Abstract
A bacterium capable of degrading microcystin-RR (MC-RR) was isolated from a Saudi eutrophic lake which was previously reported to have microcystin-producing cyanobacteria. Based on the analysis of the 16S rRNA gene sequence, the isolated strain SSZ01, most likely belong to the genus Bacillus with a highest sequence similarity (99%) with Bacillus flexus strain EMGA5. It was found that B. flexus strain SSZ01, possesses an mlrA gene encoding the most important enzyme for MC degradation. This strain was capable of degrading MC-RR, at a concentration of 10 mg l−1, in batch experiments under environmentally relevant conditions. The degradation of MC-RR was completely removed within 4 d. The degradation of MC-RR by this strain occurred in a rich medium nutrient broth (NB), indicating that this could likely occur along with other organic compounds found in the environment. Therefore, the coexistence of such bacteria with MCs in the same environment can contribute to the self-purification of the ecosystem from such potent toxins. This is the first study to report that B. flexus can degrade MCs.
Keywords: Bacillus, Biodegradation, Microcystins, 16S rRNA gene, mlrA gene
1. Introduction
Microcystins (MCs) are cyclic heptapeptide hepatotoxins produced by some cyanobacteria, such as Anabaena, Nostoc, and Planktothrix/Oscillatoria (Carmichael, 2001; Baldia et al., 2003). These toxins show a potent acute hepatotoxicity and tumor-promotion in animals and humans through inhibition of protein phosphatases 1 and 2A (Dawson, 1998; Imanishi and Harada, 2004). In addition, MCs were implicated in the death of about 50 persons using contaminated water for hemodialysis in Caruaru, Brazil (Jochimsen et al., 1998). Over 70 structural analogues of MCs have been identified (Ame et al., 2006). They have the general structure of cyclo-(D-Ala-X-D-MeAsp-Z-Adda-DGlu-Mdha-), in which X and Z represent variable L-amino acids, and Adda refers to the b-amino acid residue of 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid (Carmichael, 1992). MCs have characteristic UV absorbance at 238 nm originating from the conjugated double bond in the Adda structure (Oksanen et al., 2004). The LD50 value for the most toxic compound among the MCs, microcystin-LR (MC-LR), is 50 mg kg−1 (i.p. mouse) (Dawson, 1998). MCs are structurally stable against physicochemical and biological factors such as temperature, sunlight, and enzymes, under natural conditions (Jones and Orr, 1994; Tsuji et al., 1994; Ame et al., 2006).
Although several chemical treatments of water were proposed, it is possible that the chemical treatments sometimes produce carcinogenic substances and other mutagens (Ishii et al., 2004). Biodegradation could be one of the safest and mildest treatments for removing cyanobacterial toxins from water. As possible carbon and nitrogen supply, MC may have a high nutritional value as a source of either amino acids, or energy to microorganisms. However, the cyclic structure of MC makes them resistant to many common bacterial proteases (Harada, 1996), indicating that the capability to degrade MCs by microorganisms requires rather specific enzymes.
Recently, Bourne et al. (1996, 2001) and Saito et al. (2003) identified a gene cluster, mlrA, mlrB, mlrC and mlrD, responsible for the degradation of MC-LR. The authors determined that the mlrA gene encoded an enzyme responsible for the hydrolytic cleaving of the cyclic structure of MC-LR. The resultant linear MC-LR molecule was then sequentially hydrolyzed by peptidases encoded by the mlrB and mlrC genes. The final gene, mlrD, encoded for a putative transporter protein that may have allowed for active transport of MC and/or its degradation products into, or out of, the cell. It was found that these degradation products, including the linearized MC-LR, the tetrapeptide and Adda, are essentially non-toxic, which strongly indicated that the microbial degradation by MC-degrading bacteria is quite effective for the detoxification of MCs (Tsuji et al., 2006). There are a growing number of isolated bacteria reported as having the capacity to degrade MC in water, and most strains appear to be limited to the family Sphingomonadaceae (Bourne et al., 2001; Saito et al., 2003). However, other bacteria were successively discovered in many lakes, ponds, reservoirs, and rivers all over the world (Jones et al., 1994; Bourne et al., 1996, 2001; Takenaka and Watanabe, 1997; Park et al., 2001; Maruyama et al., 2003; Saito et al., 2003; Harada et al., 2004; Imanishi et al., 2005; Ishii et al., 2004; Maruyama et al., 2006; Ame et al., 2006; Kato et al., 2007; Lemes et al., 2008; Nybom et al., 2007, 2008; Hu et al., 2009; Manage et al., 2009).
