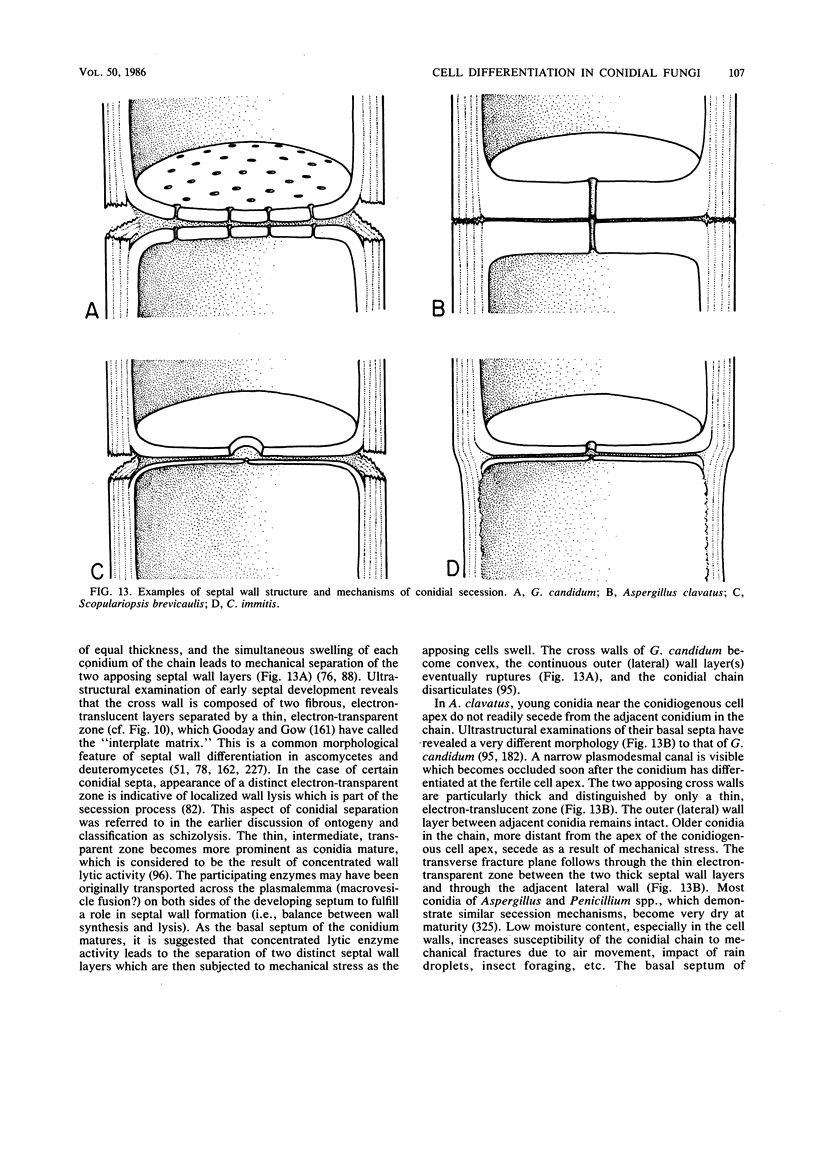
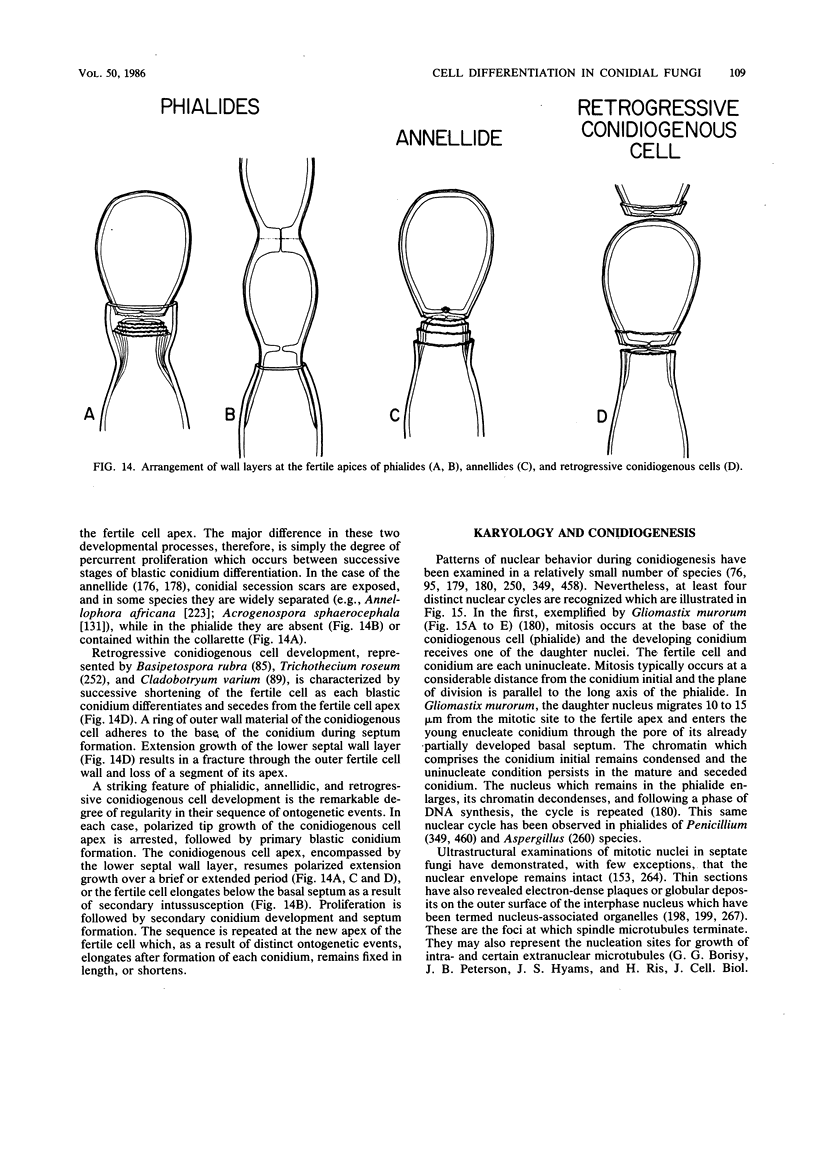
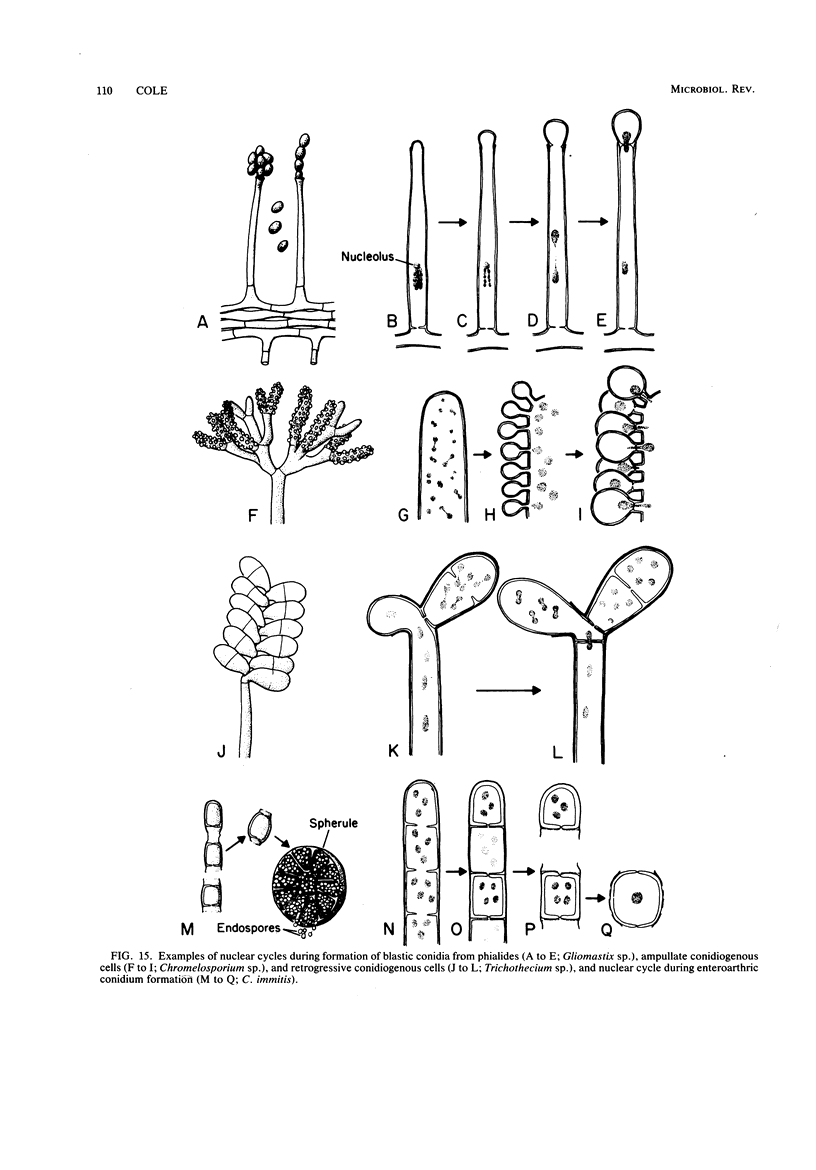
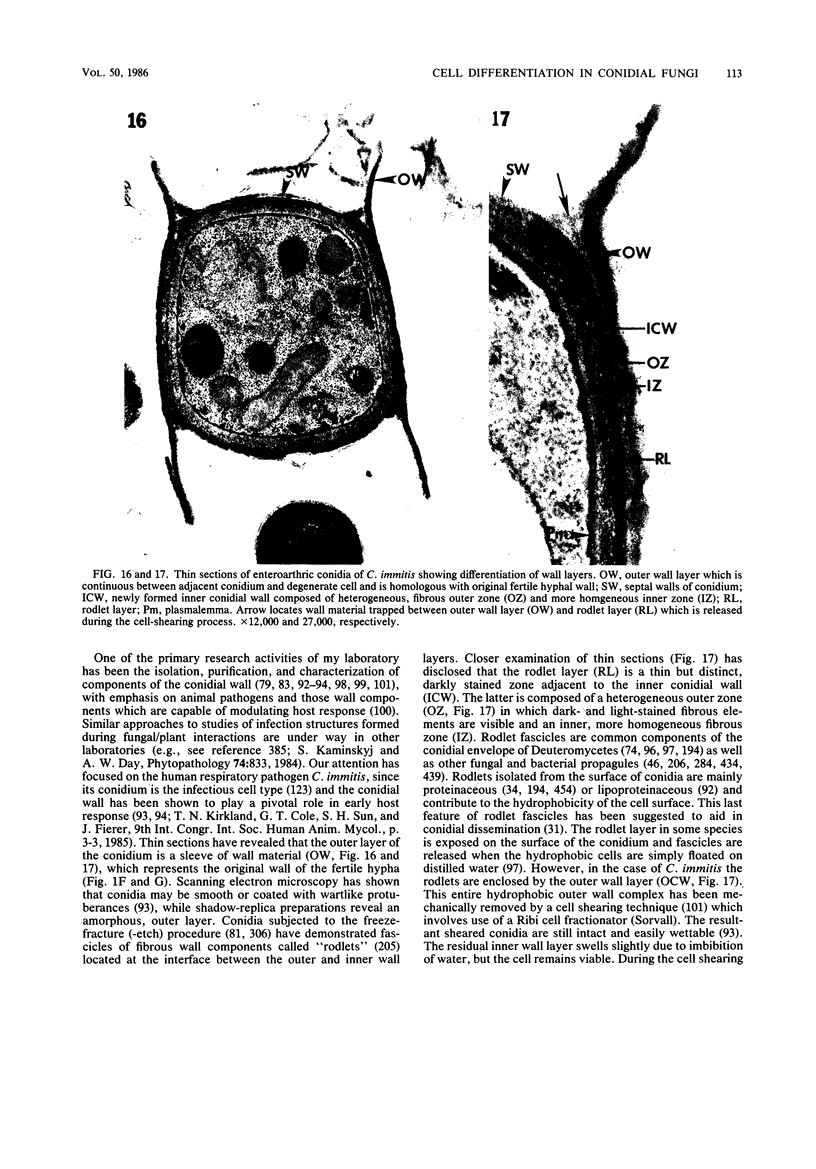
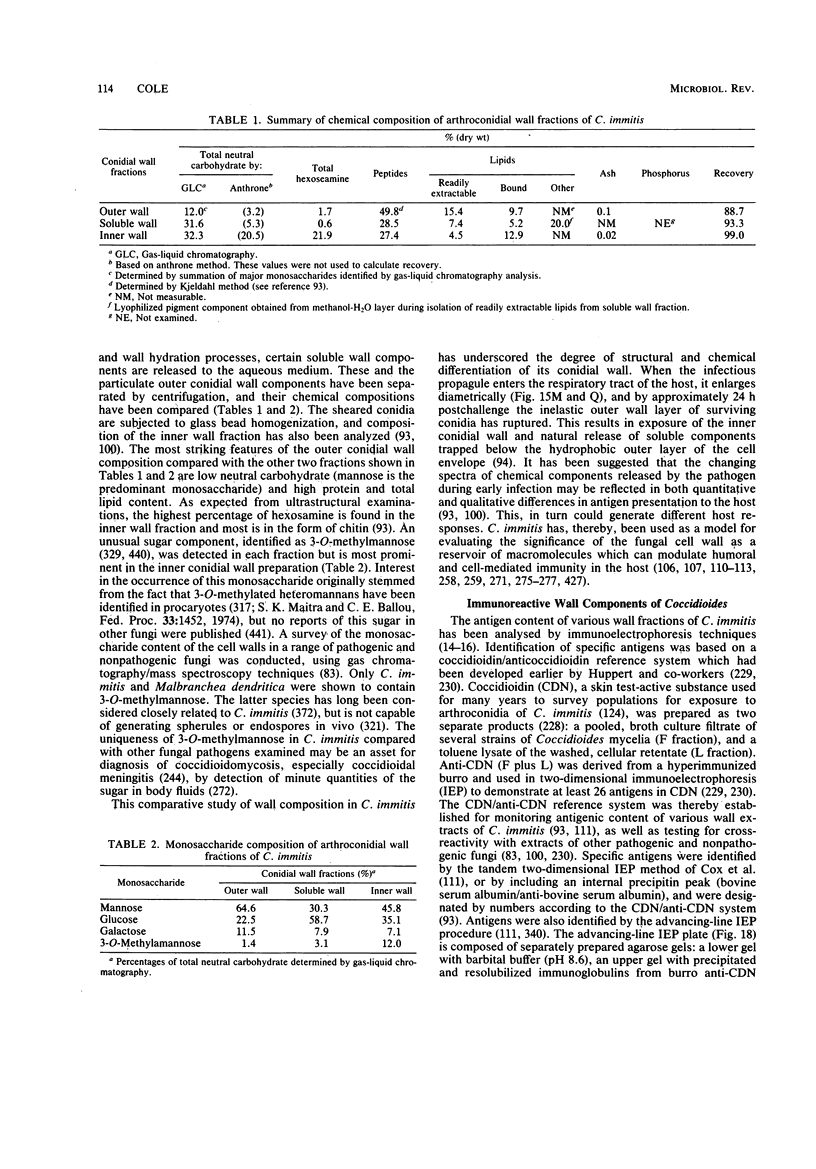
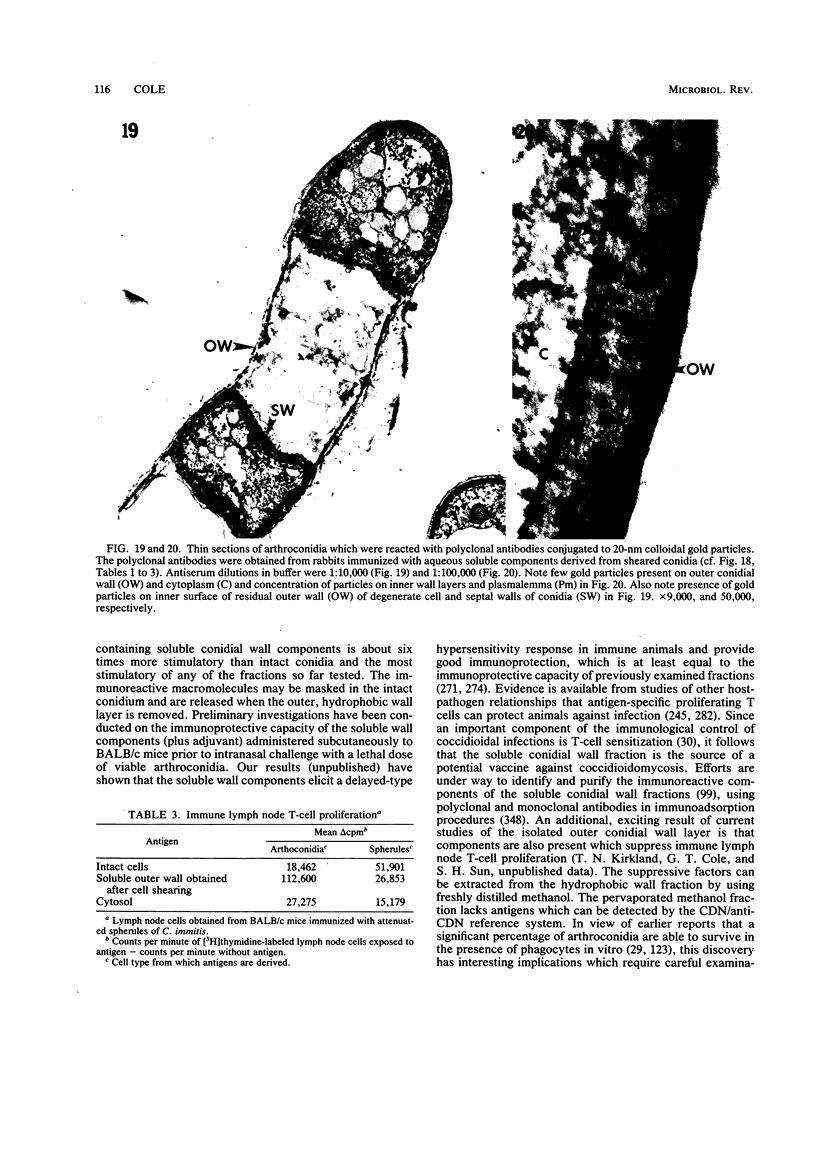
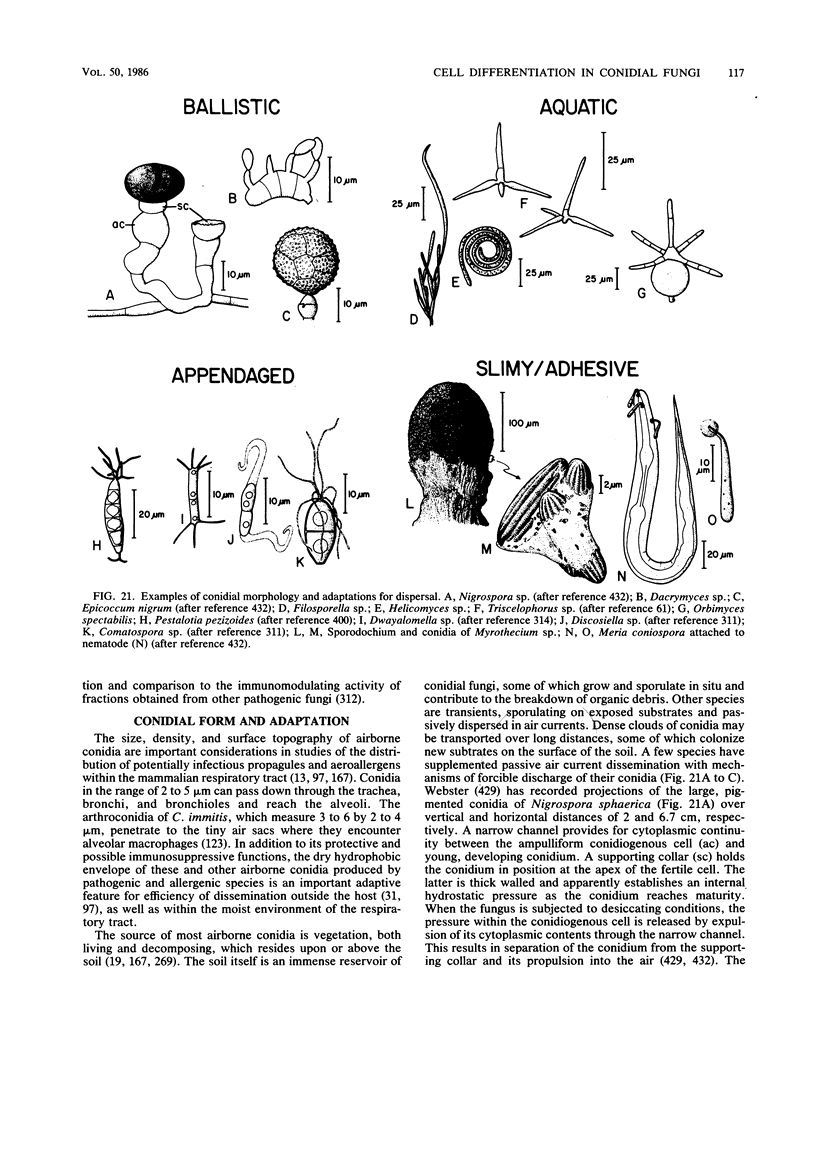
Full text
PDF


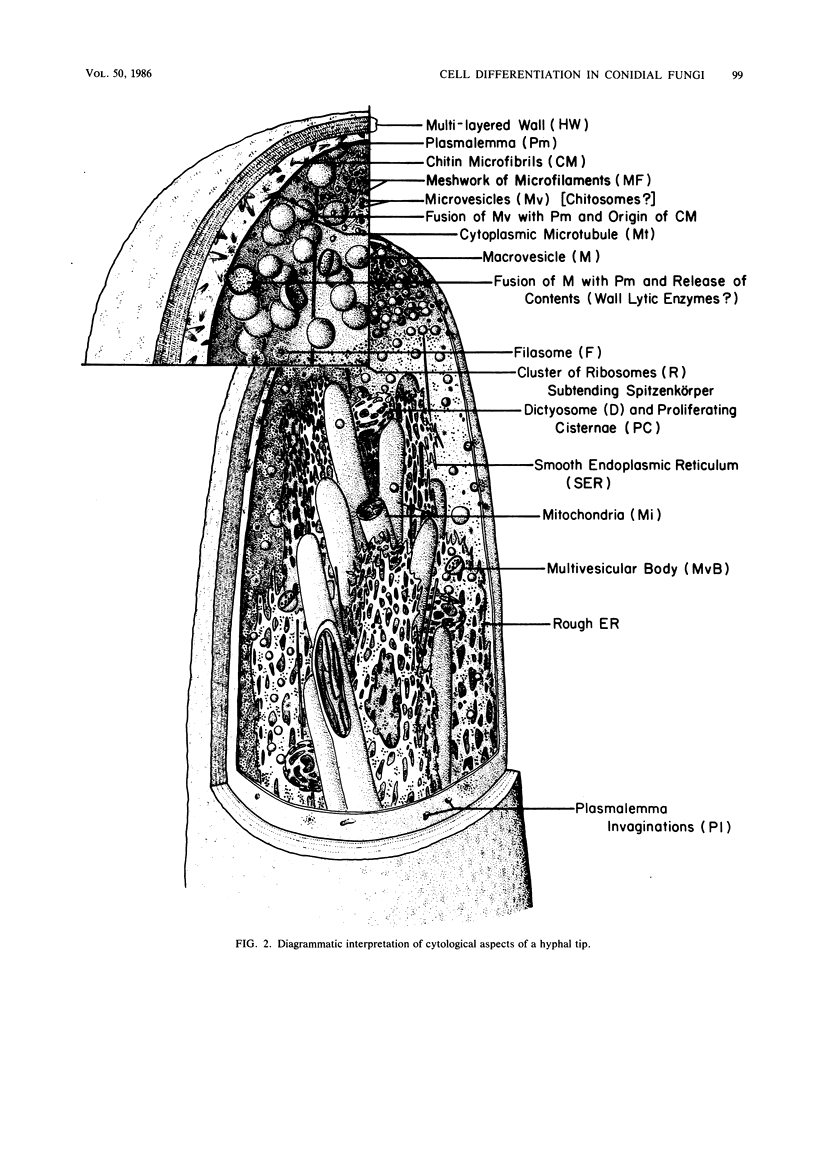

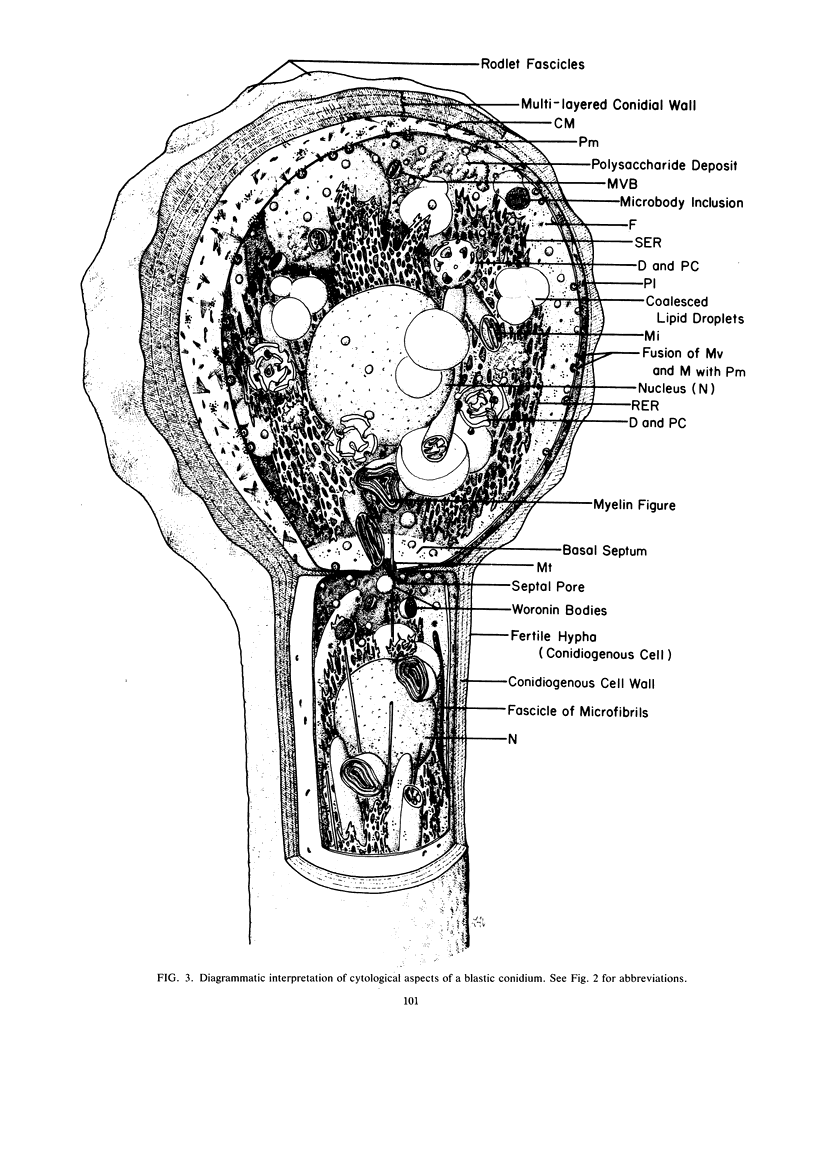
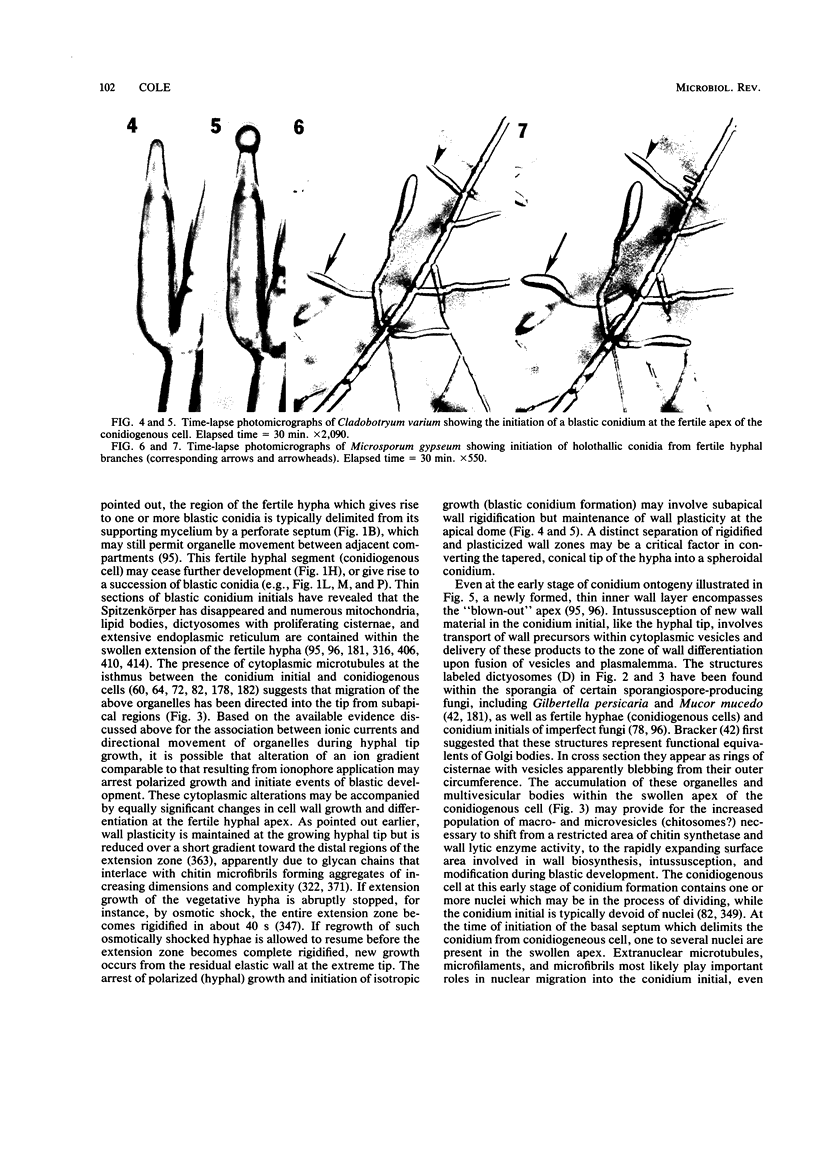



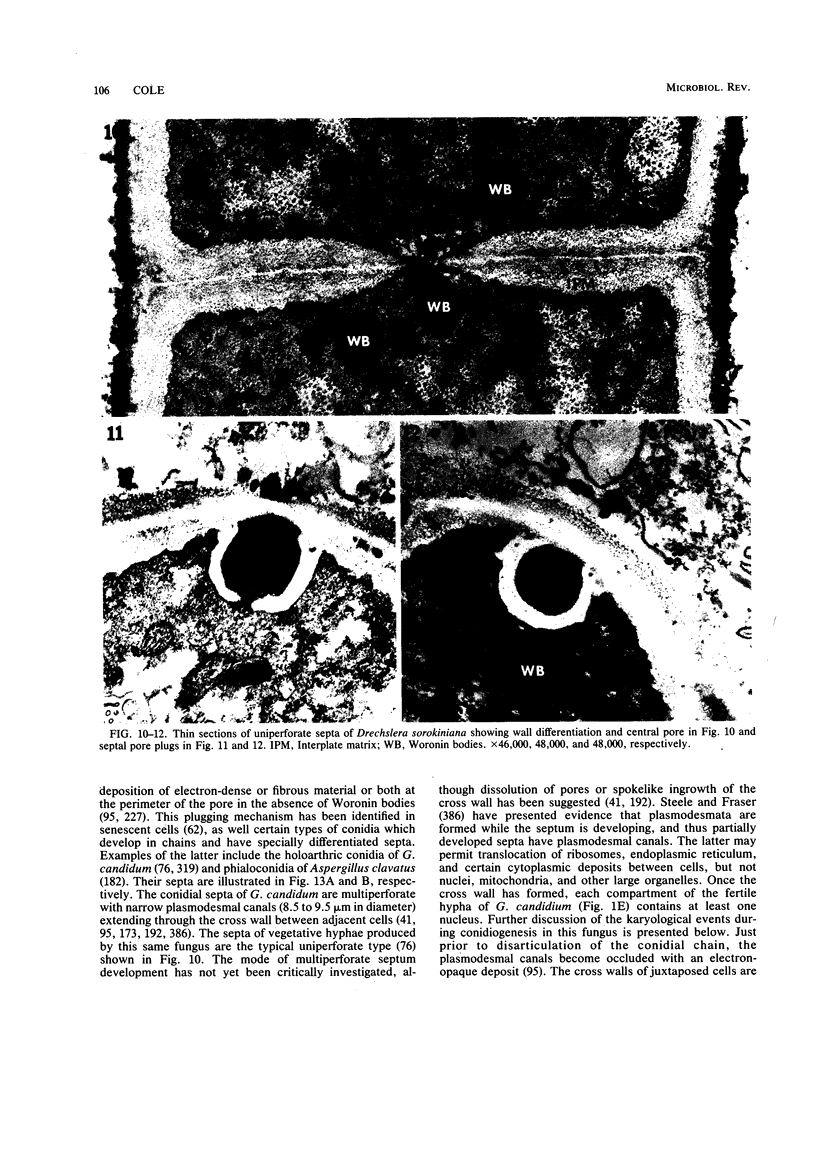



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. D., Aiuto R., Sussman A. S. Effects of cytochalasins on Neurospora crassa. I. Growth and ultrastructure. Protoplasma. 1980;102(1-2):63–75. doi: 10.1007/BF01276948. [DOI] [PubMed] [Google Scholar]
- Anderson J. G., Smith J. E. The effects of elevated temperatures on spore swelling and germination in Aspergillus niger. Can J Microbiol. 1972 Mar;18(3):289–297. doi: 10.1139/m72-045. [DOI] [PubMed] [Google Scholar]
- Anderson J. G., Smith J. E. The production of conidiophores and conidia by newly germinated conidia of Aspergillus niger (microcycle conidiation). J Gen Microbiol. 1971 Dec;69(2):185–197. doi: 10.1099/00221287-69-2-185. [DOI] [PubMed] [Google Scholar]
- Axelsen N. H. Antigen-antibody crossed electrophoresis (Laurell) applied to the study of the antigenic structure of Candida albicans. Infect Immun. 1971 Nov;4(5):525–527. doi: 10.1128/iai.4.5.525-527.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen N. H. Quantitative immunoelectrophoretic methods as tools for a polyvalent approach to standardization in the immunochemistry of Candida albicans. Infect Immun. 1973 Jun;7(6):949–960. doi: 10.1128/iai.7.6.949-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRACKER C. E., BUTLER E. E. FUNCTION OF THE SEPTAL PORE APPARATUS IN RHIZOCTONIA SOLANI DURING PROTOPLASMIC STREAMING. J Cell Biol. 1964 Apr;21:152–157. doi: 10.1083/jcb.21.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barran L. R., Schneider E. F., Seaman W. L. Requirements for the rapid conversion of macroconidia of Fusarium sulphureum to chlamydospores. Can J Microbiol. 1977 Feb;23(2):148–151. doi: 10.1139/m77-021. [DOI] [PubMed] [Google Scholar]
- Barrera C. R. Formation and ultrastructure of Mucor rouxii arthrospores. J Bacteriol. 1983 Aug;155(2):886–895. doi: 10.1128/jb.155.2.886-895.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Bracker C. E., Lippman E., Ruiz-Herrera J. Chitosomes from the wall-less "slime" mutant of Neurospora crassa. Arch Microbiol. 1984 Oct;139(2-3):105–112. doi: 10.1007/BF00401983. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Beaman L., Holmberg C. A. In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect Immun. 1980 May;28(2):594–600. doi: 10.1128/iai.28.2.594-600.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Pappagianis D., Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977 Sep;17(3):580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beever R. E., Dempsey G. P. Function of rodlets on the surface of fungal spores. Nature. 1978 Apr 13;272(5654):608–610. doi: 10.1038/272608a0. [DOI] [PubMed] [Google Scholar]
- Beever R. E., Redgwell R. J., Dempsey G. P. Purification and chemical characterization of the rodlet layer of Neurospora crassa conidia. J Bacteriol. 1979 Dec;140(3):1063–1070. doi: 10.1128/jb.140.3.1063-1070.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. A., Puhalla J. E., Tolmsoff W. J., Stipanovic R. D. Use of mutants to establish (+)-scytalone as an intermediate in melanin biosynthesis by Verticillium dahliae. Can J Microbiol. 1976 Jun;22(6):787–799. doi: 10.1139/m76-115. [DOI] [PubMed] [Google Scholar]
- Benítez T., Villa T. G., García Acha I. Some chemical and structural features of the conidial wall of Trichoderma viride. Can J Microbiol. 1976 Feb;22(2):318–321. doi: 10.1139/m76-046. [DOI] [PubMed] [Google Scholar]
