Abstract
Intraguild predation theory centres on two predictions: (i) for an omnivore and an intermediate predator (IG-prey) to coexist on shared resources, the IG-prey must be the superior resource competitor, and (ii) increasing resource productivity causes the IG-prey's equilibrium abundance to decline. I tested these predictions with a series of species-rich food webs along New Zealand's rocky shores, focusing on two predatory whelks, Haustrum haustorium, a trophic omnivore, and Haustrum scobina, the IG-prey. In contrast to theory, the IG-prey's abundance increased with productivity. Furthermore, feeding rates and allometric considerations indicate a competitive advantage for the omnivore when non-shared prey are considered, despite the IG-prey's superiority for shared prey. Nevertheless, clear and regular cross-gradient changes in network structure and interaction strengths were observed that challenge the assumptions of current theory. These insights suggest that the consideration of consumer-dependent functional responses, non-equilibrium dynamics, the dynamic nature of prey choice and non-trophic interactions among basal prey will be fruitful avenues for theoretical development.
Keywords: interaction strength, per capita attack rate, alternative prey, handling times, adaptive foraging, consumer-dependence
1. Introduction
Trophic omnivores—species that feed at multiple trophic levels—are central to our understanding of food webs. Their ubiquity in food webs complicates the predictive power of trophic cascades and undermines the utility of the trophic level concept itself [1]. This is particularly true when omnivores engage in intraguild predation (IGP) by feeding on a second consumer with whom they share prey [2].
IGP theory offers several predictions regarding the mechanisms governing species coexistence and how community structure should change with enrichment [3,4]. Two predictions have been a focal point: (i) for three species—an omnivore, an intermediate predator and a shared prey—to coexist at equilibrium, the intermediate predator (IG-prey) must be the superior competitor for the shared prey, and (ii) that when all three species coexist, increasing productivity in the resource causes the IG-prey's equilibrium abundance to decline. Models that include refuges for the IG-prey using predation-free time periods, prey vigilance or nonlinear functional responses, as well as models incorporating stage-structured life-history omnivory, or consumer-specific differences in prey quality, diet requirements or mortality rates, may prevent the high-productivity extinction of IG-prey, but, by and large, do not alter the qualitative predictions for how equilibrium species abundance should change with enrichment (for exceptions, see section Discussion and the review of models in the electronic supplementary material, appendix S1).
Theory on IGP has far outpaced its empirical assessment. A meta-analysis of manipulative experiments indicates that IG-prey typically are superior competitors, reducing the shared prey's abundance to lower levels than the omnivore [5]. However, the relevance of these transient time-scale experiments to models of long-term conditions has been questioned [4,6]. A number of studies testing equilibrium conditions have also shown the IG-prey to depress the shared prey's abundance more so than the omnivore, and have observed a decline in the IG-prey's abundance with enrichment [7], but other such studies have not [8,9].
A greater question is: whether models and experiments of tightly coupled three-species IGP modules capture the complexities of natural, species-rich communities where omnivory is more diffuse. Theory has begun to address this issue by adding two alternative prey to the basic three-species IGP module [10,11]. Alternative prey exclusive to the omnivore strengthen the predictions of three-species models, requiring a greater competitive advantage for the IG-prey and decreasing the productivity range over which coexistence is feasible. Prey exclusive to the IG-prey, on the other hand, can make unnecessary the IG-prey's competitive superiority on the shared prey and permit indefinite coexistence at high productivity. However, the IG-prey must still be the overall superior competitor [11,12], and enrichment remains favourable to the omnivore such that the IG-prey's equilibrium abundance still declines [10].
Here, I assess the robustness of current IGP theory by testing its two focal predictions with a series of food webs situated along a strong gradient of productivity on New Zealand's rocky shores. I focused my study on two dominant predatory whelks, Haustrum haustorium, the trophic omnivore, and Haustrum scobina, the IG-prey, and collected data to document the structure and strengths of their trophic interactions. In conjunction with allometric considerations of the two species' mortality rates, my results offer little support for the predictive power of current IGP theory in species-rich communities, showing the abundance of the IG-prey to increase with enrichment and the omnivore to have the competitive advantage when both shared and unshared prey are considered. Insights afforded by the observational approach of the study offer additional evidence countering the mechanisms of the few models in which increases in the IG-prey's abundance with enrichment have been observed. Nevertheless, my data reveal clear and remarkably regular cross-gradient changes in the structure and strengths of trophic interactions which theory has largely assumed are constant. These insights indicate that the consideration of consumer-dependent functional responses, non-equilibrium dynamics, optimal foraging decisions on evolutionary scales and non-trophic interactions among basal species may be important for furthering our understanding of omnivorous food webs.
2. Methods
To document cross-productivity changes in community and food web structure, and to assess the relative competitive superiority of H. haustorium and H. scobina, I determined species abundances, each whelk's diet and the richness of their potential prey pool, as well as their prey-specific handling times, per capita attack rates and feeding rates (these being primary determinants of a species pair's trophic interaction strength [13,14]). I did this at six study sites on New Zealand's South Island around which Menge and colleagues have documented a strong gradient of effective basal productivity as expressed in the growth and recruitments rates of the primary prey shared between the two whelks: barnacles and mussels (see [15,16] and electronic supplementary material, appendix S2 for system details). Two sites were chosen within each of three productivity levels: two low-productivity east coast sites (PP, Paia Point and Rk, Rakautara), two mid-productivity southwest coast sites (JH, Jackson Head and OP, Okahu Point) and two high-productivity northwest coast sites (TH, Tauranga Head and CF, Cape Foulwind).
Consistent with theoretical models of IGP, the framework I assumed was that competitive superiority could be achieved by several non-exclusive mechanisms: having sufficiently (i) shorter handling times or (ii) higher per capita attack rates on shared prey species, either of which could lead to higher feeding rates, (iii) higher feeding rates on non-shared alternative prey, (iv) a lower total rate of mortality or (v) a higher prey-to-predator conversion rate. The field and laboratory methods I used to estimate these variables were as follows, with additional details provided in [17] and the electronic supplementary material, appendix S2. Lacking system-specific empirical estimates for the latter two variables, I used analytical means to solve for possible values that would confer the predicted overall advantage on the IG-prey and thereby make coexistence theoretically feasible.