Most lakes and reservoirs in the south west of Saudi Arabia show cyanobacterial blooms (Mohamed and Al-Shehri, 2009). Most of these blooms present evidence of hepatotoxicity, with the production of MC-RR as a prevalent toxin. Since MC-RR is the most abundant MC in these water bodies, the main goal of the present study was to examine the capability of its native bacteria to degrade this cyanotoxin.
2. Materials and methods
2.1. Isolation of microcystin-degrading bacteria
Water samples were collected during May 2011 from Tendaha Lake, which has been previously reported to have MC-producing blooms of cyanobacteria (Mohamed and Al-Shehri, 2009). Diluted samples of lake water were inoculated onto the Nutrient Broth Agar (Difco) medium plates containing 0.3% Beef Extract, 0.5% Peptone, 0.5% NaCl and 1.5% Agar. Single colonies from these plates were transferred to liquid nutrient broth medium. To screen the isolated bacteria for the ability of MC degradation, the grown cells of morphologically different five bacterial isolates were inoculated (5 ml) separately to 50 ml NB medium containing MC-RR (1 mg l−1). All cultures of bacterial strains were maintained in incubator at 28 °C (the temperature of lake water during the sampling time), with shaking (120 rpm) in the dark for 5 d. The remaining concentrations of MC-RR in the medium were monitored by Enzyme-Linked Immunosorbent Assay (ELISA), using the Envirologx kit for MC as described in biodegradation of MC-RR section. Among the tested isolates, only one bacterium strain showed MC degrading activity. The isolate was then identified by 16S rRNA gene sequencing according to Neilan (1995).
2.2. DNA extraction and 16S rRNA -PCR analysis
Bacterial DNA of the isolate, capable of degrading MC, was extracted from 5 ml overnight culture by using QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA). A 350 bp of the 16S rRNA gene was amplified by polymerase chain reaction (PCR) using specific universal primers (Poppert et al., 2005). A 350-bp product was amplified using forward primer (5′-AACTGGAGGAAGGTGGGGAT-3′) and reverses primer (5′-AGGAGGTGATCCAACCGCA-3′). The PCR mixture consisted of 30 pmol of each primer, 10 ng of chromosomal DNA, 200 μM dNTPs and 2.5 units of Taq polymerase with 10 μl of polymerase buffer containing MgCl2. The PCR was carried out for 35 cycles of 94 °C for 1 min, 55 °C for 1 min and then 72 °C for 2 min (Alamri, 2010). The PCR product was analyzed on 1.0% agarose gel containing 0.5 μg ml−1 ethidium bromide and visualized by BioRad Gel Documentation System 2000. Electrophoresis was carried out for 20 min at 150 V. Sequencing steps were performed at Macrogen Comp., Korea. The 16S rRNA gene PCR product was sequenced by Macrogen Company, Korea. Homology of the 16S rRNA gene sequence of the isolates, with reference 16S rRNA gene sequences, was analyzed using the BLAST algorithm in GenBank available in the National Centre for Biotechnology Information (NCBI).
2.3. Detection of gene involved in microcystin degradation
Specific oligonucleotide primer MF, 5′-GACCCGATGTTCAAGATACT-3′ and MR, 5′-CTCCTCCCACAAATCAGGAC-3′ (Saito et al., 2003) were used in PCR to screen bacterial isolates containing the mlrA gene (800 bp). Amplifications were conducted on a BioRad MyCycler thermocycler under conditions previously documented by Ho et al. (2006). PCR product was analyzed by electrophoresis on a 1% agarose gel containing 0.5 μg ml−1 ethidium bromide and visualized by BioRad Gel Documentation System 2000.