- Bibel D. J., Crumrine D. A., Yee K., King R. D. Development of arthrospores of Trichophyton mentagrophytes. Infect Immun. 1977 Mar;15(3):958–971. doi: 10.1128/iai.15.3.958-971.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbitt T. F., Nordin J. H., Roux M., Revol J. F., Marchessault R. H. Distribution and conformation of crystalline nigeran in hyphal walls of Aspergillus niger and Aspergillus awamori. J Bacteriol. 1977 Nov;132(2):691–703. doi: 10.1128/jb.132.2.691-703.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzola J. J., Mehta R. J., Nisbet L. J., Valenta J. R. The effect of aculeacin A and papulacandin B on morphology and cell wall ultrastructure in Candida albicans. Can J Microbiol. 1984 Jun;30(6):857–863. doi: 10.1139/m84-133. [DOI] [PubMed] [Google Scholar]
- Bracker C. E. The ultrastructure and development of sporangia in Gilbertella persicaria. Mycologia. 1968 Sep-Oct;60(5):1016–1067. [PubMed] [Google Scholar]
- Brenner D. M., Carroll G. C. Fine-structural correlates of growth in hyphae of Ascodesmis sphaerospora. J Bacteriol. 1968 Feb;95(2):658–671. doi: 10.1128/jb.95.2.658-671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G., Walker L., Ling N. R., Richardson P., Johnson G. D., Guy K., Steel C. M. T-cell proliferation and expression of MHC class II antigens. Scand J Immunol. 1984 Apr;19(4):373–377. doi: 10.1111/j.1365-3083.1984.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Bull A. T. Inhibition of polysaccharases by melanin: enzyme inhibition in relation to mycolysis. Arch Biochem Biophys. 1970 Apr;137(2):345–356. doi: 10.1016/0003-9861(70)90448-0. [DOI] [PubMed] [Google Scholar]
- Byers B., Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976 Jun;69(3):717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1974;38:123–131. doi: 10.1101/sqb.1974.038.01.016. [DOI] [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. A molecular model for morphogenesis: the primary septum of yeast. Curr Top Cell Regul. 1974;8(0):1–32. doi: 10.1016/b978-0-12-152808-9.50008-0. [DOI] [PubMed] [Google Scholar]
- Carroll F. E., Carroll G. C. Senescence and death of the conidiogenous cell in Stemphylium botryosum Wallroth. Arch Mikrobiol. 1973 Dec 21;94(2):109–124. doi: 10.1007/BF00416686. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. J. A mutational analysis of conidial development in Aspergillus nidulans. Genetics. 1969 Oct;63(2):317–327. doi: 10.1093/genetics/63.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- Cochrane V. W., Cochrane J. C. Chlamydospore development in the absence of protein synthesis in Fusarium solani. Dev Biol. 1970 Nov;23(3):345–354. doi: 10.1016/0012-1606(70)90103-x. [DOI] [PubMed] [Google Scholar]
- Cochrane V. W., Cochrane J. C. Chlamydospore induction in pure culture in Fusarium solani. Mycologia. 1971 May-Jun;63(3):462–477. [PubMed] [Google Scholar]
- Cole G. T. Microfibrils in the cytoplasm of fertile hyphae of the imperfect fungus, Drechslera sorokiniana. J Ultrastruct Res. 1972 Dec;41(5):563–571. doi: 10.1016/s0022-5320(72)90057-3. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Sun S. H., Huppert M. Isolation and ultrastructural examination of conidial wall components of Coccidioides and Aspergillus. Scan Electron Microsc. 1982;(Pt 4):1677–1685. [PubMed] [Google Scholar]
- Collinge A. J., Trinci A. P. Hyphal tips of wild-type and spreading colonial mutants of Neurospora crassa. Arch Microbiol. 1974;99(4):353–368. doi: 10.1007/BF00696249. [DOI] [PubMed] [Google Scholar]
- Collins M., Pappagianis D. Effects of lysozyme and chitinase on the spherules of Coccidioides immitis in vitro. Infect Immun. 1973 May;7(5):817–822. doi: 10.1128/iai.7.5.817-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortat M., Turian G. Conidiation of Neurospora crassa in submerged culture without mycelial phase. Arch Mikrobiol. 1974 Feb 13;95(4):305–309. doi: 10.1007/BF02451771. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Huppert M., Starr P., Britt L. A. Reactivity of alkali-soluble, water-soluble cell wall antigen of Coccidioides immitis with anti-Coccidioides immunoglobulin M precipitin antibody. Infect Immun. 