Graphical abstract
Keywords: Piper betle, Ethanolic leaf extract, Cadmium, Oxidative stress, Antioxidant, Liver, Rats
Abstract
The present study was undertaken to examine the attenuative effect of Piper betle leaf extract (PBE) against cadmium (Cd) induced oxidative hepatic dysfunction in the liver of rats. Pre-oral supplementation of PBE (200 mg/kg BW) treated rats showed the protective efficacy against Cd induced hepatic oxidative stress. Oral administration of Cd (5 mg/kg BW) for four weeks to rats significantly (P > 0.05) elevated the level of serum hepatic markers such as serum aspartate transaminase (AST), serum alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), gamma-glutamyl transpeptidase (GGT), bilirubin (TBRNs), oxidative stress markers viz., thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides (LOOH), protein carbonyls (PC) and conjugated dienes (CD) and significantly (P > 0.05) reduced the enzymatic antioxidants viz., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GR) and glucose-6-phosphate dehydrogenase (G6PD) and non-enzymatic antioxidants Viz., reduced glutathione (GSH), total sulfhydryls (TSH), vitamin C and vitamin E in the liver. Pre-oral supplementation of PBE (200 mg/kg BW) in Cd intoxicated rats, the altered biochemical indices and pathological changes were recovered significantly (P > 0.05) which showed ameliorative effect of PBE against Cd induced hepatic oxidative stress. From the above findings, we suggested that the pre-administration of P. betle leaf extract exhibited remarkable protective effects against cadmium-induced oxidative hepatic injury in rats.
1. Introduction
It has become evident that increasing human activities have modified the global cycle of heavy metals and metalloids, including the toxic non-essential elements like cadmium. Thus there is ample opportunity for exposure to cadmium both in and outside the work place (Vinoth Kumar et al., 2010). Cadmium (Cd) is one of toxic heavy metal and it is reported that increased concentration of Cd in agricultural soils is known to come from the application of phosphate fertilizers, sewage sludge, waste water and pesticides (Limei et al., 2008). Further sources of cadmium derived from mining activities at mines, smelting of metalliferous ores with high Cd content and industrial application of Cd in pigments, plastic stabilizers, nickel cadmium batteries resulted in wide spread agricultural soil pollution (Liao et al., 2005). The dispersed Cd in the soil can persist for decades and can be taken up by plants, from soil since; this metal is water soluble and easily and efficiently transfers from soil to plants. This may affect the target species if there is intake of feed ingredients from Cd contaminated plant sources (Satarug et al., 2003). Cd accumulates in the biological system because of its long biological half-life (10–30 years) (Jarup, 2002). Cd causes poisoning in various tissues of humans and animals (Ramesh and Satakopan, 2010). Prolonged exposure to Cd results in injury to the liver, lungs, kidney and testes (Zitkevicius et al., 2011).
The toxic effect of Cd is due to its inhibition of liver metabolic enzyme systems containing sulfhydryl groups and uncoupling of oxidative phosphorylation in the mitochondria (Williams et al., 1999). This results in increased lipid peroxidation, DNA damage, depletion of sulfhydryls, altered calcium homeostasis, hepatic congestion, ischemia and hypoxia (Bharavi et al., 2010; Habeebu et al., 1998). Several mitigative measures have been suggested to explain the damage induced by Cd. Sulfhydryl rich proteins are the major target of oxidative damage induced by Cd and loss of their function is usually a consequence of their modification by Cd. It has a special affinity toward sulfhydryl groups of proteins by covalent binding. Cd can block the functional sites of the catalytic (or) binding domains of enzyme or modify the protein conformation (Stohs and Bagchi, 1995). The second possible mechanism of Cd toxicity is the displacement of essential metals such as zinc and selenium requiring enzymes that are inactivated through direct displacement from their binding site by Cd (Gupta et al., 1991). Finally Cd exerts its toxic effect via oxidation damage to cellular organelles by inducing the generation of reactive oxygen species (ROS) (Stohs et al., 2000; Bagchi et al., 1996), which consist mainly of and OH−. The mechanisms through which this happens are not well understood but reports have indicated that Cd does this via an indirect phenomenon (Watkin et al., 2003). Reactions of these ROS with cellular biomolecules have been shown to lead to lipid peroxidation, membrane protein and DNA damage (Bharavi et al., 2010). This possibly leads to the depletion of the body’s endogenous antioxidants which serve as a premier source of protection against free radicals and other oxidative stressors to which it invariably becomes exposed (Cross et al., 1987).
Traditionally, ethnomedicines are extensively used in India and elsewhere due to their low cost, easy accessibility to everyone and perceived fewer side effects (Rathee et al., 2006). In many respects, the mechanism of action of the herbal drugs differs from that of the synthetic drugs (or) pure compounds. This can be characterized as a polyvalent action and interpreted as an additive or, in some cases, potentiating. P. betle is used to treat alcoholism, bronchitis, asthma, leprosy and dyspepsia (Chakraborthy and Shah, 2011). Earlier, anti-ulcerogenic activity of P. betle was attributed to its antioxidative property. A preliminary study has reported P. betle leaves extract contains a large number of bioactive molecules like polyphenols, alkaloids, steroids, saponins and tannins (Koff et al., 1971). Some of the chemical constituents isolated from P. betle leaves include estragole, catechols, terpenes, lominene and cardinene (Ponglux et al., 1987). The leaf extract of p. betle has also been reported to exhibit biological capabilities of detoxification, antioxidative and antimutagenic activities (Chakraborthy and Shah, 2011). P. betle leaves are also known to contain significant amounts of antioxidants like hydroxychavicol, eugenol, ascorbic acid and β-carotene (Wealth of India, 1992; Daniel, 1991). Biologically P. betle is an aromatic, stimulant, carminative, astringent and antiseptic (Kirtikar and Basu, 1987; Panda, 2004). Leaf possesses activities like antifungal, hypotensive, respiratory depressant, antihelminthic, cardiotonic, antiplatelet, antifertility, antitumour, antiulcer and antibacterial (Manigauha et al., 2009). The possible hepatoprotective activity of P. betle leaves against Cd induced hepatotoxicity has not been reported so far. Therefore, in this study, we aimed to evaluate the hepatoprotective effect of the P. betle leaves by using the Cd-induced sub-chronic oxidative liver injury model in rats.