(a). Consumer diets
I determined each whelk's diet by conducting systematic searches of predefined areas at each site. Surveys were performed seasonally during day- and night-time low tides between 2004 and 2007 (see electronic supplementary material, table S2.2). I measured and carefully examined all individuals to determine whether or not they were feeding and recorded the identity and size of all prey items.
(b). Community structure
I estimated the density and biomass of whelks and their putative prey using quadrat surveys repeated three times between 2005 and 2007 (see electronic supplementary material, table S2.3). Mobile species were counted directly, whereas percentage-cover estimates of sessile species were converted to densities using site- and species-specific cover–count relationships (see electronic supplementary material, appendix S3). Densities were converted to biomass estimates using concurrently performed size-frequency surveys and allometric relationships between size and wet weight (see electronic supplementary material, appendix S3). Cross-gradient changes in abundance were assessed within the five-species framework of IGP theory by assigning prey into three groups—the core-shared prey, the omnivore's alternative prey and the IG-prey's alternative prey—using their presence in the two consumers' diets at the high-productivity sites (see electronic supplementary material, appendix S2). The prey shared at high productivity were a subset of those shared at other productivity levels.
(c). Handling times
To estimate the handling time of each feeding event observed in the field, I measured the temperature-dependent time required for a whelk of a given size to drill and ingest a prey item of a given size in the laboratory. This was carried out by housing whelks individually in aquaria, providing them focal prey, and subsequently classifying them as either feeding or not feeding on a near hourly basis or with video surveillance. Whelk and prey size combinations were varied to maximize the range of relative sizes. Temperatures varied between 10 and 18°C. Because monitoring was not continuous, exact handling times were unknown. I therefore regressed feeding duration midpoints on predator size, prey size and temperature using log-transformed data weighted by the inverse of the difference between the minimum and maximum possible duration [17]. Laboratory-based regression coefficients were used to calculate the expected handling time of feeding events observed in the field using whelk and prey identity and sizes and the mean field temperature observed during the month of a feeding survey.
(d). Interaction strengths
Per capita attack rates (cij)—the number of prey eaten per predator per prey per m2 per day—are an absolute measure of a predator's prey preferences [18] and are a primary determinant of trophic interaction strengths, both in models and their empirical measurement [13,14]. In this study, per capita attack rates were estimated using the observational method of Novak & Wootton [19], the efficacy of which has been substantiated by independent manipulative experiments [17] and stable isotopes [20]. The method estimates attack rates by
| 2.1 |
where Fij is the proportion of feeding predator j individuals observed to be feeding on the focal prey i, Axj is the proportion of all predator individuals (feeding and not feeding) observed to be feeding on prey species x, hij is the mean estimated field handling time of the focal predator–prey pair and Ni is the focal prey's mean density. I used the species observed most frequently in a predator's diet at a site as prey x, although the choice is arbitrary as long as the same species is used for all calculations [17,19].
Feeding rates (fij)—the grams of prey consumed per predator per day—were calculated as
| 2.2 |
where wij is the mean weight of the prey i individuals fed on by predator j, estimated from observed prey sizes using allometric relationships (see electronic supplementary material, appendix S3). Equation (2.2) reflects the multispecies type II functional response on which the derivation of equation (2.1) is based [19].
(e). Competitive superiority
In most three-species models of IGP theory, the competitive superiority of the IG-prey is conferred by a higher attack rate or a shorter handling time on the shared prey, rather than by differences in the two predator's conversion or mortality rates. I therefore contrasted my estimates of these variables between the two whelks for the set of prey species on which they were both observed feeding at all levels of productivity. Owing to potential differences in prey size selectivity, I also compared the two whelks by their feeding rates with these prey.
While a direct comparison of each consumer's attack rates and handling times on shared prey permits insights into the mechanisms of their potential competitive superiority, this assumes both that non-shared prey are unimportant and that predator mortality and prey-to-predator conversion efficiencies do not differ. Indeed, five-species models including alternative prey demonstrate that the IG-prey need not be the superior competitor for shared prey as long as the supplements it receives from its own alternative prey are sufficiently large [10–12]. Specifically, the IG-prey is the overall superior competitor only when
| 2.3 |
(see the electronic supplementary material, appendix S4) where fij is predator j's feeding rate summed across all species in prey group i (P, IG-prey; O, omnivore; S, shared prey; A, alternative prey; see electronic supplementary material, tables S3.1 and S3.2), wij is the weight of prey i individuals eaten by predator j, ej is the predator's conversion rate (number of predators produced per gram of prey consumed), Nj is its density and mj is its mortality rate that includes both intrinsic mortality and that due to unconsidered top predators. That is, for the IG-prey to have the overall competitive advantage, the difference between its gains from feeding on alternative prey and its total mortality losses must be greater than the difference between the omnivore's gains from feeding and its mortality losses, relative to their respective gains from feeding on shared prey.
Having obtained site-specific estimates for all but the conversion and mortality rates, I estimated the omnivore's mortality rates using an allometric relationship that is general to invertebrates [21], solved for its conversion rate assuming steady-state conditions, and determined how much smaller the mortality rate or larger the conversion rate of the IG-prey would have to be for the IG-prey to achieve overall competitive superiority at each site (see electronic supplementary material, appendix S4). This allowed me to evaluate the biological likelihood that the IG-prey's superiority (equation (2.3)) could be achieved. For example, given physiological constraints and the congeneric status of the two whelks, it is unlikely that their conversion rates differ greatly [22]. Similarly, given the much larger size of H. haustorium (see electronic supplementary material, appendix S1) and the negative relationship between body size and mortality rates [21], it is unlikely that the omnivore's mortality rate would exceed that of the IG-prey.
