Wilson DS. 1994. Character release and displacement in fishes: a neglected literature. Am. Nat. 144, 596–627 (doi:10.1086/285696) [Google Scholar]
- 23.Dayan T, Simberloff D. 1994. Character displacement, sexual size dimorphism, and morphological variation among the mustelids of the British Isles. Ecology 75, 1063–1073 (doi:10.2307/1939430) [Google Scholar]
- 24.Feinsinger P, Swarm L. 1982. ‘Ecological release,’ seasonal variation in food supply, and the hummingbird Amazilia tobaci on Trinidad and Tobago. Ecology 63, 1574–1587 (doi:10.2307/1938881) [Google Scholar]
- 25.Burns JH, Strauss SY. 2011. More closely related species are more ecologically similar in an experimental test. Proc. Natl Acad. Sci. USA 108, 5302–5307 (doi:10.1073/pnas.1013003108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blount ZD, Borland CZ, Lenski RE. 2008. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc. Natl Acad. Sci. USA 105, 7899–7906 (doi:10.1073/pnas.0803151105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cahill JF, Jr, Kembel SW, Lamb EG, Keddy PA. 2008. Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspect. Plant Ecol. Evol. Syst. 10, 41–50 (doi:10.1016/j.ppees.2007.10.001) [Google Scholar]
- 28.Violle C, Nemergut DR, Pu Z, Jiang L. 2011. Phylogenetic limiting similarity and competitive exclusion. Ecol. Lett. 14, 782–787 (doi:10.1111/j.1461-0248.2011.01644.x) [DOI] [PubMed] [Google Scholar]
- 29.Brauer F, Castillo-Chavez C. 2001. Mathematical models in population biology and epidemiology. New York, NY: Springer [Google Scholar]
- 30.Zhang QG, Ellis RJ, Godfray HC. 2012. The effect of a competitor on a model adaptive radiation. Evolution 66, 1985–1990 (doi:10.1111/j.1558-5646.2011.01559.x) [DOI] [PubMed] [Google Scholar]
- 31.Kassen R, Buckling A, Bell G, Rainey PB. 2000. Diversity peaks at intermediate productivity in a laboratory microcosm. Nature 406, 508–512 (doi:10.1038/35020060) [DOI] [PubMed] [Google Scholar]
- 32.Meyer JR, Kassen R. 2007. The effects of competition and predation on diversification in a model adaptive radiation. Nature 446, 432–435 (doi:10.1038/nature05599) [DOI] [PubMed] [Google Scholar]
- 33.Koza A, Moshynets O, Otten W, Spiers AJ. 2010. Environmental modification and niche construction: developing O2 gradients drive the evolution of the wrinkly spreader. ISME J. 5, 665–673 (doi:10.1038/ismej.2010.156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainey PB, Rainey K. 2003. Evolution of co-operation and conflict in experimental bacterial populations. Nature 425, 72–74 (doi:10.1038/nature01906) [DOI] [PubMed] [Google Scholar]
- 35.Spiers AJ, Kahn SG, Bohannon J, Travisano M, Rainey PB. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bantinaki E, Kassen R, Knight CG, Robinson Z, Spiers AJ, Rainey PB. 2007. Adaptive divergence in experimental populations of Pseudomonas fluorescens. III. Mutational origins of wrinkly spreader diversity. Genetics 176, 441–453 (doi:10.1534/genetics.106.069906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson GC, Bertels F, Rainey PB. Submitted Adaptive divergence in experimental populations of Pseudomonas fluorescens. V. Genetic and phenotypic bases of fuzzy spreader fitness. [Google Scholar]
- 38.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 39.Kassen R, Bataillon T. 2006. Distribution of fitness effects among beneficial mutations prior to selection in experimental populations of bacteria. Nat. Genetics 38, 484–488 (doi:10.1038/ng1751) [DOI] [PubMed] [Google Scholar]
- 40.McDonald MJ, Gehrig SM, Meintjes PL, Zhang X-X, Rainey PB. 2009. Adaptive divergence in experimental populations of Pseudomonas fluorescens. IV. Genetic constraints guide evolutionary trajectories in a parallel adaptive radiation. Genetics 183, 1041–1053 (doi:10.1534/genetics.109.107110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey SF, Kassen R. 2012. Spatial structure of ecological opportunity drives adaptation in a bacterium. Am. Nat. 180, 270–283 (doi:10.1086/666609) [DOI] [PubMed] [Google Scholar]
- 42.Meyer JR, Schoustra SE, Lachapelle J, Kassen R. 2011. Overshooting dynamics in a model adaptive radiation. Proc. R. Soc. B 278, 392–398 (doi:10.1098/rspb.2010.0640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X-X, Rainey PB. 2007. Construction and validation of a neutrally-marked strain of Pseudomonas fluorescens SBW25. J. Microbiol. Meth. 71, 78–81 (doi:10.1016/j.mimet.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 44.Bailey SF, Dettman JR, Rainey PB, Kassen R. 2013. Data from: Competition both drives and impedes diversification in a model adaptive radiation. Dryad Digital Repository (doi:10.5061/dryad.bb2p8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall AR, Meyer JR, Kassen R. 2008. Selection for predator resistance varies with resource supply in a model adaptive radiation. Evol. Ecol. Res. 10, 735–746 [Google Scholar]
- 46.Seehausen O. 2006. African cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B 273, 1987–1998 (doi:10.1098/rspb.2006.03539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forbes AA, Powell THQ, Stelinski LL, Smith JJ, Feder JL. 2009. Sequential sympatric speciation across trophic levels. Science 323, 776–779 (doi:10.1126/science.1166981) [DOI] [PubMed] [Google Scholar]
- 48.Emerson BC, Kolm N. 2005. Species diversity can drive speciation. Nature 434, 1015–1017 (doi:10.1038/nature03450) [DOI] [PubMed] [Google Scholar]