1984 Feb;43(2):502–507. doi: 10.1128/iai.43.2.502-507.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Larsh H. W. Isolation of skin test-active preparations from yeast-phase cells of Blastomyces dermatitidis. Infect Immun. 1974 Jul;10(1):42–47. doi: 10.1128/iai.10.1.42-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Mead C. G., Pavey E. F. Comparisons of mycelia- and spherule-derived antigens in cellular immune assays of Coccidioides immitis-infected guinea pigs. Infect Immun. 1981 Feb;31(2):687–692. doi: 10.1128/iai.31.2.687-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E., Swatek F. E. Pigment production by Basidiobolus in the presence of tyrosine. Mycologia. 1969 Jan-Feb;60(1):130–135. [PubMed] [Google Scholar]
- Drutz D. J., Huppert M. Coccidioidomycosis: factors affecting the host-parasite interaction. J Infect Dis. 1983 Mar;147(3):372–390. doi: 10.1093/infdis/147.3.372. [DOI] [PubMed] [Google Scholar]
- Durán A., Bowers B., Cabib E. Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3952–3955. doi: 10.1073/pnas.72.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS M. R., HAZEN E. L., EDWARDS G. A. The micromorphology of the tuberculate spores of Histoplasma capsulatum. Can J Microbiol. 1960 Feb;6:65–70. doi: 10.1139/m60-009. [DOI] [PubMed] [Google Scholar]
- Edwards M. R., Fritz K. E. Detection of an antigenic cell wall layer in Histoplasma capsulatum. An immunoelectron microscopic study. Arch Microbiol. 1985 Aug;142(3):242–247. doi: 10.1007/BF00693397. [DOI] [PubMed] [Google Scholar]
- Ellis D. H., Griffiths D. A. The fine structure of conidial development in the genus Torula. I. T. herbarum (Pers.) Link ex S. F. Gray and T. herbarum f. quaternella Sacc. Can J Microbiol. 1975 Nov;21(11):1661–1675. doi: 10.1139/m75-244. [DOI] [PubMed] [Google Scholar]
- Emyanitoff R. G., Hashimoto T. The effects of temperature, incubation atmosphere, and medium composition on arthrospore formation in the fungus Trichophyton mentagrophytes. Can J Microbiol. 1979 Mar;25(3):362–366. doi: 10.1139/m79-056. [DOI] [PubMed] [Google Scholar]
- Farkas V. Biosynthesis of cell walls of fungi. Microbiol Rev. 1979 Jun;43(2):117–144. doi: 10.1128/mr.43.2.117-144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet G. H. Composition and structure of yeast cell walls. Curr Top Med Mycol. 1985;1:24–56. doi: 10.1007/978-1-4613-9547-8_2. [DOI] [PubMed] [Google Scholar]
- Galun M., Braun A., Frensdorff A., Galun E. Hyphal walls of isolated lichen fungi: autoradiographic localization of precursor incorporation and binding of fluorescein-conjugated lectins. Arch Microbiol. 1976 May 3;108(1):9–16. doi: 10.1007/BF00425087. [DOI] [PubMed] [Google Scholar]
- Geis P. A., Wheeler M. H., Szaniszlo P. J. Pentaketide metabolites of melanin synthesis in the dematiaceous fungus Wangiella dermatitidis. Arch Microbiol. 1984 Apr;137(4):324–328. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- Gopal P. K., Shepherd M. G., Sullivan P. A. Analysis of wall glucans from yeast, hyphal and germ-tube forming cells of Candida albicans. J Gen Microbiol. 1984 Dec;130(12):3295–3301. doi: 10.1099/00221287-130-12-3295. [DOI] [PubMed] [Google Scholar]
- Griffiths D. A. Development and structure of the aleuriospores of Humicola grisea Traaen. Can J Microbiol. 1974 Jan;20(1):55–58. doi: 10.1139/m74-009. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J Bacteriol. 1970 Nov;104(2):989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Ion currents and physiological functions in microorganisms. Annu Rev Microbiol. 1977;31:181–203. doi: 10.1146/annurev.mi.31.100177.001145. [DOI] [PubMed] [Google Scholar]