(f). Food web structure
Finally, to gain insights into the discrepancies I observed with IGP theory's predictions, I investigated cross-gradient changes in food web structure and per capita attack rates. Theory has largely assumed these to be constant. I did this within the framework of five-species IGP models by categorizing prey into core-shared and alternative prey groups according to each consumer's high-productivity diet, as I had performed when assessing community structure.
3. Results
(a). Consumer diets
Across all sites, an average of 9.3% of H. haustorium individuals (0–41%, 2142 of 21 028 individuals) and 21.0% of H. scobina individuals (0–49%, 3526 of 17 293) were observed in the act of feeding (see electronic supplementary material, appendix S3). Overall, H. scobina's diet consisted of 19 species, whereas H. haustorium's diet consisted of 44 species, including H. scobina at all sites and its own juveniles at the low-productivity sites. Sampling effort was sufficient to characterize diet diversity and to permit the accurate estimation of the attack rates [19] (see electronic supplementary material, figure S2.2). Mussels and barnacles comprised 28% of the feeding observations made for H. haustorium and 89% of the observations made for H. scobina (see electronic supplementary material, appendix S3). However, diet richness varied markedly across sites, with both whelks exhibiting maximum diet richness at low-productivity sites (see electronic supplementary material, figure S2.2). Haustrum scobina's diet richness decreased monotonically with productivity, consisting of half as many species at high-productivity than at low-productivity.
(b). Community structure
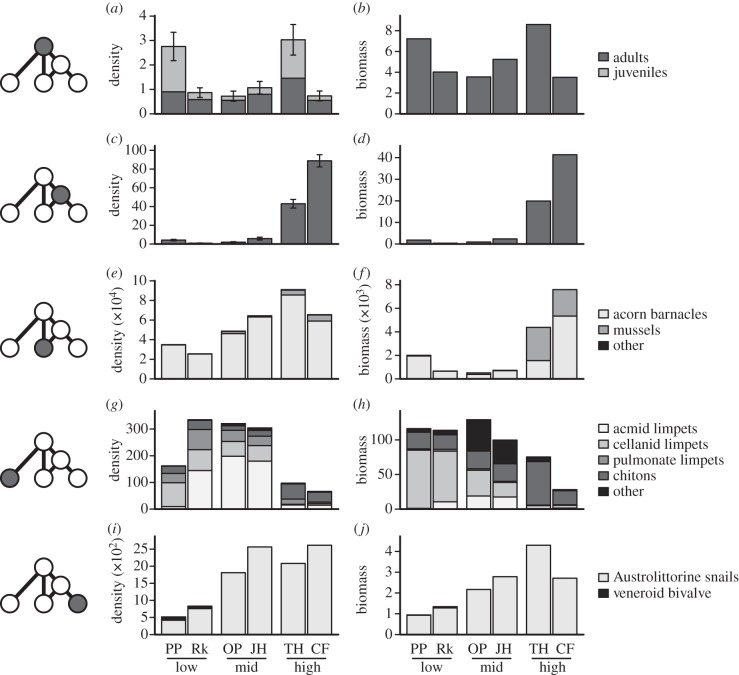
The abundance of the omnivore (H. haustorium) did not change directionally across the gradient, with site-to-site variation driven largely by differences in the number of juveniles (figure 1). By contrast, the abundance of the IG-prey (H. scobina) changed dramatically, with densities being up to 110 times higher at high- than at low-productivity sites. The abundance of core-shared prey also increased with productivity, particularly in the density and biomass of barnacles and mussels. The abundance of the omnivore's alternative prey was similar at low- and mid-productivity sites but decreased markedly at high-productivity, whereas the abundance of the IG-prey's alternative prey increased from low- to mid-productivity and was similar at mid- and high-productivity sites. Despite these cross-gradient changes in species abundances, the estimated richness of the potential prey pool remained constant (see electronic supplementary material, figure S2.4), with only eight of 46 species not being observed at all sites.
Figure 1.
Cross-gradient changes in the mean density (m−2) and biomass (g m−2) of (a,b) the omnivore, Haustrum haustorium; (c,d) the IG-prey, Haustrum scobina; (e,f) their core-shared prey; (g,h) the omnivore's alternative prey and (i,j) the IG-prey's alternative prey. Omnivore densities are split into adults and juveniles (less than or equal to 25 mm shell length, see electronic supplementary material, appendix S2). Error bars indicate±1 s.e.
(c). Competitive superiority
The IG-prey's cumulative feedings rates on core-shared prey exceeded those of the omnivore (see electronic supplementary material, figure S2.6), thereby conferring it a competitive advantage for these prey. It achieved this advantage largely by exhibiting significantly higher per capita attack rates and, to a lesser extent, shorter handling times on barnacles and mussels, particularly the two most abundant species (Chamaesipho columna and Xenostrobus pulex; electronic supplementary material, figure S2.6).
By contrast, consideration of the gains from alternative prey and total mortality losses indicates that the IG-prey was not the overall superior competitor at any site (figure 2). In fact, in order to achieve competitive superiority—such that the difference between the IG-prey's feeding gains and mortality losses exceeded that of the omnivore's, relative to their respective gains from shared prey—would require the IG-prey to exhibit a combination of significantly lower mortality (mP < mO) and higher conversion rates (eP > eO). The inferred minimum magnitude of these required mortality and conversion rate advantages for the IG-prey increased with enrichment. Thus, for example, theory would predict a feasible coexistence given equal mortality rates, only if the IG-prey's conversion rate were at least six times greater than the omnivore's at mid- and high-productivity sites. Similarly, coexistence would be predicted given equal conversion rates, only if the omnivore's mortality rate were at least an order-of-magnitude greater than the IG-prey's at these sites. By contrast, allometric expectations [21], based on their respective site-specific mean body sizes, are for the IG-prey's mortality rate to be 1.36–1.58 times higher than the omnivore's.