2. Materials and methods
2.1. Chemicals
Cadmium chloride, 2-thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), reduced glutathione (GSH), 2,2-dipyridyl, xylenol orange, 2,4-dinitrophenylhydrazine (DNPH), γ-glutamyl-p-nitroanilide, 5,5-dithiobis-2-nitrobenzoic acid were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The rest of the chemicals utilized were obtained from a local firm (India) and were of analytical grade.
2.2. Plant material and extract preparation
Fresh P. betle leaves were collected during the month of March, 2008 from kumbakonam near chidambaram. The leaves were identified and authenticated by a herbalist, Department of Botany, Annamalai University. The voucher specimen (Herbarium No: 113/2008) was stored in the Department of Botany.
2.2.1. Piper betle
The P. betle plant belongs to the family Piperaceae and is widely growing in the tropical humid climates of Southeast Asia and its leaves, with a strong pungent and aromatic flavor are widely consumed as a mouth freshener. The leaves are credited with wound healing, digestive and pancreatic lipase stimulant activities in the traditional medicine which has also been proved in experimental animals (Chatterjee and Pakrashi, 1995; Santhanam and Nagarajan, 1990; Prabhu et al., 1995). In addition the leaf extract has also been reported to have antioxidant, antimicrobial, antifungal, anti-inflammatory and radio- protective properties (Ramji et al., 2002; Majumdar et al., 2003; Bhattacharya et al., 2005).
The principle chemical constituents of P. betle were found to be polyphenols like eugenol, chavicol, charvacrol, chevibetol, catechol and allyl pyrocatechol and vitamin C, which were reported to exhibit strong antioxidant activity. Further these polyphenols exert their protective activities through their superior radical scavenging and immune modulating potentials (Bhattacharya et al., 2005).
2.2.2. Preparation of ethanolic extract of the leaves of P. betle
The fresh leaves of P. betle were washed and shade dried for one week. The air dried leaves were milled into fine powder in a commercial blender. The powder (500 g) was Soxhlet extracted with 95% ethanol (1:3, w/v) at 37 °C for 2 days. The resultant extract was concentrated to dryness under reduced pressure and was freeze dried. The total yield was 9.38 g (1.87%, w/w) of light greenish brown extract. The ethanolic leaf extract of P. betle (PBE) was reconstituted to a final concentration of 5% (w/v) using aqueous solution of gum acacia (5%) for further treatments.
2.3. Dosage fixation
The oral LD50 dose of CdCl2 in rat was 75 mg/kg BW (Weil, 1952). In the present study, we have selected 5 mg/kg BW of CdCl2 which was 1/15 of the LD50 dose of CdCl2. And this particular dose level of CdCl2 has been reported to produce hepato toxicity in rats (EI-demerdash et al., 2004).
According to the available literature, the LD50 value of the ethanolic extract of P. betle was found to be above 3 g/kg and has been proved to be non-toxic to rats. The oral dose of 200 mg/kg of the ethanolic extract of P. betle was reported to be more effective in reducing the hepatotoxicity induced by alcohol in rats (Saravanan et al., 2004). A pilot study was conducted with three different doses of PBE (100, 200 and 400 mg/kg) to determine the dose dependent effect of PBE in Cd treated hepatotoxic rats. After 4 weeks of experiment, it was observed that PBE pretreatment at the doses of 100, 200 and 400 mg/kg significantly (p < 0.05) lowered the levels of serum transaminases, thiobarbituric acid reactive substances and elevated the levels of reduced glutathione in the liver of Cd intoxicated rats (data have not shown). 200 mg/kg of PBE showed higher significant protective effect than the other doses 100 and 400 mg/kg against Cd intoxication. Hence, we have chosen the 200 mg/kg of PBE for our further biochemical and histological studies.
2.4. Determination of total phenolic and total flavonoid contents
Total phenolic content in the extract was determined by Folin–Ciocalteu method (Sirivasan et al., 2007), and it was expressed as gallic acid equivalents (GAE) (mg/g). Total flavonoids content in the extract was measured as described previously by Piccolella et al. (2008) and it was calculated as rutin equivalents (mg/g).
2.5. Animals
Male albino Wistar rats, body weight of 180–200 g bred in Central Animal House, Rajah Muthiah Medical College, Annamalai University, were used in this study. Throughout the study, the animals were housed six animals per each polypropylene cage and were maintained in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India, and approved by the Institutional Animal Ethical Committee (Vide No. 531/2008), Annamalai University. The rats were allowed standard rat pellet diet (Lipton India Ltd., Mumbai, India) and water ad libitum for the duration of the experiment.
2.6. Experimental design
The rats were randomly divided into four groups of six rats in each group
-
•
Group 1: Control rats intragastrically administered with normal saline.
-
•
Group 2: Rats intragastrically administered with PBE (200 mg/kg/day) for 28 days using intragastric tube.
-
•
Group 3: Rats orally received Cd as cadmium chloride (5 mg/kg/day) (Renugadevi and Milton Prabu, 2010) in saline for 28 days.
-
•
Group 4: Rats received PBE (200 mg/kg/day) followed by oral administration of Cd as cadmium chloride (5 mg/kg/day) for 28 days.
Food and water intake was recorded and rats were weighed every week. Forty-eight hours after the administration of the last dose, the animals were anesthetized with an intramuscular injection of ketamine hydrochloride (24 mg/kg) and sacrificed by decapitation. Blood was collected in tubes for the separation of serum. The liver tissue was dissected out, weighed and washed using chilled saline solution. Tissue was minced and homogenized (10%, w/v) in appropriate buffer (pH 7.4) and centrifuged (3000g for 10 min). The resulting clear supernatant was used for various enzymatic and non-enzymatic biochemical assays. Six rats from each group were sacrificed and used for analyzing serum and tissue biochemical assays.
2.7. Biochemical assays
2.7.1. Activities of serum marker enzymes
The activities of serum aspartate aminotransferase (EC. 2.6.1.1), alanine aminotransferase (EC. 2.6.1.2), alkaline phosphatase (EC. 3.1.3.1), lactate dehydrogenase (EC. 3.1.3.1) and total bilirubin were assayed using commercially available diagnostic kits (Sigma diagnostics (I) Pvt. Ltd., Baroda, India). Gamma-glutamyl transferase (EC. 2.3.2.2) activity was determined by the method of Rosalki et al. (1970) using γ-glutamyl-p-nitroanilide as substrate.