2.4. Biodegradation of microcystin-RR
To study degradation of MC, the MC degrading bacterium was cultured overnight on an orbital shaker at 140 rpm in NB medium at 28 °C, harvested at the exponential phase and used for biodegradation experiment. The grown cells were inoculated into three sterile 100 ml Erlenmeyer flasks containing 50 ml NB liquid medium, and MC-RR (95%, provided by Prof. Dr. Zakaria A. Mohamed, King Khalid University) at a concentration of 10 mg l−1. Flasks with NB medium containing 10 mg l−1 MC-RR without bacterial cells were used as control. Treated and control cultures were shaken (140 rpm) in the dark at 28 °C. One ml of samples was withdrawn carefully under sterile conditions at zero time and 1-d intervals for 10 d. Each sample was monitored for bacterial growth by measuring the optical density (OD) spectrophotometrically at 600 nm wavelength. The samples were then centrifuged (10,000g) and the supernatants were kept in glass tubes for the determination of non-degraded MC by ELISA. ELISA was used with no further sample preparation and run using the Envirologix kit for MCs according to Envirologix manufacturer protocol (Carmichael and An, 1999).
An ELX800 microplate reader (BioTek, Highland Park, Winooski, VT 05404, USA) was used for the absorbance measurement at 450 nm. For quantification, the competitive calibration curve (B/Bo% versus concentration of MC-RR on a semi-log scale, where B and Bo are the absorbance values of the sample and the blank, respectively), was used. The curve was drawn using the calibrator solutions contained in the kit (0.16–2.50 μg l−1). All standards and samples were analyzed in triplicate. Samples with CV > 15% were always rejected.
2.5. Biodegradation rate calculation
The average biodegradation rate was calculated according to Lemes et al. (2008) by dividing the concentration of MC-RR, as initially spiked into the samples, by the number of the days, until MC was no longer detected by ELISA. The half life of toxin degradation was calculated from the linear regression of the toxin decay curve. The loss of toxicity of bacterial degradation by-products was assessed by protein phosphatase inhibition assay (PPIA), using protein phosphatase 2A (PP2A) enzyme and p-nitrophenyl phosphate substrate purchased from Sigma. Enzyme and substrate solutions were prepared according to the known procedure (Heresztyn and Nicholson, 2001). Samples (20 μl) were combined with PP2A (0.25 U ml−1) solutions (20 μl) in microplate wells and incubated at 37 °C for 5 min. After the addition of 200 μl of substrate solution, the microplate was incubated at 37 °C for 1.5 h and the absorbance of the wells was measured at 405 nm using an ELX800 microplate reader.
Toxin concentrations were calculated from calibration curves which were plotted as percentage inhibition of PP2A, expressed as B/Bo%, versus MC-RR concentrations (0.01–10 μg l−1). In blanks, MC standards were replaced by high-purity water so full color development was obtained. In order to more precisely determine toxin concentrations, only the linear region of the calibration curve, i.e., the region between 20% and 80% activity, was used for quantification. The IC50 was the toxin concentration which resulted in 50% inhibition, i.e., 50% activity.
2.6. Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS, version 10.0, Chicago, IL, USA). Data are presented as means with their standard error. Statistical evaluation of the data was performed using one-way ANOVA test. Values were considered statistically significant when p ⩽ 0.05.
3. Results
3.1. Molecular identification of the selected bacterial isolates
Among different bacterial strains isolated from Tendaha Lake, Saudi Arabia, during the present study, only strain SSZ01 showed to degrade MC-RR. For bacterial identification, the 16S rRNA gene for the isolate SSZ01 (Fig. 1) was amplified. The obtained DNA nucleotide was sequenced and then analyzed using DNA Blast search (NCBI). The highest sequence similarity value (99%) was obtained between this strain and Bacillus flexus strain EMGA5. Thus, this strain was designated to be B. flexus and deposited in the Genbank with an accession number of GU112451.
Figure 1.
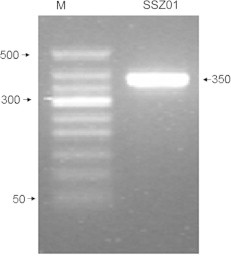
PCR amplification for the 16S rRNA gene from the isolate SSZ01, where M: Marker and SSZ01: The bacterial isolate.