Figure 2.
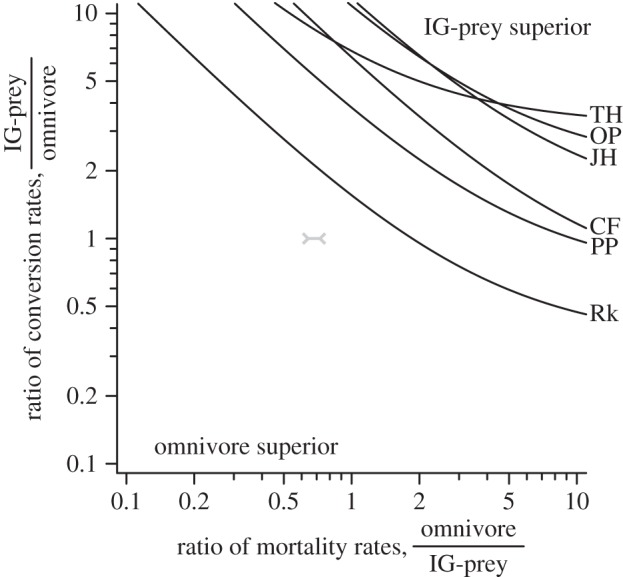
IGP theory predicts that coexistence is feasible when the IG-prey is the overall superior competitor; the difference between its feeding gains and mortality losses exceeding that of the omnivore's, relative to their respective gains from shared prey (equation (2.3)). Because Haustrum haustorium and Haustrum scobina's conversion and mortality rates are unknown, each curve reflects the values of their relative conversion and mortality rates required for neither predator to have the competitive advantage given their estimated feeding rates; regions above each curve indicate the IG-prey's overall superiority, whereas regions below each curve indicate the omnivore's overall superiority. The most likely true range of mortality rate ratios given allometric expectations [21] is also indicated.
(d). Food web structure and interaction strengths
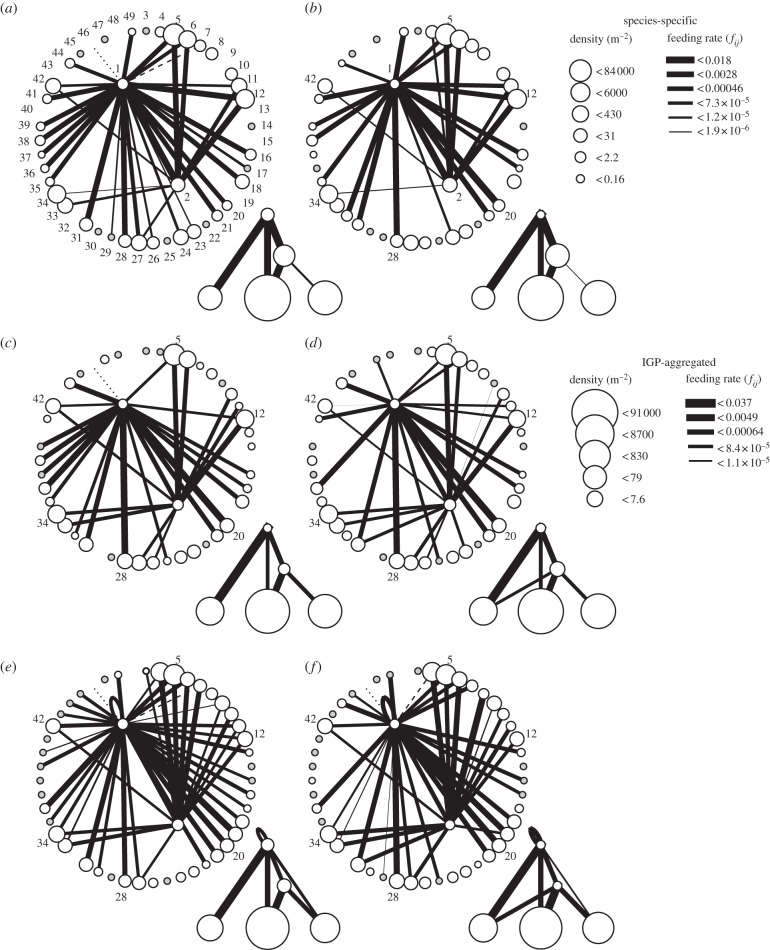
Food web structure changed in a directional manner across the productivity gradient (figure 3). Within the five-species framework of IGP theory, the simplest structure was observed at two high- and one mid-productivity site. At these sites, the omnivore fed on the IG-prey, both fed on shared prey, and each fed on its own alternative prey. Food web complexity then increased as productivity decreased, with the IG-prey first adding prey previously exclusive to the omnivore at mid-productivity, and the omnivore consuming prey previously exclusive to the IG-prey and engaging in cannibalism at low productivity.
Figure 3.
Cross-gradient changes in species-specific and IGP-aggregated food web structure, densities and feeding rates (grams of prey per predator per day) across sites of high (a, TH; b, CF), mid (c, OP; d, JH) and low (e, PP; f, Rk) shared-prey productivity. Grey-filled circles indicate species present at a site but not observed during systematic abundance surveys. Species identification codes: 1, omnivore; 2, IG-prey. See electronic supplementary material, table S2.5 for additional identification codes and IGP-aggregation assignments.
Similarly, although variation in group-summed per capita attack rates spanned over four orders-of-magnitude, the attack rates of all but one set of interactions were either constant or changed in a directional manner across the gradient (figure 4). The IG-prey's attack rates on its alternative prey showed the largest change, decreasing by nearly three orders-of-magnitude from low- to high-productivity (figure 4h). Its attack rates on core-shared prey declined 20-fold from mid- to high-productivity (figure 4f). Conversely, the omnivore's attack rates on alternative prey increased 20-fold between low- and high-productivity (figure 4c), whereas its attack rate on the IG-prey remained constant (figure 4e). Only the omnivore's attack rates on core-shared prey exhibited a non-directional change across the gradient, being two orders of magnitude higher at one mid-productivity site than at the other sites (figure 4b).