- Harold R. L., Harold F. M. Oriented growth of Blastocladiella emersonii in gradients of ionophores and inhibitors. J Bacteriol. 1980 Dec;144(3):1159–1167. doi: 10.1128/jb.144.3.1159-1167.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971 Jul 14;59(1):183–194. doi: 10.1016/0022-2836(71)90420-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971 Dec;69(2):265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Blumenthal H. J. Factors affecting germination of Trichophyton mentagrophytes arthrospores. Infect Immun. 1977 Nov;18(2):479–486. doi: 10.1128/iai.18.2.479-486.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Blumenthal H. J. Survival and resistance of Trichophyton mentagrophytes arthrospores. Appl Environ Microbiol. 1978 Feb;35(2):274–277. doi: 10.1128/aem.35.2.274-277.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Morgan J., Conti S. F. Morphogenesis and ultrastructure of Geotrichum candidum septa. J Bacteriol. 1973 Oct;116(1):447–455. doi: 10.1128/jb.116.1.447-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Pollack J. H., Blumenthal H. J. Carotenogenesis associated with arthrosporulation of Trichophyton mentagrophytes. J Bacteriol. 1978 Dec;136(3):1120–1126. doi: 10.1128/jb.136.3.1120-1126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Wu-Yuan C. D., Blumenthal H. J. Isolation and characterization of the rodlet layer of Trichophyton mentagrophytes microconidial wall. J Bacteriol. 1976 Sep;127(3):1543–1549. doi: 10.1128/jb.127.3.1543-1549.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess W. M., Sassen M. M., Remsen C. C. Surface characteristics of Penicillum conidia. Mycologia. 1968 Mar-Apr;60(2):290–303. [PubMed] [Google Scholar]
- Hess W. M., Stocks D. L. Surface characteristics of Aspergillus conidia. Mycologia. 1969 May-Jun;61(3):560–571. [PubMed] [Google Scholar]
- Hill E. P. Effect of light on growth and sporulation of Aspergillus ornatus. J Gen Microbiol. 1976 Jul;95(1):39–44. doi: 10.1099/00221287-95-1-39. [DOI] [PubMed] [Google Scholar]
- Hill T. W., Mullins J. T. Hyphal tip growth in Achlya. I. Cytoplasmic organization. Can J Microbiol. 1980 Sep;26(9):1132–1140. doi: 10.1139/m80-187. [DOI] [PubMed] [Google Scholar]
- Hill T. W., Mullins J. T. Hyphal tip growth in Achlya. II. Subcellular localization of cellulase and associated enzymes. Can J Microbiol. 1980 Sep;26(9):1141–1146. doi: 10.1139/m80-188. [DOI] [PubMed] [Google Scholar]
- Hoch H. C., Maxwell D. P. Proteinaceous hexagonal inclusions in hyphae of Whetzelinia sclerotiorum and Neurospora crassa. Can J Microbiol. 1974 Jul;20(7):1029–1035. doi: 10.1139/m74-159. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. Cytoplasmic microtubules and fungal morphogenesis: ultrastructural effects of methyl benzimidazole-2-ylcarbamate determined by freeze-substitution of hyphal tip cells. J Cell Biol. 1980 Oct;87(1):55–64. doi: 10.1083/jcb.87.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. Effects of MBC on hyphal tip organization, growth, and mitosis of Fusarium acuminatum, and their antagonism by D2O. Protoplasma. 1977;92(3-4):195–210. doi: 10.1007/BF01279458. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. Hyphal tip cell ultrastructure of the fungus Fusarium: improved preservation by freeze-substitution. J Ultrastruct Res. 1979 Mar;66(3):224–234. doi: 10.1016/s0022-5320(79)90120-5. [DOI] [PubMed] [Google Scholar]
- Howard R. J. Ultrastructural analysis of hyphal tip cell growth in fungi: Spitzenkörper, cytoskeleton and endomembranes after freeze-substitution. J Cell Sci. 1981 Apr;48:89–103. doi: 10.1242/jcs.48.1.89. [DOI] [PubMed] [Google Scholar]
- Hunsley D., Gooday G. W. The structure and development of septa in Neurospora crassa. Protoplasma. 1974;82(1):125–146. doi: 10.1007/BF01276876. [DOI] [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Harrison J. L. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia. 1982 May 22;78(2):107–122. doi: 10.1007/BF00442634. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Control of development by ionic currents. Soc Gen Physiol Ser. 1979;33:199–231. [PubMed] [Google Scholar]