3.2. Biodegradation of microcystin-RR
The degradation of MC-RR by B. flexus SSZ01, isolated from an eutrophic lake in Saudi Arabia, containing toxic blooms of cyanobacteria is shown in Fig. 2. MC-RR biodegradation occurred rapidly, where 29% of the toxin was degraded within 1 d of incubation and completely degraded within 4 d. The results of PPIA revealed a decrease in toxicity, in parallel with the decrease in MC concentrations as determined by ELISA (Fig. 2). No significant difference (P > 0.05) in the bacterial density was observed between toxin-treated and control cultures (Fig. 3). This confirms that MC-RR has no effect on bacterial growth, and that this toxin might be used as carbon and/or nitrogen source. The control (NB medium without bacteria) showed no degradation of MC-RR during all the period of incubation. The average biodegradation rate of MC-RR by B. flexus SSZ01was about 2.5 μg ml−1 d−1. The results of linear regression of the percentage of degraded MC-RR showed that the half-life (D1/2) of MC-RR degradation by this strain was 1.7 d (Fig. 4).
Figure 2.
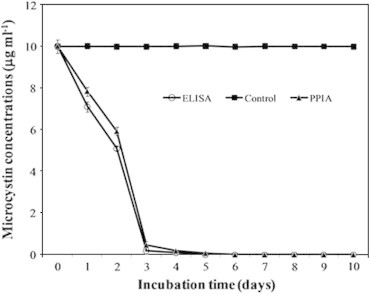
Degradation of microcystins-RR over a 10-d period with Bacillus flexus strain SSZ01 isolated from a Saudi eutrophic lake. Changes in microcystin concentrations (μg ml−1) were monitored by enzyme-linked immunosorbent assay (ELISA) and protein phosphatase inhibition assay (PPIA).
Figure 3.

Growth curve of strain SSZ01 in NB medium under toxin-treated and control conditions.
Figure 4.
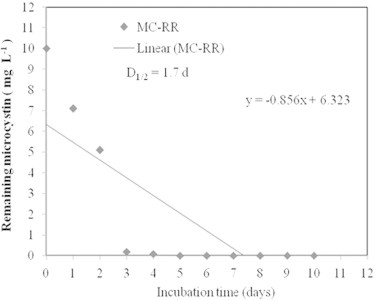
The half life time of microcystin-RR during the biodegradation by strain SSZ01.
3.3. Detection of the mlrA gene from the microcystin degrading bacteria
Fig. 5 shows the results of detection of the mlrA gene by PCR. Bands of the expected size (approximately 800 bp) were detected in B. flexus SSZ01 by the PCR employing the primer set MF-MR. This confirmed that mlrA homologues exist in this strain.
Figure 5.
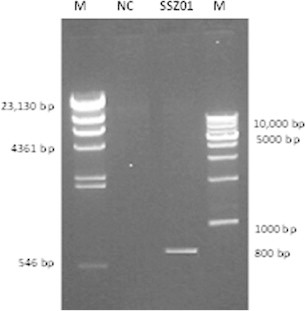
Detection of mlrA gene by PCR, where M: Marker, NC: negative control and SSZ01: the bacterial isolate (microcystin-degrading strain).
4. Discussion
Microcystins are very stable compounds, but they disappear rapidly in water (Takenaka and Tanaka, 1995) as a result of biological degradation by aquatic bacteria as a major route of their detoxification (Lahti et al., 1997; Miller and Fallowfield, 2001; Ishii et al., 2004). Although most MC-degrading bacteria are confined to the genus Sphingomonas (Bourne et al., 1996; Park et al., 2001; Harada et al., 2004), MC degradation has been reported for different bacterial strains of other genera including Pseudomonas (Takenaka and Watanabe, 1997), Paucibacter (Rapala et al., 2005), Methylobacillus (Hu et al., 2009), Lactobacillus and Bifidobacterium (Nybom et al., 2007), Burkholderia (Lemes et al., 2008), Sphingosinicella (Maruyama et al., 2006), Arthrobacter, Brevibacterium and Rhodococcus (Manage et al., 2009). However, MC degradation by B. flexus has not been addressed yet.