2.7.2. Determination of lipid peroxidation and oxidative stress markers
Lipid peroxidation in the liver was estimated spectrophotometrically by measuring thiobarbituric acid reactive substances and lipid hydroperoxides by the method of Niehaus and Samuelsson (1968) and Jiang et al. (1992) respectively. Protein carbonyl content was determined by the method of Levine et al. (1999). The levels of conjugated dienes were assessed by the method of Rao and Recknagel (1968).
2.7.3. Determination of non-enzymatic and enzymatic antioxidants
Reduced glutathione was determined by the method of Ellman (1959). Oxidized glutathione was estimated by the method of Hissin and Hilf (1976). Total sulfhydryl groups were measured by the method of Sedlak and Lindsay (1958). Vitamin C concentration was measured as previously reported (Omaye et al., 1979). Vitamin E (α-tocopherol) was estimated by the method of Desai (1984). Superoxide dismutase activity was determined by the method of Kakkar et al. (1984). The activity of catalase was determined by the method of Sinha (1972). Glutathione peroxidase activity was estimated by the method of Rotruck et al. (1973). Glutathione S-transferase activity was determined by the method of Habig et al. (1974). Glutathione reductase was assayed by the method of Horn and Burns (1978). The estimation of glucose-6-phosphate dehydrogenase was carried out by the method of Beutler (1983).
2.7.4. Estimation of membrane-bound ATPase
The sediment after centrifugation was resuspended in ice-cold Tris–HCl buffer (0.1 M) pH 7.4. This was used for the estimations of membrane-bound enzymes and protein content. The membrane bound enzymes such as Na+/K+-ATPase, Ca2+-ATPase and Mg2+-ATPase activity were assayed by estimating the amount of phosphorous liberated from the incubation mixture containing tissue homogenate, ATP and the respective chloride salt of the electrolytes (Bonting, 1970; Hjerten and Pan, 1983; Ohnishi et al., 1982). Total protein content was estimated by the method described by Lowry et al. (1951).
2.8. Histopathological studies
For qualitative analysis of liver histology, the tissue samples were fixed for 48 h in 10% formalin-saline and dehydrated by passing successfully in different mixture of ethyl alcohol, water, cleaned in xylene and embedded in paraffin. Sections of the tissues (5–6 μm thick) were prepared by using a rotary microtone and stained with hematoxylin and eosin dye, which was mounted in a neutral deparaffined xylene medium for microscopical observations. Six rats from each group were sacrificed for analyzing the hepatic histological examinations.
2.9. Statistical analyses
Data were analyzed by one way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using a commercially available statistics software package (SPSS® for Windows, V. 13.0, Chicago, USA). Results were presented as mean ± SD. p values <0.05 were regarded as statistically significant.
3. Results
3.1. Total phenolic and total flavonoid contents
Total phenolic content estimated as 257.2 ± 10.5 mg gallic acid equivalent/g dry weight of the extract. Total flavonoids content estimated as 212.7 ± 9.7 mg rutin equivalents/g dry weight of the extract.
3.2. Body weight gain, relative liver weight, feed and water intake
The effect of Cd and PBE on food and water intake, body weight gain and liver-body weight ratio (%) in normal and experimental animals were shown in Table.1. In Cd treated rats, water and pellet diet consumption significantly (p < 0.05) decreased with decrease in body weight gain. A significant (p < 0.05) increase in liver-body weight ratio was noticed in Cd treated rats. No significant changes were observed between control and PBE administered rats. All these alterations induced by Cd intoxication were significantly (p < 0.05) restored to near normal levels upon pre-administration of PBE.
Table 1.
Effect of Cd and PBE on body weight gain, food intake, water intake and organ-body weight ratio in control and experimental rats.
| Variables | Control | PBE | Cd | Cd + PBE | |
|---|---|---|---|---|---|
| Body weight | Initial (g) | 190 ± 10.58a | 191 ± 9.87a | 189 ± 9.71 a | 188 ± 11.18a |
| Final (g) | 227 ± 12.47a | 228 ± 11.68a | 211 ± 10.17 b | 221 ± 10.79c | |
| Body weight gain (%) | 19.47 | 19.37 | 11.64 | 17.55 | |
| Food intake (g/100 g bw/day) | 12.15 ± 0.93a | 8.09 ± 0.71b | 11.89 ± 0.87a | 10.51 ± 0.81c | |
| Water intake (mL/rat/day) | 18.20 ± 2.50a | 13.50 ± 1.25b | 17.50 ± 2.50a | 16.10 ± 1.50c | |
| Organ-body weight ratio (%) | 2.94 ± 0.33a | 3.85 ± 0.42b | 3.01 ± 0.41a | 3.27 ± 0.36c | |
Values are expressed as mean ± SD for six rats in each group.
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
3.3. Effects on serum hepatic marker enzymes and bilirubin
Fig. 1 shows the status of serum AST, ALT, ALP, LDH, GGT and bilirubin levels of control and experimental animals. Cd induced hepatotoxicity is shown by the elevated levels of serum hepatic markers as compared to vehicle treated normal control (Group I). This elevated level of serum hepatic markers due to Cd challenge were significantly (p < 0.05) decreased upon the pre-treatment with PBE.
Figure 1.
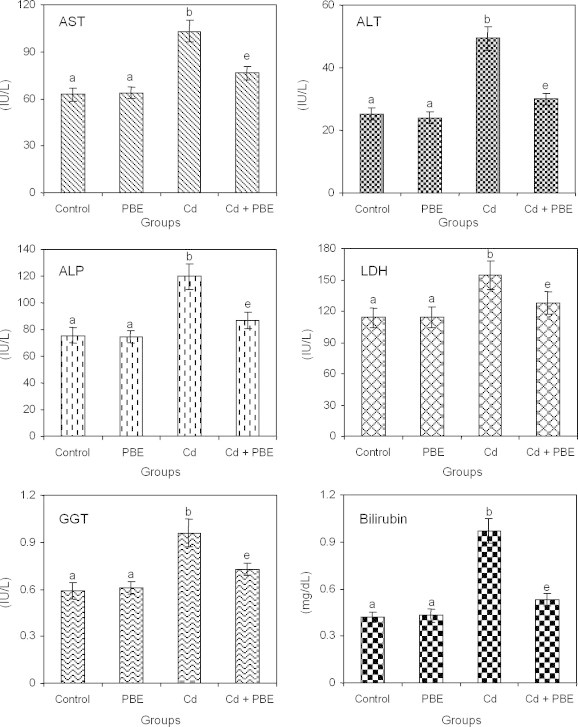
Changes in the activities of serum hepatic markers in control and experimental rats.