- Jaffe L. F. Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- Jansons V. K., Nickerson W. J. Chemical composition of chlamydospores of Candida albicans. J Bacteriol. 1970 Nov;104(2):922–932. doi: 10.1128/jb.104.2.922-932.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG Y. C., LEVINE H. B., SMITH C. E. Immunogenic properties of nondisrupted and disrupted spherules of Coccidioides immitis in mice. Sabouraudia. 1963 Feb;2:131–142. [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. C., Levine H. B. Experimentally induced immunity in the mycoses. Bacteriol Rev. 1967 Mar;31(1):35–53. doi: 10.1128/br.31.1.35-53.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzman G., Chet I., Henis Y. Localization of beta-(1,3)-glucanase in the mycelium of Sclerotium rolfsii. J Bacteriol. 1978 May;134(2):470–475. doi: 10.1128/jb.134.2.470-475.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künkel W., Hädrich H. Ultrastrukturelle Untersuchungen zur antimitotischen Aktivität von Methylbenzimidazol-2-ylcarbamat (MBC) und seinen Einfluss auf die Replikation des Kern-assoziierten Organells ("centriolar plaque", "MOTC", "KCE") bei Aspergillus nidulans. Protoplasma. 1977;92(3-4):311–323. doi: 10.1007/BF01279467. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B., KONG Y. C., SMITH C. IMMUNIZATION OF MICE TO COCCIDIOIDES IMMITIS: DOSE, REGIMEN AND SPHERULATION STAGE OF KILLED SPHERULE VACCINES. J Immunol. 1965 Jan;94:132–142. [PubMed] [Google Scholar]
- Lecara G., Cox R. A., Simpson R. B. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect Immun. 1983 Jan;39(1):473–475. doi: 10.1128/iai.39.1.473-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. B., Aukrust L., Salvaggio J. E. Respiratory allergy induced by fungi. Clin Chest Med. 1983 Jan;4(1):23–41. [PubMed] [Google Scholar]
- Levine H. B., Pappagianis D., Cobb J. M. Development of vaccines for coccidioidomycosis. Mycopathol Mycol Appl. 1970;41(1):177–185. doi: 10.1007/BF02051493. [DOI] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli S. D., Clutterbuck A. J. A quantitative survey of conidiation mutants in Aspergillus nidulans. J Gen Microbiol. 1971 Dec;69(2):261–268. doi: 10.1099/00221287-69-2-261. [DOI] [PubMed] [Google Scholar]
- McKeen W. E. Woronin bodies in Erysiphe graminis DC. Can J Microbiol. 1971 Dec;17(12):1557–1560. doi: 10.1139/m71-248. [DOI] [PubMed] [Google Scholar]
- Miller S. E., Spurlock B. O., Michaels G. E. Electron microscopy of young Candida albicans chlamydospores. J Bacteriol. 1974 Sep;119(3):992–999. doi: 10.1128/jb.119.3.992-999.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M., Nurse P., Thuriaux P., Mitchison J. M. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1979 Jan;137(1):440–446. doi: 10.1128/jb.137.1.440-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M., Kitamura J., Miyata H. Lysis of growing fissin-yeast cells induced by aculeacin A, a new antifungal antibiotic. Arch Microbiol. 1980 Aug;127(1):11–16. doi: 10.1007/BF00414349. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano J., Polacheck I., Duran A., Cabib E. An endochitinase from wheat germ. Activity on nascent and preformed chitin. J Biol Chem. 1979 Jun 10;254(11):4901–4907. [PubMed] [Google Scholar]
- Mullins J. T. A freeze-fracture study of hormone-induced branching in the fungus Achlya. Tissue Cell. 1979;11(3):585–595. doi: 10.1016/0040-8166(79)90064-8. [DOI] [PubMed] [Google Scholar]
- Müllbacher A., Waring P., Eichner R. D. Identification of an agent in cultures of Aspergillus fumigatus displaying anti-phagocytic and immunomodulating activity in vitro. J Gen Microbiol. 1985 May;131(5):1251–1258. doi: 10.1099/00221287-131-5-1251. [DOI] [PubMed] [Google Scholar]
- Najim L., Turian G. Conidiogenous loss of structuro-functional polarity in the hyphal tips of Sclerotinia fructigena. Eur J Cell Biol. 1979 Oct;20(1):24–27. [PubMed] [Google Scholar]
- Nimmich W. Occurrence of 3-O-methylmannose in lipopolysaccharides of Klebsiella and Escherichia coli. Biochim Biophys Acta. 1970 Jul 21;215(1):189–191. doi: 10.1016/0304-4165(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Nolan R. A., Bal A. K. Cellulase localization in hyphae of Achlya ambisexualis. J Bacteriol. 1974 Feb;117(2):840–843. doi: 10.1128/jb.117.2.840-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Morphogenesis in Candida albicans. Crit Rev Microbiol. 1985;12(1):45–93. doi: 10.3109/10408418509104425. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G. The parasexual cycle in fungi. Annu Rev Microbiol. 1956;10:393–400. doi: 10.1146/annurev.mi.10.100156.002141. [DOI] [PubMed] [Google Scholar]