Figure 4.

Cross-gradient changes in the per capita attack rates of the omnivore on (a) itself, (b) the shared prey, (c) its own alternative prey, (d) the IG-prey's alternative prey and (e) the IG-prey, and similarly, of the IG-prey on (f) the shared prey, (g) the omnivore's alternative prey and (h) its own alternative prey. Note the variable scales on the y-axes. Prey species not observed in systematic abundance surveys excluded (see electronic supplementary material, appendix S2).
4. Discussion
IGP theory predicts that the IG-prey must be a superior competitor to the omnivore for coexistence to be feasible. The IG-prey's competitive superiority is necessary to compensate for the additional predation pressure it incurs from the omnivore. With few exceptions, IGP models also predict that the IG-prey's equilibrium abundance will decline as the productivity of the shared prey increases. This occurs through a shift in the balance of stronger competitive effects at low productivity to stronger top-down effects of predation at high productivity.
In this study, the IG-prey, H. scobina, demonstrated a competitive advantage over the omnivore, H. haustorium, for the core prey species they shared. It exhibited this advantage by having generally higher attack rates, lower handling times and, as a result, higher feeding rates on these prey species. However, given estimated feeding rates when both shared and non-shared prey are considered would require the IG-prey to exhibit a combination of significantly higher conversion rates and lower mortality rates than the omnivore in order for it to achieve its predicted overall competitive superiority. Because the two whelk's physiologically governed conversion rates are unlikely to differ significantly given their congeneric relationship and the similarity of their life histories and ecologies, and because allometric expectations are for the omnivore's mortality rates to be lower than that of the IG-prey's, the predicted overall superiority of the IG-prey seems improbable. In further contrast to IGP theory's predictions, the IG-prey's abundance increased, rather than decreased, across the gradient of increasing productivity in the prey species the two predators share.
Only a few published models produce predictions consistent with these observations of the New Zealand system (see electronic supplementary material, appendix S1). The mechanisms these models incorporate limit the omnivore's top-down control of the IG-prey by having the omnivore exhibit either movement between spatially heterogeneous productivity levels, cannibalism, adaptive foraging, a consumer-dependent functional response or by non-equilibrium population dynamics. As described below, these mechanisms fail to explain H. haustorium's apparent competitive advantage or are largely inconsistent with the biology of the study system. Moreover, each of these models is inconsistent with the cross-gradient changes in food web structure and per capita attack rates I observed. These empirical patterns, however, do offer direction for further theoretical development.
(a). Equilibrium conditions
First, Amarasekare [23,24] has shown that the IG-prey's abundance may increase with enrichment in three-species metacommunity patch dynamic models. This occurs when the omnivore emigrates from high-productivity patches at high enough rates to preclude it from controlling the more slowly dispersing IG-prey. The IG-prey must still be the superior within-patch competitor. The crawl-away larval life history of the two Haustrum species, the large distances between New Zealand's productivity regimes and the observation that populations of similar, Northern Hemisphere whelks show low levels of gene flow [25], indicate that this hypothesis is unfitting to the New Zealand system.
Second, Rudolf [26] has shown the IG-prey's equilibrium abundance to increase with enrichment when the omnivore engages in size-structured cannibalism. This mechanism also permits the omnivore to be the superior competitor. However, in order to limit its top-down effect at high productivity, the omnivore must exhibit higher cannibalistic attack rates than on the IG-prey. While H. haustorium did exhibit cannibalisms, cannibalistic attack rates were higher than attack rates on H. scobina at only one low-productivity site (cf. figure 4a,e) and did not occur at the mid- and high-productivity sites where this mechanism would need to be strongest to explain the patterns observed in New Zealand. Age-structured models lacking cannibalism do not show increases in the IG-prey's equilibrium abundance (see electronic supplementary material, appendix S1).
Third, Křivan and co-workers [27–29] have shown the equilibrium abundance of the IG-prey to increase with enrichment when the omnivore forages adaptively, shifting its feeding from the IG-prey to the shared prey as the latter becomes the more profitable prey to consume. The mechanism for this shift is a trade-off between feeding on the two species and predicts that the omnivore's attack rate on the IG-prey will decline with enrichment [27]. Such a decline was not observed in the New Zealand system (figure 4e). Furthermore, such a trade-off is unlikely to occur in New Zealand where prey species are encountered within the same fine-grained environment [29,30]. Adaptive foraging does not affect the IG-prey's equilibrium coexistence window in models where course-grained trade-offs are not incorporated and, furthermore, does not remove the need for the IG-prey's competitive superiority [28]. Current models of adaptive foraging assessing equilibrium conditions on ecological time scales thus also fail to explain the patterns observed in New Zealand.
Finally, Hart [31] has shown the IG-prey's equilibrium abundance to increase with enrichment when the omnivore exhibits a ratio-dependent functional response. Hart's [31] study is noteworthy for being the only study to consider such consumer-dependence (see electronic supplementary material, table S1.1) which makes the omnivore's feeding rate a saturating function of both its prey's and its own abundances. The negative density-dependence introduced by the conspecific interference entailed therein prevents the omnivore from controlling the IG-prey at high productivity, consistent with H. scobina's cross-gradient increase in abundance. However, the IG-prey must still be the superior competitor [31], a prediction that is inconsistent with H. haustorium's apparent competitive advantage. How likely this mechanism is to occur among New Zealand's whelks is thus unclear. Further, although such consumer-dependent functional responses may arise by a variety of mechanisms [31,32], they have been difficult to assess empirically [33]. Even for tightly coupled specialist predator–prey interactions, their prevalence remains debated [34,35]. The few studies that have examined consumer-dependence in intertidal whelks have also seen mixed interpretation [36–39]. Relative to these other whelk species, H. haustorium's densities are low and dispersed (figure 1). Thus, although consumer-dependence should have little effect on H. haustorium's feeding rates within the range of predator and prey abundances observed in the field [17,32,40], further work on the strength of consumer-dependence in generalist predators is required to assess its importance.