3.4. Effects on hepatic oxidative stress markers
The levels of lipid peroxidation and protein oxidation in the liver tissue of control and experimental rats are illustrated in Table 2. The levels of lipid peroxidation products viz., thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides (LOOH), protein carbonyl contents (PCC) and conjugated dienes (CD) significantly (p < 0.05) increased in Cd intoxicated rats when compared with control rats. Treatment with PBE afforded significant (p < 0.05) hepatoprotection against Cd induced elevation of lipid peroxidation and protein carbonylation as evidenced by a significant fall in their levels.
Table 2.
Effect of Cd and PBE on hepatic TBARS, LOOH, PCC and CD of control and experimental rats.
| Groups | Control | PBE | Cd | Cd + PBE |
|---|---|---|---|---|
| TBARS (mg/g) | 6.84 ± 0.48a | 6.32 ± 0.67a | 18.27 ± 1.72b | 9.62 ± 0.97c |
| LOOH (mmol/g) | 0.87 ± 0.08a | 0.79 ± 0.09a | 1.64 ± 0.15b | 1.07 ± 0.11c |
| PCC (nmol/mg) | 2.57 ± 0.25a | 2.48 ± 0.23a | 6.87 ± 0.65b | 3.28 ± 0.27c |
| CD (mmol/mg) | 52.17 ± 0.34a | 51.03 ± 0.45a | 87.42 ± 0.77b | 65.32 ± 0.56c |
Values are expressed as mean ± SD for six rats in each group.
Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
3.5. Effects on hepatic non-enzymatic antioxidants
Alterations in the levels of non-enzymatic antioxidants namely reduced vitamins C and E, reduced glutathione (GSH), oxidized glutathione (GSSG), total sulfhydryls (TSH), in the liver of control and experimental animals are shown in Table 3. A significant depletion in the levels of these non-enzymatic antioxidants and increased level of oxidized glutathione in the liver was observed in Cd intoxicated rats when compared with normal control rats. Upon Pre-administration with PBE along with Cd for 4 weeks, the levels of these non-enzymatic antioxidants and oxidized glutathione were significantly (p < 0.05) restored to their near normal levels when compared with Cd treated rats.
Table 3.
Effect of Cd and PBE on vitamin ‘C’, vitamin ‘E’, reduced glutathione (GSH), oxidized glutathione (GSSG) and total sulfhydryl groups (TSH) in the liver of control and experimental rats.
| Parameters | Control | PBE | Cd | Cd + PBE |
|---|---|---|---|---|
| Vitamin ‘C’ (μmol/mg) | 1.94 ± 0.18a | 2.01 ± 0.21a | 1.53 ± 0.11b | 1.76 ± 0.17c |
| Vitamin ‘E’ (μmol/mg) | 1.37 ± 0.10a | 1.39 ± 0.11a | 0.93 ± 0.07 b | 1.24 ± 0.08c |
| GSH (μg/mg) | 20.35 ± 1.27a | 22.74 ± 1.51a | 14.51 ± 1.15b | 18.03 ± 1.21c |
| GSSG (nmol/mg | 0.36 ± 0.03 a | 0.34 ± 0.02a | 0.69 ± 0.08 b | 0.47 ± 0.05c |
| TSH (μg/mg) | 14.52 ± 0.81a | 15.78 ± 0.89a | 9.81 ± 0.69b | 12.45 ± 0.74c |
Values are expressed as mean ± SD for six rats in each group.
Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
3.6. Effects on hepatic enzymatic antioxidants
The activities of enzymatic antioxidants namely SOD, CAT, GPx, GST, GR and G6PD in the liver of control and experimental rats are shown in Table 4. A significant (p < 0.05) decrease in the activities of these antioxidant enzymes was recorded in Cd intoxicated rats when compared with normal control rats. Prior oral pre-administration of PBE along with Cd for 4 weeks significantly (p < 0.05) restored the activities of these antioxidant enzymes to their near normal levels as compared with Cd treated rats.
Table 4.
Effect of Cd and PBE on superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GR) and glucose 6-phosphate dehydrogenase (G6PD) in the liver of control and experimental rats.
| Groups | Control | PBE | Cd | Cd + PBE |
|---|---|---|---|---|
| SOD (Units/mg protein) | 7.81 ± 0.53a | 8.29 ± 0.67a | 5.27 ± 0.43b | 7.08 ± 0.17c |
| CAT (μmol/min mg protein) | 93.25 ± 5.84a | 96.21 ± 5.26a | 55.18 ± 4.26b | 78.34 ± 5.81c |
| GPx (μg/min mg protein) | 9.67 ± 0.62a | 9.83 ± 0.71a | 5.35 ± 0.41b | 8.89 ± 0.72c |
| GST (μmol/min mg protein) | 7.83 ± 0.52a | 8.26 ± 0.57a | 5.71 ± 0.27b | 7.15 ± 0.41c |
| GR (nmol/min mg protein) | 0.48 ± 0.04a | 0.51 ± 0.04a | 0.29 ± 0.02b | 0.40 ± 0.03c |
| G6PD (nmol/min mg protein) | 2.07 ± 0.11a | 2.12 ± 0.15a | 1.43 ± 0.07b | 1.83 ± 0.10c |
Values are expressed as mean ± SD for six rats in each group.
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
3.7. Effects on hepatic membrane bound ATPases
The activities of Na+, K+, Ca2+ and Mg2+–ATPases significantly (p < 0.05) decreased in the liver tissue of Cd treated rats as compared to normal controls (Table 5). Administration of PBE significantly (p < 0.05) prevented the decrease in the activities of all ATPases in Cd intoxicated rats. PBE alone treated group did not show any alteration in the activities of these ATPases in the liver.
Table 5.