- Pancaldi S., Poli F., Dall'Olio G., Vannini G. L. Morphological anomalies induced by Congo red in Aspergillus niger. Arch Microbiol. 1984 Mar;137(3):185–187. doi: 10.1007/BF00414540. [DOI] [PubMed] [Google Scholar]
- Pappagianis D., Hector R., Levine H. B., Collins M. S. Immunization of mice against coccidioidomycosis with a subcellular vaccine. Infect Immun. 1979 Jul;25(1):440–445. doi: 10.1128/iai.25.1.440-445.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Rosenberger R. F. Distribution of autolysins in hyphae of Aspergillus nidulans: evidence for a lipid-mediated attachment to hyphal walls. J Bacteriol. 1978 Sep;135(3):741–747. doi: 10.1128/jb.135.3.741-747.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. F., Scheer E. R., Wheat R. W. Characterization of 3-O-methylmannose from Coccidioides immitis. Infect Immun. 1971 Nov;4(5):660–661. doi: 10.1128/iai.4.5.660-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudaskoski M., Koltin Y. Ultrastructural aspects of a mutant of Schizophyllum commune with continuous nuclear migration. J Bacteriol. 1973 Nov;116(2):981–988. doi: 10.1128/jb.116.2.981-988.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudaskoski M. Occurrence of microtubules in the hyphae of Schizophyllum commune during intercellular nuclear migration. Arch Mikrobiol. 1972;86(2):91–100. doi: 10.1007/BF00413365. [DOI] [PubMed] [Google Scholar]
- Regúlez P., Pontón J., Domínguez J. B., Goñi F. M., Uruburu F. Lipid composition and the transition from yeast-like to chlamydospore cells of Pullularia pullulans. Can J Microbiol. 1980 Dec;26(12):1428–1437. doi: 10.1139/m80-238. [DOI] [PubMed] [Google Scholar]
- Reiss E., Huppert M., Cherniak R. Characterization of protein and mannan polysaccharide antigens of yeasts, moulds, and actinomycetes. Curr Top Med Mycol. 1985;1:172–207. doi: 10.1007/978-1-4613-9547-8_7. [DOI] [PubMed] [Google Scholar]
- Reiss E., Nickerson W. J. Control of dimorphism in Phialophora verrucosa. Sabouraudia. 1974 Jul;12(2):202–213. [PubMed] [Google Scholar]
- Riggsby W. S. Some recent developments in the molecular biology of medically important Candida. Microbiol Sci. 1985 Sep;2(9):257-8, 261-3. [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow C. F., Caten C. E. Mitosis in Aspergillus nidulans. J Cell Sci. 1969 Sep;5(2):403–431. doi: 10.1242/jcs.5.2.403. [DOI] [PubMed] [Google Scholar]
- Robinow C. F., Marak J. A fiber apparatus in the nucleus of the yeast cell. J Cell Biol. 1966 Apr;29(1):129–151. doi: 10.1083/jcb.29.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S., Winnik H. Z. Motivation for the addiction to amphetamine and reducing drugs. Isr Ann Psychiatr Relat Discip. 1969 Nov;7(2):213–222. [PubMed] [Google Scholar]
- Roobol A., Gull K., Pogson I. Evidence that griseofulvin binds to a microtubule associated protein. FEBS Lett. 1977 Mar 15;75(1):149–153. doi: 10.1016/0014-5793(77)80073-2. [DOI] [PubMed] [Google Scholar]
- San-Blas G. Paracoccidioides brasiliensis: cell wall glucans, pathogenicity, and dimorphism. Curr Top Med Mycol. 1985;1:235–257. doi: 10.1007/978-1-4613-9547-8_9. [DOI] [PubMed] [Google Scholar]
- San-Blas G. The cell wall of fungal human pathogens: its possible role in host-parasite relationships. Mycopathologia. 1982 Sep 17;79(3):159–184. doi: 10.1007/BF01837196. [DOI] [PubMed] [Google Scholar]
- Saëz H. Formation d'endospores chez Geotrichum candidum. Ann Parasitol Hum Comp. 1969 Mar-Apr;44(2):197–204. doi: 10.1051/parasite/1969442197. [DOI] [PubMed] [Google Scholar]
- Schneider E. F., Seaman W. L. Development of conidial chlamydospores of Fusarium sulphureum in distilled water. Can J Microbiol. 1974 Feb;20(2):247–254. doi: 10.1139/m74-039. [DOI] [PubMed] [Google Scholar]