(b). Non-equilibrium conditions
IGP theory has overwhelmingly dealt with models of equilibrium conditions which, as such, were the primary consideration of this study; species abundances were not sampled long or frequently enough to document temporal fluctuations (see the electronic supplementary material, figure S2.7). However, evidence does suggest that non-equilibrium whelk–prey dynamics are possible: whelks are able to regulate prey populations [17] and are known to affect prey declines following episodic prey recruitment events [37,41,42]. Recent theory indicates that non-equilibrium dynamics such as limit cycles and chaos can affect predictions that counter those of equilibrium models [43,44].
To date, three processes have been shown to promote non-equilibrium coexistence and permit the IG-prey's time-averaged abundance to increase with enrichment [43,44]. The underlying mechanism is for fluctuations in the prey abundances to decrease the omnivore's average growth rate more so than the IG-prey's. This reduces and may even remove the IG-prey's need for a competitive advantage in a manner analogous to the well-known Armstrong–McGehee [45] mechanism of competitive coexistence. The first two processes require the IG-prey to be important for the omnivore's own persistence, either by the omnivore having nutritional requirements satisfied only by the IG-prey [43] or because the omnivore exhibits a life-history diet switch by having adults that consume the IG-prey exclusively [44]. Neither scenario applies to New Zealand's whelks where the IG-prey and many shared prey are consumed by and are nutritionally substitutable for adult H. haustorium. The third is for the omnivore to exhibit an imperfectly adaptive functional response that is more saturated (less linear) than the IG-prey's [43]. Consistent with this third mechanism, H. scobina did exhibit shorter handling times than H. haustorium for most shared prey species (see electronic supplementary material, figure S2.6). Nonetheless, attack rates and handling times taken together indicate greater saturation in H. scobina's feeding rates than in H. haustorium's [40]. These, furthermore, are not high enough to affect saturation-driven limit cycles in an empirically parametrized model of New Zealand's whelk–prey interactions [40].
(c). Future directions for intraguild predation theory
In summary, there is little support for the mechanisms of current IGP theory in explaining the patterns observed in the food webs of New Zealand's intertidal. Although this study made several assumptions in quantifying parameters and arriving at this conclusion (e.g. type II functional responses, allometry-derived mortality rates), their empirical justification is arguably quite strong. Other IGP systems have certainly also shown unexplained coexistence in the presence of a competitively superior omnivore [46,47], but have not been able to investigate its possible mechanisms. The consideration of these mechanisms suggests several directions for additional research. In New Zealand's intertidal, for example, recruitment-driven dynamics may offer an alternative mechanism affecting non-equilibrium dynamics independent of predator saturation. Empirical estimates of the conversion and mortality rates, as well as theoretical investigations into the effects of consumer-dependent functional responses on the need for the IG-prey's competitive superiority will also be fruitful. Indeed, most of the prey-dependent model variations considered by current IGP theory (see electronic supplementary material, appendix S1) deserve consideration under a starting assumption of consumer- and ratio-dependence [32].
However, my data also suggest that IGP theory should consider systems with alternative prey in more detail and should focus in particular on the potential for non-trophic interactions to occur between basal prey species [48], particularly for models of adaptively foraging predators and under non-equilibrium conditions. Changes in community structure observed across the New Zealand productivity gradient suggest that such basal interactions could be important: mussels and barnacles (the core-shared prey) are likely to have affected the increased abundance H. scobina's alternative prey (small Austrolittorine snails) at high-productivity levels through the facilitative provisioning of structural substrate complexity [49]. They are also likely to have affected the reduced abundance of the species comprising H. haustorium's alternative prey group (large limpets and snails) which typically prefer less complex surfaces on which to graze [49]. The potential for such interactions among shared and non-shared prey has not been considered by current models whose basal species compete for a fixed proportion of a total carrying capacity [10], or have equally large but independent carrying capacities [11].
I also suggest that future modelling consider that both the structure and interaction strengths of nature's food webs are dynamic, particularly over evolutionary spatio-temporal scales larger than those considered by current theory. That the components of species interactions need not be constant has largely been ignored, but is clearly evidenced by my data. Indeed, the cross-gradient changes in food web structure and per capita attack rates observed in New Zealand provide strong evidence that such processes have played a role in structuring this system. Classic optimal foraging theory predicts, for example, that a predator's diet richness will increase as the availability of its primary prey decreases. Such a response is clear in the cross-gradient changes of the IG-prey's diet (see electronic supplementary material, figure S2.2). Additional empirical studies that partition the components of species interactions have much to contribute in this regard. For example, the magnitude by which H. scobina's per capita attack rates on shared prey differed from those of H. haustorium, relative to the magnitude by which their handling times on these prey differed (see electronic supplementary material, figure S2.6), suggests that per capita attack rates are more easily evolved. This pattern is consistent with the observation that behavioural traits (i.e. prey preferences) are more labile than physiological traits (i.e. digestion and drilling rates) [50], and should therefore be considered in future studies of IGP systems.
5. Conclusions
The reticulate nature of food webs requires us to better understand the role that trophic omnivores play in their communities. No longer in its infancy, IGP theory has contributed much to our understanding of food webs by integrating our knowledge of how the direct and indirect effects of predation and competition can affect a community's structure and dynamics. While the two key predictions of current theory for three- to five-species IGP systems were not supported by this study of more species-rich IGP food webs, the consistent and unidirectional nature of the cross-productivity changes observed in New Zealand indicate that hope is nonetheless warranted for these discrepancies to be explained.