Effect of Cd and PBE on membrane bound adenosine triphosphatases (ATPase) in the liver of control and experimental rats.
| Groups | Control | PBE | Cd | Cd + PBE |
|---|---|---|---|---|
| Total ATPases | 1.76 ± 0.19a | 1.79 ± 0.18a | 1.19 ± 0.10b | 1.57 ± 0.15c |
| Na+K+–ATPases | 0.44 ± 0.04a | 0.47 ± 0.04a | 0.28 ± 0.02b | 0.38 ± 0.03c |
| Ca2+–ATPases | 0.58 ± 0.07a | 0.56 ± 0.07a | 0.34 ± 0.03b | 0.53 ± 0.06c |
| Mg2+– ATPases | 0.74 ± 0.08a | 0.76 ± 0.07a | 0.57 ± 0.05b | 0.66 ± 0.06c |
Values are expressed as mean ± SD for six rats in each group.
Units for ATPases μg pi liberated/min/mg protein.
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
Values are not sharing a common superscript letter (a–c) differ significantly at p < 0.05 (DMRT).
3.8. Histological examination of liver tissue
Histopathological investigations showed that the administrations of As in rats produced severe hepatic damage including the extensive degeneration of hepatocytes with necrosis, inflammation, vacuolization, inflammatory cell infiltration and fatty degenerative changes (Figs. 2B and 2C) when compared with control rats (Fig. 2A). The histopathological abnormalities induced by Cd were significantly (p < 0.05) ameliorated in the liver of rats administered with SB (Fig. 2D). The histoarchitectural pattern of the liver was almost normal in SB administered rats (Fig. 2E).
Figure 2.
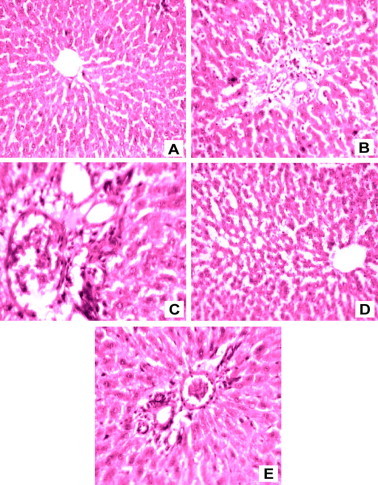
Liver section of rats: (A) control showing normal arrangement of hepatocytes and central vein (H&E ×100); (B) cadmium treated rats showing periportal inflammation, degeneration of hepatocytes and necrosis (H&E ×100); (C) cadmium treated rats showing micro vesicular steatosis, balloon degeneration and inflammatory cell infiltration (H&E ×200); (D) cadmium and PBE treated rats showing almost normal appearance of hepatocytes and central vein with mild derangement of hepatic cords (H&E ×100); (E) PBE alone treated rats showing normal hepatocytes and portal triad (H&E ×100).
4. Discussion
Environmental contamination by cadmium is recognized as a global problem. Cd is a potent inducer of oxidative stress and affects cellular antioxidant defense potential biphasically by inhibition and enhancement of several antioxidant enzymatic and non-enzymatic molecules activity. In the present study, the highly potent chelation therapy of PBE was examined against Cd induced toxicity in the liver of rats.
The mean body weight of Cd exposed group decreased along with the increase in relative liver weight, which is in agreement with the findings of EI-demerdash et al. (2009). Rahman et al. (1998) suggest that prolonged exposure of Cd is associated with an increased risk of diabetes mellitus, which explains the weight loss in rats. Kaltreider et al. (2001) reported that exposure to low level of heavy metals impairs the glucocorticoid system. The glucocorticoid hormones play a vital role in glucose regulation as well as carbohydrate, lipid and protein metabolism. Dysfunction in the glucocorticoid system has been linked to weight gain/loss. Cd intoxicated rats pre-treated with PBE, the altered body weight and liver weight parameters were recovered to near normal levels due to the antioxidant effects of polyphenols found in PBE.
Liver dysfunction is accompanied by elevated levels of serum hepatic marker enzymes which are indicative of cellular leakage and loss of functional integrity of cell membrane in the liver (Zimmerman and Seef, 1970). High levels of cytosolic and mitochondrial enzymes AST and ALT are better parameters to detect liver damage (Williamson et al., 1996). The membrane bound enzyme ALP and bilirubin levels are also related to the status and function of hepatic cells. Increased serum ALP is due to increased synthesis in the presence of increasing biliary pressure (Burtis and Ashwood, 1986). LDH is an intracellular enzyme. Increased levels of serum LDH is an indicator of hepato cellular damage (Kim et al., 2001). GGT is a microsomal brush border enzyme found notably in liver. It is involved in the transfer of aminoacids across the cellular membrane. It is also involved in GSH metabolism by transferring the glutamyl moiety to a variety of acceptors leaving the cysteine product to preserve the intracelluluar homeostasis of oxidative stress (Meister, 1992). Serum GGT level is considered as a better index and to be highly sensitive to biliary tract damage (Rosalki and Rao, 1972). Several reports have explained the elevated levels of serum hepatic marker enzymes due to Cd exposure (Amin et al., 2006; Selvarajan et al., 2007; Milton Prabu et al., 2007). In the present study, the increased levels of serum hepatic market enzymes and bilirubin were released into the blood from the membrane of hepatocytes due to the extensive injury of the liver that was caused by chronic exposure to Cd. Significant restoration of hepatic serum marker enzymes and bilirubin were observed in the animals pre-treated with PBE offering protection against Cd toxicity in rats. This protective effect of PBE may be due to its active components such as polyphenols, β-carotene and vitamin-C. Polyphenols have been reported to possess a membrane stabilizing activity (Chen et al., 1990) by inhibiting the Cd induced generation of reactive oxygen species and maintain the structural integrity of the membrane.
Lipid peroxidation is one of the main manifestations of oxidative damage and has been found to play an important role in the toxicity of cadmium (Stohs and Bagchi, 1995). Cd induced oxidative stress by producing hydroxyl radicals, superoxide anions, nitric oxide and hydrogen peroxide (Koizumi and Li, 1992; Weisberg et al., 2003). Significant increase in the level of hepatic TBARS in Cd intoxicated rats could be possibly due to excessive formation of free radicals which leads to the deterioration of biological macromolecules (Stohs et al., 2000). Milton Prabu et al. (2007) have reported that lipid peroxidation is considered a sensitive marker of Cd hepato toxicity. Hussain et al. (1987) and Manca (1991) have also reported the increased level of LPO in the various tissues of Cd treated rats. In the present investigation Cd intoxicated rats pretreated with PBE showed a marked decrease in the levels of TBARS, LOOH, PCC and CD. This may be due to the presence of polyphenols, β-carotene and vitamin-C, which have been recognized as excellent scavengers of free radicals thereby inhibiting lipid peroxidation and protein carbonylation (Chakraborthy and Shah, 2011; EI-demerdash et al., 2004).