- Schneider E. F., Seaman W. L. Ontogeny of lipid bodies in the endoplasmic reticulum of Fusarium sulphureum. Can J Microbiol. 1977 Feb;23(2):190–196. doi: 10.1139/m77-027. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M., Costerton J. W. Microcycle conidiation in Penicillium urticae: an ultrastructural investigation of spherical spore growth. Can J Microbiol. 1975 Dec;21(12):2048–2058. doi: 10.1139/m75-294. [DOI] [PubMed] [Google Scholar]
- Selitrennikoff C. P., Nelson R. E., Siegel R. W. Phase-specific genes for macroconidiation in Neurospora crassa. Genetics. 1974 Oct;78(2):679–690. doi: 10.1093/genetics/78.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloat B. F., Adams A., Pringle J. R. Roles of the CDC24 gene product in cellular morphogenesis during the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1981 Jun;89(3):395–405. doi: 10.1083/jcb.89.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smail E. H., Jones J. M. Demonstration and solubilization of antigens expressed primarily on the surfaces of Candida albicans germ tubes. Infect Immun. 1984 Jul;45(1):74–81. doi: 10.1128/iai.45.1.74-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. T., Batenburg-Van der Vegte W. H. Ultrastructure in septa in Blastobotrys and Sporothrix. Antonie Van Leeuwenhoek. 1985;51(1):121–128. doi: 10.1007/BF00444233. [DOI] [PubMed] [Google Scholar]
- Smucker R. A., Pfister R. M. Characteristics of Streptomyces coelicolor A3(2) aerial spore rodlet mosaic. Can J Microbiol. 1978 Apr;24(4):397–408. doi: 10.1139/m78-066. [DOI] [PubMed] [Google Scholar]
- Soll D. R. The role of zinc in Candida dimorphism. Curr Top Med Mycol. 1985;1:258–285. doi: 10.1007/978-1-4613-9547-8_10. [DOI] [PubMed] [Google Scholar]
- Staples R. C. The development of infection structures by the rusts and other fungi. Microbiol Sci. 1985 Jul;2(7):193-4, 197-8. [PubMed] [Google Scholar]
- Steele S. D., Fraser T. W. The ultrastructure of Geotrichum candidum hyphae. Can J Microbiol. 1973 Dec;19(12):1507–1512. doi: 10.1139/m73-245. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L., Becker S. A. The fine structure of mature and germinating chlamydospores of Fusarium oxysporum. Can J Microbiol. 1979 Jul;25(7):808–817. doi: 10.1139/m79-119. [DOI] [PubMed] [Google Scholar]
- Stipanovic R. D., Bell A. A. Pentaketide metabolites of Verticillium dahliae. 3. Identification of (-)-3,4-dihydro-3,8-dihydroxy-1(2h)-naphtalenone((-)-vermelone) as a precursor to melanin. J Org Chem. 1976 Jul 9;41(14):2468–2469. doi: 10.1021/jo00876a026. [DOI] [PubMed] [Google Scholar]
- Stipanovic R. D., Bell A. A. Pentaketide metabolites of Verticillium dahliae. II. Accumulation of naphthol derivatives by the aberrant-melanin mutant BRM-2. Mycologia. 1977 Jan-Feb;69(1):164–172. [PubMed] [Google Scholar]
- Sun S. H., Huppert M. A cytological study of morphogenesis in Coccidioides immitis. Sabouraudia. 1976 Jul;14(2):185–198. [PubMed] [Google Scholar]
- Sun S. H., Huppert M., Vukovich K. R. Rapid in vitro conversion and identification of Coccidioides immitis. J Clin Microbiol. 1976 Feb;3(2):186–190. doi: 10.1128/jcm.3.2.186-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. H., Sekhon S. S., Huppert M. Electron microscopic studies of saprobic and parasitic forms of Coccidioides immitis. Sabouraudia. 1979 Sep;17(3):265–273. doi: 10.1080/00362177985380391. [DOI] [PubMed] [Google Scholar]
- That T. C., Turian G. Ultrastructural study of microcyclic macroconidiation in Neurospora crassa. Arch Microbiol. 1978 Mar;116(3):279–288. doi: 10.1007/BF00417852. [DOI] [PubMed] [Google Scholar]
- Turian G., Oulevey N., Cortat M. Recherches sur la différenciation conidienne de Neurospora crassa. V. Ultrastructure de la sequence macroconidiogène. Ann Microbiol (Paris) 1973 Jun;124A(4):443–458. [PubMed] [Google Scholar]
- WHITE L. P. Melanin: a naturally occurring cation exchange material. Nature. 1958 Nov 22;182(4647):1427–1428. doi: 10.1038/1821427a0. [DOI] [PubMed] [Google Scholar]