In the present study, a Bacillus strain (SSZ01) isolated from an eutrophic lake (Tendaha Lake) containing toxic blooms of cyanobacteria was found to degrade MC-RR (the most prevalent toxin in this lake). The results of phylogentic analysis based on the 16S rRNA gene sequence data, showed a homology (99%) with B. flexus. Therefore, the present study is the first to demonstrate the ability of this species to degrade the hepatotoxin MC.
B. flexus SSZ01 strain started to degrade MC-RR rapidly, without a delay in the lag phase. These results contrast other studies reporting longer lag phases, ranging from 3 d to 20 d, in the experiments of bacterial derogation of MC (Jones et al., 1994; Lam et al., 1995; Hyenstrand et al., 2003). However, Christoffersen et al. (2002) reported that the degradation of MCs in natural waters with previous cyanobacterial histories can occur without lag phases. This could be true in this study, where strain SSZ01 was isolated from a lake containing MC-producing blooms. Therefore, this strain could be already adapted to grow under toxin conditions in nature, and therefore a lag phase is not required during culturing in a medium containing MC. MC-RR was no longer detected within 4 d. Such a short time of MC degradation was previously reported by Ame et al. (2006), who found that MC-RR had completely disappeared within 48 h of incubation with Sphingomonas sp. CBA4 isolated from San Roque reservoir, Argentina.
In addition to rapid degradation of toxin, strain SSZ01 had a high degradation rate of MC-RR (2.5 mg l−1 d−1 at initial concentration 10 mg l−1) compared to a degradation rate (0.12 mg l−1 d−1) of MC-RR by Sphingomonas CBA4 (Ame et al., 2006). The biodegradation rate of MC-RR by B. flexus strain afforded a calculated half-life of 1.7 d in this study. These results can be compared to a half-life time of 18 h for MC-RR degradation by Sphingomonas CBA4 using 200 μg l−1 initial concentration of MCs (Ame et al., 2006). The difference in half-life times of toxin degradation between the two studies may be due to the difference in bacterial species used in these studies. Analysis of the sterile control (containing MC-RR without bacteria), showed no loss of MC-RR confirming that the observed degradation was due to the bacterium present in the culture, and there was no abiotic degradation due to physical, and/or chemical factors.
The results of present study also show that the degradation of MC-RR by B. flexus SSZ01 has occurred in the rich medium (NB medium). This indicates that this strain was able to degrade MC-RR irrespective of the presence of carbon and nitrogen sources. These results agree with those of Ishii et al. (2004), reporting that Sphingomonas 7CY, isolated from Suwa Lake, can degrade MCs in both minimal medium (M90), and LB medium containing nitrogen and carbon sources. The results of present study along with those of Ishii et al. (2004) indicate that the bacterial enzyme involved in MC-RR degradation is constitutive. This hypothesis is not surprising as bacteria can degrade MC along with other organic compounds frequently found in the environment (Christoffersen et al., 2002).
The results of this study also reveal that B. flexus SSZ01 possess a mlrA gene which encodes a hydrolytic enzyme to open the cyclic peptide of MCs. The detection of the homologous mlrA gene in this strain, suggests that MC-RR degradation by B. flexus most likely follow a degradation pathway similar to one previously reported by Bourne et al. (1996, 2001), where the enzyme encoded by the mlrA gene cleaves the cyclic structure of MC. The presence of this gene in Bacillus spp. also confirms the hypothesis that mlrA gene is unique to MC degraders but not to the genus Sphingomonas (Saito et al., 2003). The resultant linear structure of MC is then sequentially degraded by two additional enzymes, encoded by the mlrB and mlrC genes, respectively.
It is also found that the degradation products are essentially non-toxic, which strongly indicates that the microbial degradation using MC-degrading bacteria, is quite effective for the detoxification of MCs (Tsuji et al., 2006; Hu et al., 2009). Similarly, the current study showed that the toxicity of by-products as determined by PPIA, decreased gradually with incubation time and ultimately no detectable amounts of MC were found in the culture medium. Previously, Ho et al. (2007) reported a good conformity between the results of PPIA and HPLC, as methods for determination of the toxicity of by-products generated from the biodegradation of MC-LR and MC-LA by Sphingopyxis sp. LH21.