GSH a tripeptide (L-r-glutamylcysteinyl glycine) and a cysteine rich protein participates in the maintenance of cytoplasmic and membrane thiol status. It is an antioxidant and a powerful nucleophile, critical for cellular protection such as detoxification of ROS. In the present investigation, the reduced level of hepatic GSH by Cd could be probably due to either increased utilization of GSH by the cells act as scavengers of free radicals produced by Cd or increased utilization of GSH for the activity of GPx forming oxidized GSH (GSSG) due to increased generation of ROS (Larson, 1988). Significant depletion of hepatic GSH has also been reported by Jeyaprakash and Chinnaswamy (2005) in cadmium intoxicated rats. Vitamin C along with vitamin E and GSH that participates in the scavenging of ROS (Niki et al., 1986). Significant enhancement and restoration of reduced GSH, TSH, vitamin ‘C’ and vitamin ‘E’ in rats treated with PBE definitely revealed the protective nature of the PBE against Cd hepatotoxicity. This possible protective effect may be due to antioxidant and free radical scavenging activity of polyphenols found in PBE (Rathee et al., 2006; Chakraborthy and Shah, 2011).
Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) constitute a mutually supportive team of antioxidant defense against reactive oxygen species. SOD is a metalloenzyme that catalyzes the dismutation of superoxide radicals (McCord, 1987). CAT is a hemeprotein which catalyses the reduction of H2O2 to water and oxygen and thus protects the cells from the oxidative damage of H2O2 and OH– (Chance et al., 1952). GPx is a seleno enzyme, two thirds of which is present in the cytosol and one-thirds in the mitochondria. It plays a major role in the reduction of hydrogen peroxide and hydroperoxides. GST catalyzes the Conjugation of xenobiotic electrophilic substances with GSH to form the corresponding GSH-S-conjugate. GR utilizes the NADPH and maintains the GSH in a reduced form (Moron et al., 1979). Glucose-6-phosphate dehydrogenase (G6PDH) is responsible for generating the NADPH, required for the recycling reaction of GSSG to GSH (Reed, 1990). In the present investigation Cd intoxicated rats showed a significant decrease in the activity of these antioxidant enzymes in liver tissue might be due to the over production of ROS, the primary mechanism of Cd toxicity (Casalino et al., 2002; Amin et al., 2006). Pretreatment with PBE in Cd intoxicated rats show a significant recovery of hepatic enzymatic antioxidant systems. Polyphenols were shown to be potent scavengers of superoxide anions, peroxides, hydroxyl and peroxyl radicals (Chakraborthy and Shah, 2011). Polyphenols have also been reported to increase the activities of antioxidant enzymes (Daniel et al., 1998). These findings support our observation of the fact that elevated activities of enzymatic antioxidants in the supplementation of PBE in Cd intoxicated rats.
The determination of membrane associated enzyme activities like adenosine triphosphatases (ATPases) indicates the changes in membranes under pathological conditions (Kempaiah and Srinivasan, 2006). ATPases are lipid dependent membrane-bound enzymes, which play a central role in the active transport of ions, maintenance of cellular homeostasis and are also involved in neurotransmission process, maintenance of ion gradients and regulation of cell volume. The peroxidation of membrane lipids not only alters the structural as well as functional integrity of cell membranes, but also affects the activities of various membrane-bound enzymes including Mg2+-ATPase, Ca2+-ATPase and Na+/K+-ATPase, which in turn lead to disruption in cellular homeostasis (Hazarika et al., 2003).
In the present study significantly decreased activities of membrane bound total ATPases in the liver were observed in Cd treated rats. Decreased activity of Na+/K+-ATPase could be due to enhanced lipid peroxidation by free radicals on Cd intoxication, since Na+/K+-ATPase is a ‘SH’ group containing enzyme and is lipid dependent (Ithayarasi and Devi, 1997). Decreased activity of Na+, K+-ATPase can lead to a decrease in sodium efflux, thereby altering membrane permeability (Finotti and Palatini, 1986). The disruption of membrane permeability or fragmentation of the membrane leads to the leakage of Ca2+ ions into cells thereby potentiating irreversible cell destruction. The Ca2+ overload medicated Cd also decreased the Ca2+-ATPase activity in cell membrane. It is generally accepted that due to high affinity for SH groups, Cd binds avidly to various enzyme proteins and inactivates them. Mg2+-ATPase activity is involved in other energy requiring processes in the cell and its activity is sensitive to lipid peroxidation. Administration of SB in Cd intoxicated rats significantly reduced the lipid peroxidation in liver tissue and sustained the activities of membrane bound enzymes. This could be due to the ability of PBE to protect the sulfhydryl groups from the oxidative damage through the inhibition of peroxidation of membrane lipids. This effect is due to the membrane stabilizing properties of flavonoids in PBE. It may also be due to the blocking nature of lipid peroxidation by polyphenols present in PBE. The preservation of cellular membrane integrity of PBE depends on its antioxidant properties that neutralize the oxidative reactions. In addition PBE administration significantly improved the levels of endogenous enzymatic and non-enzymatic antioxidants involved in membrane protection, which in turn reduced the Cd induced alterations in membrane bound enzymes as well as ionic gradients within the cell.
Histopathological results also supported our biochemical findings and confirmed the protective nature of PBE against Cd intoxication. According to light microscopic examination, the oxidative hepatic damage induced by Cd in rats was greatly reduced by the pre-administration of PBE, which was in good correlation with the results of the serum hepatic markers, oxidative stress markers, antioxidant enzyme activities and membrane bound ATPases.
The PBE contained high concentration of polyphenols. Polyphenols have been reported to possess metal chelating property, in addition to their high antioxidant activity. Protective effects of polyphenols on human health are partly explained by their metal chelating and antioxidant property (Gautam and Flora, 2010). The literature indicated that the major bioactive compound found in high concentration in PBE was allylpyrocatechol (Chakraborthy and Shah, 2011). Allylpyrocatechol is a well known antioxidant, which has shown a high degree of antioxidant power through invitro assays (Rathee et al., 2006; Goncalo et al., 2006). This may be the main reason for the antihepatotoxic activity of PBE against Cd induced oxidative hepatic dysfunction. However, further studies are necessary to find out the actual mechanism of action of phytochemicals and their doses in the presence of oxidative stress due to Cd intoxication.