- Ward E. R., Jr, Cox R. A., Schmitt J. A., Jr, Huppert M., Sun S. H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975 Nov;12(5):1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli E., Scherrer P., Kübler O. The crystalline layers in spores of Bacillus cereus and Bacillus thuringiensis studied by freeze-etching and high resolution electron microscopy. Eur J Cell Biol. 1980 Feb;20(3):283–289. [PubMed] [Google Scholar]
- Weisberg S. H., Turian G. Ultrastructure of Aspergillus nidulans conidia and conidial lomasomes. Protoplasma. 1971;72(1):55–67. doi: 10.1007/BF01281011. [DOI] [PubMed] [Google Scholar]
- Wergin W. P. Development of Woronin bodies from microbodies in Fusarium oxysporum f.sp. lycopersici. Protoplasma. 1973;76(2):249–260. doi: 10.1007/BF01280701. [DOI] [PubMed] [Google Scholar]
- Wessels J. G., Kreger D. R., Marchant R., Regensburg B. A., De Vries O. M. Chemical and morphological characterization of the hyphal wall surface of the basidiomycete Schizophyllum commune. Biochim Biophys Acta. 1972 Jul 19;273(2):346–358. doi: 10.1016/0304-4165(72)90226-7. [DOI] [PubMed] [Google Scholar]
- Wheat R. W., Woodruff W. W., 3rd, Haltiwanger R. S. Occurrence of antigenic (species-specific?) partially 3-O-methylated heteromannans in cell wall and soluble cellular (nonwall) components of Coccidioides immitis mycelia. Infect Immun. 1983 Aug;41(2):728–734. doi: 10.1128/iai.41.2.728-734.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat R., Scheer E. Cell walls of Coccidioides immitis: neutral sugars of aqueous alkaline extract polymers. Infect Immun. 1977 Jan;15(1):340–341. doi: 10.1128/iai.15.1.340-341.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. H., Stipanovic R. D. Melanin biosynthesis and the metabolism of flaviolin and 2-hydroxyjuglone in Wangiella dermatitidis. Arch Microbiol. 1985 Aug;142(3):234–241. doi: 10.1007/BF00693396. [DOI] [PubMed] [Google Scholar]
- Wheeler M. H., Tolmsoff W. J., Bell A. A., Mollenhauer H. H. Ultrastructural and chemical distinction of melanins formed by Verticillium dahliae from (+)-scytalone, 1,8-dihydroxynaphthalene, catechol, and L-3,4-dihydroxyphenylalanine. Can J Microbiol. 1978 Mar;24(3):289–297. doi: 10.1139/m78-049. [DOI] [PubMed] [Google Scholar]
- Wheeler M. H., Tolmsoff W. J., Meola S. Ultrastructure of melanin formation in Verticillium dahliae with (+)-scytalone as a biosynthetic intermediate. Can J Microbiol. 1976 May;22(5):702–711. doi: 10.1139/m76-103. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Variable nature of the bacterial cell surface. Aust J Biol Sci. 1984;37(5-6):315–322. doi: 10.1071/bi9840315. [DOI] [PubMed] [Google Scholar]
- Wilsenach R., Kessel M. The role of lomasomes in wall formation in Penicillium vermiculatum. J Gen Microbiol. 1965 Sep;40(3):401–404. doi: 10.1099/00221287-40-3-401. [DOI] [PubMed] [Google Scholar]
- Wu-Yuan C. D., Hashimoto T. Architecture and chemistry of microconidial walls of Trichophyton mentagrophytes. J Bacteriol. 1977 Mar;129(3):1584–1592. doi: 10.1128/jb.129.3.1584-1592.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Hiratani T., Iwata K., Yamamoto Y. Studies on the mechanism of antifungal action of aculeacin A. J Antibiot (Tokyo) 1982 Feb;35(2):210–219. doi: 10.7164/antibiotics.35.210. [DOI] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., de Souza E. R., Mullaney E. J., Timberlake W. E. Developmental regulation of the Aspergillus nidulans trpC gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7576–7580. doi: 10.1073/pnas.80.24.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariah K., Metitiri P. O. The effect of mutation on cell proliferation and nuclear behaviour in Penicillium claviforme Bainier. Protoplasma. 1970;69(3):331–339. doi: 10.1007/BF01320298. [DOI] [PubMed] [Google Scholar]
- van Eck W. H. Lipid body content and persistence of chlamydospores of Fusarium solani in soil. Can J Microbiol. 1978 Jan;24(1):65–69. doi: 10.1139/m78-012. [DOI] [PubMed] [Google Scholar]