5. Conclusion
In the present study, a native bacterium was isolated from Saudi freshwater lakes which could be adapted to perform MC degradation. Based on 16S rRNA gene analysis, this strain was identified as B. flexus SSZ01. It was able to biodegrade MC-RR at initial concentration of 10 mg l−1 as it owns an mlrA gene, which encodes the most important enzyme for MC degradation. Furthermore, this study is the first to report that B. flexus can degrade MCs. B. flexus SSZ01 was able to degrade MC in rich medium containing nitrogen and carbon sources. This could likely occur along with other organic compounds found in the environment. Therefore, the coexistence of such bacteria with MCs in the same environment can contribute to the self-purification of the ecosystem from such potent toxins.
Acknowledgment
The author would like to acknowledge and thank King Khalid University for supporting this study. This appreciation is extended to the Chemist Department, College of Science, King Khalid University, for the technical help in chemical analyses.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alamri S. Isolation, phylogeny and characterization of new α-amylase producing thermophilic Bacillus sp. from the Jazan region, Saudi Arabia. Int. J. Biochem. Biotech. 2010;6:537–547. [Google Scholar]
- Ame M.V., Echenique J.R., Pflugmacher S., Wunderlin D.A. Degradation of microcystin-RR by Sphingomonas sp. CBA4 isolated from San Roque reservoir (Cordoba–Argentina) Biodegradation. 2006;17:447–455. doi: 10.1007/s10532-005-9015-9. [DOI] [PubMed] [Google Scholar]
- Baldia S.F., Conaco C.G., Nishijima T., Imanishi S., Harada K.I. Microcystin production during algal bloom occurrence in Laguna de Bay, the Philippines. Fish Sci. 2003;69:110–116. [Google Scholar]
- Bourne D.G., Jones G.J., Blakeley R.L., Jones A., Negri A.P., Riddles P. Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin-LR. Appl. Environ. Microbiol. 1996;62:4086–4094. doi: 10.1128/aem.62.11.4086-4094.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne D.G., Riddles P., Jones G.J., Smith W., Blakeley R.L. Characterization of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin-LR. Environ. Toxicol. 2001;16:523–534. [PubMed] [Google Scholar]
- Carmichael W.W. Health effects of toxin-producing cyanobacteria: the CyanoHABs. Hum. Ecolog. Risk Assess. 2001;7:1393–1407. [Google Scholar]
- Carmichael W.W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Bacteriol. 1992;72:445–459. doi: 10.1111/j.1365-2672.1992.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Carmichael W.W., An J. Using of enzyme linked immunosorbent assay (ELISA) and a protein phosphatase inhibition assay (PPIA) for the detection of MCYST and Nodularin. J. Nat. Toxins. 1999;7:377–385. doi: 10.1002/1522-7189(199911/12)7:6<377::aid-nt80>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Christoffersen K., Lyck S., Winding A. Microbial activity and bacterial community structure during degradation of microcystins. Aquat. Microb. Ecol. 2002;27:125–136. [Google Scholar]
- Dawson R.M. The toxicology of microcystins. Toxicon. 1998;36:953–962. doi: 10.1016/s0041-0101(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Harada K.I. Chemistry and detection for microcystins. In: Watanabe M.F., Harada K., Carmichael W.W., Fujiki H., editors. Toxic Microcystis. CRC Press; Boca Raton, MI USA: 1996. pp. 103–148. [Google Scholar]
- Harada K.I., Imanishi S., Kato H., Mizuno M., Ito E., Tsuji K. Isolation of Adda from microcystin-LR by microbial degradation. Toxicon. 2004;44:107–109. doi: 10.1016/j.toxicon.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Heresztyn T., Nicholson B.C. Determination of cyanobacterial toxins directly in water using a protein phosphatase inhibition assay. Water Res. 2001;35:3049–3056. doi: 10.1016/s0043-1354(01)00018-5. [DOI] [PubMed] [Google Scholar]