Acknowledgements
I am grateful for the assistance and logistical support provided by David Schiel, the members of the Marine Ecology Research Group at the University of Canterbury, and the staff at the Edward Percival Field Station. Thanks to Paul Durst, Chris Gibbons, and Kenan Matterson for their efforts in the field and laboratory, to Tomoyuki Nakano for his help with Notoacmid identification and to Priyanga Amarasekare, Greg Dwyer, Michael Foote, Lev Ginzburg, Orlando Sarnelle, David Schiel, Cathy Pfister, Rebecca Terry, and especially Tim Wootton for discussions and suggestions at all stages of this research.
Funding statement
This work was supported by an NSF DDIG (no. DEB-0608178), an EPA STAR fellowship, a DOE GAANN training grant and the University of Chicago Hinds Fund.
References
- 1.Cousins S. 1987. The decline of the trophic level concept. Trends Ecol. Evol. 2, 312–316 (doi:10.1016/0169-5347(87)90086-3) [DOI] [PubMed] [Google Scholar]
- 2.Polis GA, Myers CA, Holt RD. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst. 20, 297–330 (doi:10.1146/annurev.es.20.110189.001501) [Google Scholar]
- 3.Polis GA, Holt RD. 1992. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol. Evol. 7, 151–154 (doi:10.1016/0169-5347(92)90208-S) [DOI] [PubMed] [Google Scholar]
- 4.Holt RD, Polis GA. 1997. A theoretical framework for intraguild predation. Am. Nat. 149, 745–764 (doi:10.1086/286018) [Google Scholar]
- 5.Vance-Chalcraft HD, Rosenheim JA, Vonesh JR, Osenberg CW, Sih A. 2007. The influence of intraguild predation on prey suppression and prey release: a meta-analysis. Ecology 88, 2689–2696 (doi:10.1890/06-1869.1) [DOI] [PubMed] [Google Scholar]
- 6.Briggs CJ, Borer ET. 2005. Why short-term experiments may not allow long-terms predictions about intraguild predation? Ecol. Appl. 15, 1111–1117 (doi:10.1890/04-1776) [Google Scholar]
- 7.Diehl S, Feissel M. 2001. Intraguild prey suffer from enrichment of their resources: a microcosm experiment with ciliates. Ecology 82, 2977–2983 (doi:10.1890/0012-9658(2001)082[2977:IPSFEO]2.0.CO;2) [Google Scholar]
- 8.Liess A, Diehl S. 2006. Effects of enrichment on protist abundances and bacterial composition in simple microbial communities. Oikos 114, 15–26 (doi:10.1111/j.2006.0030-1299.14516.x) [Google Scholar]
- 9.Montserrat M, Magalhaes S, Sabelis MW, de Roos AM, Janssen A. 2008. Patterns of exclusion in an intraguild predator–prey system depend on initial conditions. J. Anim. Ecol. 77, 624–630 (doi:10.1111/j.1365-2656.2008.01363.x) [DOI] [PubMed] [Google Scholar]
- 10.Holt RD, Huxel GR. 2007. Alternative prey and the dynamics of intraguild predation: theoretical perspectives. Ecology 88, 2706–2712 (doi:10.1890/06-1525.1) [DOI] [PubMed] [Google Scholar]
- 11.Daugherty MP, Harmon JP, Briggs CJ. 2007. Trophic supplements to intraguild predation. Oikos 116, 662–677 (doi:10.1111/j.2006.0030-1299.15378.x) [Google Scholar]
- 12.Kondoh M. 2008. Building trophic modules into a persistent food web. Proc. Natl Acad. Sci. USA 105, 16 631–16 635 (doi:10.1073/pnas.0805870105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wootton JT, Emmerson M. 2005. Measurement of interaction strength in nature. Annu. Rev. Ecol. Evol. Syst. 36, 419–444 (doi:10.1146/annurev.ecolsys.36.091704.175535) [Google Scholar]
- 14.Novak M, Wootton JT. 2010. Using experimental indices to quantify the strength of species interactions. Oikos 119, 1057–1063 (doi:10.1111/j.1600-0706.2009.18147.x) [Google Scholar]
- 15.Menge BA, Daley BA, Lubchenco J, Eric S, Dahlhoff E, Halpin PM, Hudson G, Burnaford JL. 1999. Top-down and bottom-up regulation of New Zealand rocky intertidal communities. Ecol. Monogr. 69, 297–330 (doi:10.1890/0012-9615(1999)069[0297:TDABUR]2.0.CO;2) [Google Scholar]
- 16.Menge BA, et al. 2003. Coastal oceanography sets the pace of rocky intertidal community dynamics. Proc. Natl Acad. Sci. USA 100, 12 229–12 234 (doi:10.1073/pnas.1534875100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak M. 2010. Estimating interaction strengths in nature: experimental support for an observational approach. Ecology 91, 2394–2405 (doi:10.1890/09-0275.1) [DOI] [PubMed] [Google Scholar]
- 18.Chesson J. 1983. The estimation and analysis of preference and its relationship to foraging models. Ecology 64, 1297–1304 (doi:10.2307/1937838) [Google Scholar]
- 19.Novak M, Wootton JT. 2008. Estimating nonlinear interaction strengths: an observation-based method for species-rich food webs. Ecology 89, 2083–2089 (doi:10.1890/08-0033.1) [DOI] [PubMed] [Google Scholar]
- 20.Yeakel JD, Novak M, Guimarães PR, Jr, Dominy NJ, Koch PL, Ward EJ, Moore JW, Semmens BX. 2011. Merging resource availability with isotope mixing models: the role of neutral interaction assumptions. PLoS ONE 6, e22015 (doi:10.1371/journal.pone.0022015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCoy MW, Gillooly JF. 2008. Predicting natural mortality rates of plants and animals. Ecol. Lett. 11, 710–716 (doi:10.1111/j.1461-0248.2008.01190.x) [DOI] [PubMed] [Google Scholar]