In conclusion, our results demonstrated that Cd is capable of causing marked oxidative stress in addition to deplete the antioxidants and inhibiting the activities of antioxidant enzymes. The treatment with PBE could significantly attenuate the Cd induced oxidative hepatotoxicity and shows its therapeutic potential to be used as a cost effective safe herbal antioxidative agent in the treatment of Cd toxicity.
References
- Amin A., Hamza A.A., Daoud S., Hamsa W. Spirulina protects against cadmium induced hepatotoxicity in rats. American Journal of Pharmacology and Toxicology. 2006;1:21–25. [Google Scholar]
- Bagchi D., Bafchi E.A., Hassoun, Stohs S.J. Cadmium induced excretion of urinary lipid metabolite, DNA damage, glutathione depletion and hepatic lipid peroxidation in Sprague–Dawley rats. Biological Trace Element Research. 1996;52:143–154. doi: 10.1007/BF02789456. [DOI] [PubMed] [Google Scholar]
- Beutler E. Active transport of glutathione disulfide from erythrocytes. In: Larson A., Orrenius S., Holmgren A., Mannerwik B.E.D.S., editors. Functions of Glutathione, Biochemical, Physiological, Toxicological and Clinical Aspects. Raven Press; New York: 1983. p. 65. [Google Scholar]
- Bharavi K., Gopala Reddy A., Rao G.S., Ravikumar P., Rajasekhar Reddy A., Rama Rao S.V. Reversal of cadmium induced oxidative stress and its bioaccumulation by culinary herbs Murraya koenigii and Allium sativum. Research Journal of Pharmacology. 2010;4:60–65. [Google Scholar]
- Bhattacharya S., Subramanian M., Bauri A., Kamat J.P. Radioprotecting property of the ethonolic extract of the piper betel leaf. Journal of Radiation Research. 2005;46:165–171. doi: 10.1269/jrr.46.165. [DOI] [PubMed] [Google Scholar]
- Bonting S.L. Wiley Interscience; London: 1970. Membrane and ion transport, presence of enzyme systems in mammalian tissues. pp. 257–263. [Google Scholar]
- Burtis C.A., Ashwood E.R. W.B. Saunders Company; Philadelphia, Pennsylvania: 1986. Text book of clinical chemistry. [Google Scholar]
- Casalino E., Calzaterri C., Sblano C., Landriscina Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology. 2002;179:37–50. doi: 10.1016/s0300-483x(02)00245-7. [DOI] [PubMed] [Google Scholar]
- Chakraborthy D., Shah B. Antimicrobial, antioxidative and anti-hemolytic activity of Piper betle leaf extracts. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3:192–199. [Google Scholar]
- Chance B., Green Stein D.S., Roughton R.J.W. The mechanism of catalase action 1-steady state analysis. Archives of Biochemistry and Biophysics. 1952;37:301–339. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Pakrashi S.C. vol. 1. CSIR publication; New Delhi: 1995. (Treatise of Indian Medical Plants). p. 26. [Google Scholar]
- Chen Y.T., Zheng Jia Z.J., Ju Y. Flavonoids as superoxide scavengers and antioxidants. Free Radical Biology and Medicine. 1990;9:19–21. doi: 10.1016/0891-5849(90)90045-k. [DOI] [PubMed] [Google Scholar]
- Cross C.E., Halliwell B., Borish E.T. Oxygen radicals and human disease. Proceedings of a Conference. Annals of Internal Medicine. 1987;107:526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Daniel M. 1st ed. Kalyani Publishers; New Delhi, India: 1991. Method in Plant Chemistry and Economic Botany. [Google Scholar]
- Daniel R.S., Mathew B.C., Devi K.S., Augusti K.T. Antioxidant effect of two flavonoids from the bark of Ficus bengalensis Linn. in hyperlipidemic rats. Indian Journal of Experimental Biology. 1998;36:902–906. [PubMed] [Google Scholar]
- Desai I.D. Vitamin E analysis method for animal tissues. Methods in Enzymology. 1984;105:138–143. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- EI-demerdash F.M., Yousef I.M., Kedwany F.S., Baghadadi H.H. Cadmium induced changes in lipid peroxidation, blood heamatology, biochemical parameters and serum quality of male rats: protective role of vitamin E and β-carotene. Food and Chemical Toxicology. 2004;42:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- EI-demerdash F.M., Yousef I.M., Radwan M.E. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food and Chemical Toxicology. 2009;47:249–254. doi: 10.1016/j.fct.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulphydryl groups. Archives of Biochemistry and Biophysics. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Finotti P., Palatini P. Reduction of erythrocyte Na+K+ ATPase activity in type I insulin dependent diabetic subjects and its activation by homologus plasma. Diabetologia. 1986;29:623–628. doi: 10.1007/BF00869260. [DOI] [PubMed] [Google Scholar]
- Gautam P., Flora S.J.S. Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition. 2010;26:563–570. doi: 10.1016/j.nut.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Goncalo C.J., Catarina F., Correia C., Lurdes M., Rui M., Borges Dos Santos Antioxidative activity of a catechol derived from abietic acid. Journal of Agricultural Food Chemistry. 2006;14:342–348. doi: 10.1021/jf052062k. [DOI] [PubMed] [Google Scholar]
- Gupta S., Athar M., Behari J.R., Srirastava R.C. Cadmium mediated induction of cellular defence mechanism: a novel example for the development of adaptive response against a toxicant. Industrial Health. 1991;29:1–9. doi: 10.2486/indhealth.29.1. [DOI] [PubMed] [Google Scholar]
- Habeebu S.S., Liu J., Klaassen C.D. Cadmium induced apoptosis in mouse liver. Toxicology and Applied Pharmacology. 1998;149:203–209. doi: 10.1006/taap.1997.8334. [DOI] [PubMed] [Google Scholar]
- Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferase the first step in mercapturic acid formation. Journal of Biological Chemistry. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hazarika A., Sarkar S.N., Hajare S., Meena Kata ria, Malik J.K. Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology. 2003;185:1–8. doi: 10.1016/s0300-483x(02)00574-7. [DOI] [PubMed] [Google Scholar]
- Hissin P.J., Hilf R.A. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Analytical Biochemistry. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Hjerten S., Pan H. Purification and characterization of two forms of low affinity Ca++-ATPase from erythrocyte membrane. Biochimica et Biophysica Acta. 1983;728:281–288. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- Horn H.D., Burns F.H. Assay of glutathione reductase activity. In: Bergmeyer H.V., editor. Methods of Enzymatic Analysis. Academic Press; New York: 1978. pp. 142–146. [Google Scholar]