- Ho L., Gaudieux A.L., Fanok S., Newcombe G., Humpage A.R. Bacterial degradation of microcystin toxins in drinking water eliminates their toxicity. Toxicon. 2007;50:438–441. doi: 10.1016/j.toxicon.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Ho L., Meyn T., Keegan A., Hoefel D., Brookes J., Saint C.P., Newcombe G. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res. 2006;40:768–774. doi: 10.1016/j.watres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hu L.B., Yang J.D., Zhou W., Yin Y.F., Chen J., Shi Z.Q. Isolation of a Methylobacillus sp. that degrades microcystin toxins associated with cyanobacteria. New Biotechnol. 2009;26:205–211. doi: 10.1016/j.nbt.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hyenstrand P., Rohrlack T., Beattie K.A., Metcalf J.S., Codd G.A., Christoffersen K. Laboratory studies of dissolved radiolabelled microcystin-LR in lake water. Water Res. 2003;37:3299–3306. doi: 10.1016/S0043-1354(03)00180-5. [DOI] [PubMed] [Google Scholar]
- Imanishi S., Kato H., Mizuno M., Tsuji K., Harada K.I. Bacterial degradation of microcystins and nodularin. Chem. Res. Toxicol. 2005;18:591–598. doi: 10.1021/tx049677g. [DOI] [PubMed] [Google Scholar]
- Imanishi S., Harada K.I. Proteomics approach on microcystin binding proteins in mouse liver for investigation of microcystin toxicity. Toxicon. 2004;43:651–659. doi: 10.1016/j.toxicon.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Ishii H., Nishijima M., Abe T. Characterization of degradation process of cyanobacterial hepatotoxins by a gram-negative aerobic bacterium. Water Res. 2004;38:2667–2676. doi: 10.1016/j.watres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Jochimsen E.M., Carmichael W.W., An J.S., Cardo D.M., Cookson S.T., Holmes C.E., Antunes M.B., de Melo Filho D.A., Lyra T.M., Barreto V.S., Azevedo S.M., Jarvis W.R. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N. Eng. J. Med. 1998;338:873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- Jones G.J., Orr P.T. Release and degradation of microcystins following alguicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994;28:871–876. [Google Scholar]
- Jones G.J., Bourne D.G., Blakeley R.L., Doelle H. Degradation of cyanobacterial hepatotoxin microcystin by aquatic bacteria. Nat. Toxins. 1994;2:228–235. doi: 10.1002/nt.2620020412. [DOI] [PubMed] [Google Scholar]
- Kato H., Imanishi S.Y., Tsuji K., Harada K.I. Microbial degradation of cyanobacterial cyclic peptides. Water Res. 2007;41:1754–1762. doi: 10.1016/j.watres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lahti K., Rapala J., Fardag M., Maija N., Sivonen K. Persistence of cyanobacterial hepatoxin, microcystin-LR, in particulate material and dissolved in lake water. Water Res. 1997;31:1005–1012. [Google Scholar]
- Lam A.K., Fedorak P.M., Prepas E.E. Biotransformation of the cyanobacterial hepatotoxin-LR, as determined by HPLC and protein phosphatase bioassay. Environ. Technol. 1995;29:242–246. doi: 10.1021/es00001a030. [DOI] [PubMed] [Google Scholar]
- Lemes G.A., Kersanach R., Pinto L.D., Dellagostin O.A., Yunes J.S., Matthiensen A. Biodegradation of microcystins by aquatic Burkholderia sp. from a South Brazilian coastal lagoon. Ecotoxicol. Environ. Safe. 2008;69:358–365. doi: 10.1016/j.ecoenv.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Manage P.M., Edwards C., Singh B.K., Lawton L.A. Isolation and identification of novel microcystin-degrading bacteria. Appl. Environ. Microbiol. 2009;75:6924–6928. doi: 10.1128/AEM.01928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Kato K., Yokoyama A., Tanaka T., Hiraishi A., Park H.D. Dynamics of microcystin-degrading bacteria in mucilage of Microcystis. Microb. Ecol. 2003;46:279–288. doi: 10.1007/s00248-002-3007-7. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Park H.D., Ozawa K., Tanaka Y., Sumino T., Hamana K., Hiraishi A., Kato K. Sphingosinicella microcystinivorans gen. nov., sp. nov., a microcystin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2006;56:85–89. doi: 10.1099/ijs.0.63789-0. [DOI] [PubMed] [Google Scholar]