- 22.Humphreys WF. 1979. Production and respiration in animal populations. J. Anim. Ecol. 48, 427–453 (doi:10.2307/4171) [Google Scholar]
- 23.Amarasekare P. 2006. Productivity, dispersal and the coexistence of intraguild predators and prey. J. Theor. Biol. 243, 121–133 (doi:10.1016/j.jtbi.2006.06.007) [DOI] [PubMed] [Google Scholar]
- 24.Amarasekare P. 2007. Spatial dynamics of communities with intraguild predation: the role of dispersal strategies. Am. Nat. 170, 819–831 (doi:10.1086/522837). [DOI] [PubMed] [Google Scholar]
- 25.Sanford E, Roth MS, Johns GC, Wares JP, Somero GN. 2003. Local selection and latitudinal variation in a marine predator–prey interaction. Science 300, 1135–1137 (doi:10.1126/science.1083437) [DOI] [PubMed] [Google Scholar]
- 26.Rudolf VHW. 2007. The interaction of cannibalism and omnivory: consequences for community dynamics. Ecology 88, 2697–2705 (doi:10.1890/06-1266.1) [DOI] [PubMed] [Google Scholar]
- 27.Křivan V. 2000. Optimal intraguild foraging and population stability. Theor. Popul. Biol. 58, 79–94 (doi:10.1006/tpbi.2000.1480) [DOI] [PubMed] [Google Scholar]
- 28.Křivan V, Schmitz OJ. 2003. Adaptive foraging and flexible food web topology. Evol. Ecol. Res. 5, 623–652 [Google Scholar]
- 29.Křivan V, Diehl S. 2005. Adaptive omnivory and species coexistence in tri-trophic food webs. Theor. Popul. Biol. 67, 85–99 (doi:10.1016/j.tpb.2004.09.003) [DOI] [PubMed] [Google Scholar]
- 30.Holt RD. 1983. Optimal foraging and the form of the predator isocline. Am. Nat. 122, 521–541 (doi:10.1086/284153) [Google Scholar]
- 31.Hart DR. 2002. Intraguild predation, invertebrate predators, and trophic cascades in lake food webs. J. Theor. Biol. 218, 111–128 (doi:10.1006/jtbi.2002.3053) [DOI] [PubMed] [Google Scholar]
- 32.Arditi R, Ginzburg LR. 2012. How species interact: altering the standard view of trophic ecology, 170 p Oxford, UK: Oxford University Press [Google Scholar]
- 33.Abrams PA, Ginzburg LR. 2000. The nature of predation: prey dependent, ratio dependent or neither? Trends Ecol. Evol. 15, 337–341 (doi:10.1016/S0169-5347(00)01908-X) [DOI] [PubMed] [Google Scholar]
- 34.Jensen CXJ, Jeschke JM, Ginzburg LR. 2007. A direct, experimental test of resource vs. consumer dependence: comment. Ecology 88, 1600–1602 [DOI] [PubMed] [Google Scholar]
- 35.Fussmann GF, Weithoff G, Yoshida T. 2007. A direct, experimental test of resource vs. consumer dependence: reply. Ecology 88, 1603–1604 [DOI] [PubMed] [Google Scholar]
- 36.Murdoch WW. 1969. Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol. Monogr. 39, 335–354 (doi:10.2307/1942352) [Google Scholar]
- 37.Katz CH. 1985. A nonequilibrium marine predator–prey interaction. Ecology 66, 1426–1438 (doi:10.2307/1938005) [Google Scholar]
- 38.Akcakaya HR, Arditi R, Ginzburg LR. 1995. Ratio-dependent predation: an abstraction that works. Ecology 76, 995–1004 (doi:10.2307/1939362) [Google Scholar]
- 39.Abrams PA. 1994. The fallacies of ‘ratio-dependent’ predation. Ecology 75, 1842– 1850 [Google Scholar]
- 40.Novak M. 2008. Trophic omnivory and the structure, strength, and nonlinear nature of species interactions across a productivity gradient. PhD thesis, University of Chicago, Chicago [Google Scholar]
- 41.Connell JH. 1970. A predator–prey system in the marine intertidal region. I. Balanus glandula and several predatory species of Thais. Ecol. Monogr. 40, 49–78 (doi:10.2307/1942441) [Google Scholar]
- 42.Navarrete SA, Berlow EL. 2006. Variable interaction strengths stabilize marine community pattern. Ecol. Lett. 9, 526–536 (doi:10.1111/j.1461-0248.2006.00899.x) [DOI] [PubMed] [Google Scholar]
- 43.Abrams PA, Fung SR. 2010. Prey persistence and abundance in systems with intraguild predation and type-2 functional responses. J. Theor. Biol. 264, 1033–1042 (doi:10.1016/j.jtbi.2010.02.045) [DOI] [PubMed] [Google Scholar]
- 44.Abrams PA. 2011. Simple life-history omnivory: responses to enrichment and harvesting in systems with intraguild predation. Am. Nat. 178, 305–319 (doi:10.1086/661243) [DOI] [PubMed] [Google Scholar]
- 45.Armstrong RA, McGehee R. 1980. Competitive-exclusion. Am. Nat. 115, 151–170 (doi:10.1086/283553) [Google Scholar]
- 46.Navarrete SA, Menge BA, Daley BA. 2000. Species interactions in intertidal food webs: prey or predation regulation of intermediate predators? Ecology 81, 2264–2277 (doi:10.1890/0012-9658(2000)081[2264:SIIIFW]2.0.CO;2) [Google Scholar]
- 47.Amarasekare P. 2008. Coexistence of intraguild predators and prey in resource-rich environments. Ecology 89, 2786–2797 (doi:10.1890/07-1508.1) [DOI] [PubMed] [Google Scholar]
- 48.Polis GA, Strong DR. 1996. Food web complexity and community dynamics. Am. Nat. 147, 813–846 (doi:10.1086/285880) [Google Scholar]
- 49.Menge BA. 1995. Indirect effects in marine rocky intertidal interaction webs: patterns and importance. Ecol. Monogr. 65, 21–74 (doi:10.2307/2937158) [Google Scholar]
- 50.Blomberg SP, Garland T, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745 (doi:10.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]