- Miller M.J., Fallowfield H.J. Degradation of cyanobacterial hepatotoxins in batch experiments. Water Sci. Technol. 2001;43:229–232. [PubMed] [Google Scholar]
- Mohamed Z.A., Al-Shehri A.M. Microcystin-producing blooms of Anabaenopsis arnoldi in a potable mountain lake in Saudi Arabia. FEMS Microbiol. Ecol. 2009;69:98–105. doi: 10.1111/j.1574-6941.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- Neilan B.A. Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Appl. Environ. Microb. 1995;61:2286–2291. doi: 10.1128/aem.61.6.2286-2291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybom S.M.K., Salminen S.J., Meriluoto J.A.O. Specific strains of probiotic bacteria are efficient in removal of several different cyanobacterial toxins from solution. Toxicon. 2008;52:214–220. doi: 10.1016/j.toxicon.2008.04.169. [DOI] [PubMed] [Google Scholar]
- Nybom S.M., Salminen S.J., Meriluoto J.A. Removal of microcystin-LR by strains of metabolically active probiotic bacteria. FEMS Microbiol. Lett. 2007;270:27–33. doi: 10.1111/j.1574-6968.2007.00644.x. [DOI] [PubMed] [Google Scholar]
- Oksanen I., Jokela J., Fewer D.P., Wahlsten M., Rikkinen J., Sivonen K. Discovery of rare and highly toxic microcystins from lichen-associated cyanobacterium Nostoc sp. strain IO-102-I. Appl. Environ. Microbiol. 2004;70:5756–5763. doi: 10.1128/AEM.70.10.5756-5763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.D., Sasakum Y., Maruyama T., Yanagisawa E., Hiraishi A., Kato K. Degradation of the cyanobacterial hepatotoxin Microcystin by a new bacterium isolated from a hyperthophic lake. Environ. Toxicol. 2001;16:337–343. doi: 10.1002/tox.1041. [DOI] [PubMed] [Google Scholar]
- Poppert S., Essig A., Stoehr B., Steingruber A., Wirths B., Juretschko S., Reischl U., Wellinghausen N. Rapid diagnosis of bacterial meningitis by real time PCR and fluorescence in situ hybridization. J. Clin. Microbiol. 2005;43:3390–3397. doi: 10.1128/JCM.43.7.3390-3397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapala J., Berg K., Lyra C., Niemi R.M., Manz W., Suomalainen S., Paulin L., Lahti K. Paucibacter toxinivorans gen.nov., sp. nov., a bacterium that degrades cyclic cyanobacterial hepatotoxins microcystins and nodularin. Int. J. Syst. Evol. Microbiol. 2005;55(4):1563–1568. doi: 10.1099/ijs.0.63599-0. [DOI] [PubMed] [Google Scholar]
- Saito T., Okano K., Park H.D., Itayama T., Inamori Y., Neilan B.A., Burns B.P., Sugiura N. Detection and sequencing of the microcystin LR-degrading gene, mlrA, from new bacteria isolated from Japanese lakes. FEMS Microbiol. Lett. 2003;229:271–276. doi: 10.1016/S0378-1097(03)00847-4. [DOI] [PubMed] [Google Scholar]
- Takenaka S., Tanaka Y. Behavior of microcystins and its decomposition product in water treatment process. Chemosphere. 1995;31:3635–3641. [Google Scholar]
- Takenaka S., Watanabe M.F. Microcystin LR degradation by Pseudomonas aeruginosa alkaline protease. Chemosphere. 1997;34:749–757. doi: 10.1016/s0045-6535(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Asakawa M., Anzai Y., Sumino T., Harada K.I. Degradation of microcystins using immobilized microorganism isolated in an eutrophic lake. Chemosphere. 2006;65:117–124. doi: 10.1016/j.chemosphere.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Naito S., Kondo F., Ishikawa N., Watanabe F.M., Suzuki M., Harada K.I. Stability of microcystins from cyanobacteria: effect of light on decomposition and isomerization. Environ. Sci. Technol. 1994;28:173–177. doi: 10.1021/es00050a024. [DOI] [PubMed] [Google Scholar]