- Hussain T., Shukla G.S., Chandra S.V. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacology and Toxicology. 1987;60:355–358. doi: 10.1111/j.1600-0773.1987.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Ithayarasi A.P., Devi C.S. Effect of alpha-tocopherol on lipid peroxidation in isoproterenol induced myocardial infarction in rats. Indian Journal of Physiology and Pharmacology. 1997;41:369–376. [PubMed] [Google Scholar]
- Jarup L. Cadmium overload and toxicity. Nephrology Dialysis Transplantation. 2002;17:35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash K., Chinnaswamy P. Effect of Spirulina and Liv. 52 on cadmium toxicity in albino rats. Indian Journal of Experimental Biology. 2005;43:773–781. [PubMed] [Google Scholar]
- Jiang Z.Y., Hunt J.Y., Wolff S.P. Detection of lipid hydroperoxides using the Fox reagent. Analytical Biochemistry. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry and Biology. 1984;21:130–132. [PubMed] [Google Scholar]
- Kaltreider R.C., Davis A.M., Lariviere J.P., Hamilton J.W. Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environmental Health Perspectives. 2001;109:245–251. doi: 10.1289/ehp.01109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempaiah R.K., Srinivasan K. Protective effect of curcumin, capsaicin and garlic on erythrocyte integrity in high fat fed rats. Journal of Nutritional Biochemistry. 2006;17:471–478. doi: 10.1016/j.jnutbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kim K.V., Rhim T., Choi I., Kim S.S. N-Acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. Journal of Biological Chemistry. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- Kirtikar, K.R., Basu, B.D., 1987. Piperaceae. In: Indian medicinal plants. 2nd ed., International Book Distributors, Dehra Dun, pp. 2125–2238.
- Koff R.S., Gordan G., Sabesin S.M. D-Galactosamine hepatitis hepatocellular injury and fatty liver following a single dose. Proceedings of the Society for Experimental Biology and Medicine. 1971;137:696–701. [Google Scholar]
- Koizumi T., Li Z.G. Role of oxidative stress in single-dose, cadmium-induced testicular cancer. Journal of Toxicology and Environmental Health. 1992;37:25–36. doi: 10.1080/15287399209531654. [DOI] [PubMed] [Google Scholar]
- Larson R.A. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. [Google Scholar]
- Levine R.L., Garland D., Oliver C.N., Amic A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology. 1999;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Liao X.Y., Chen T.B., Xie H., Liv Y.R. Soil as contamination and its risk assessment in areas near the industrial districts of Chenzhou city. Southern China. Environmental International. 2005;31:791–798. doi: 10.1016/j.envint.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Limei Z., Xiaoyong L., Tongbin C., Xiulan Y., Hua X., Bin W., Lixia W. Regional assessment of cadmium pollution in agricultural lands and the potential health risk related to intensive mining activities. Journal of Environmental Sciences. 2008;20:696–703. doi: 10.1016/s1001-0742(08)62115-4. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with Folin-phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Majumdar B., Chaudhuri S.R., Roy A. Effect of ethanol extract of leaf of piper betel Linn. leaf on healing of NSAID-induced experimental ulcer-a novel role of free radical scavenging action. Indian Journal of Experimental Biology. 2003;41:311–315. [PubMed] [Google Scholar]
- Manca D. In vitro and in vivo response of rat tissue to cadmium induced lipid peroxidation. Bulletin of Environmental Contaminated Toxicology. 1991;46:929–936. doi: 10.1007/BF01689740. [DOI] [PubMed] [Google Scholar]
- Manigauha A., Patel S., Ali H., Chandy A., Uma Maheshwari M. Study the effect of phytochemical constituents of Piper betle leaves extracts on liver disorders by in vivo model. Journal of Pharmacy Research. 2009;2:353–356. [Google Scholar]
- McCord J.M. Oxygen derived radicals a link between reperfusion injury and inflammation. Federation Proceedings. 1987;46:2402–2406. [PubMed] [Google Scholar]
- Meister S. Commentary on the antioxidant effects of ascorbic acid and glutathione. Biochemistry and Pharmacology. 1992;44:1905–1915. doi: 10.1016/0006-2952(92)90091-v. [DOI] [PubMed] [Google Scholar]
- Milton Prabu S., Renugadevi J., Ramesh Kumar T. Ameliorative effect of selenium against cadmium induced biochemical alterations in Cirrhinus mrigala (Hamilton) Asian Journal of Biosciences. 2007;2:143–148. [Google Scholar]
- Moron M.S., Bepierre J.W., Mannerwick B. Levels of glutathione reductase and glutathione S-transferase in rat lung and liver. Biochimica Et Biophysica Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Niehaus W.G., Samuelsson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Niki E., Saito M., Yoshikawa Oxidation of lipids XII. Inhibition of oxidation of soybean phosphatidylcholine and methyl linoleate in aqueous dispersions by uric acid. Bulletin of the Chemical Society of Japan. 1986;59:471–477. [Google Scholar]
- Ohnishi T., Suzuki T., Suzuki Y., Ozawa K. A comparative study of plasma membrane Mg++-ATPase activities in normal, regenerating and malignant cells. Biochimica Et Biophysica Acta. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- Omaye S.T., Turbull T.D., Sauberlich H.C. Selected method for the determination of ascorbic acid in animal cells, tissues and fluids. In: McCormic D.B., Wright D.L., editors. Methods in Enzymology. Academic Press; New York, USA: 1979. pp. 3–11. [DOI] [PubMed] [Google Scholar]
- Panda H. NIIR; Delhi: 2004. Herbs Cultivation and Medicinal Uses. pp. 447–450. [Google Scholar]
- Piccolella S., Fiorentino A., Pacifico S., Dwbrosca B., Uzzo P., Monaco P. Antioxidant properties of sour cherries (Prunus cerasus L.): role of colorless phytochemicals from the methanolic extract of ripe fruits. Journal of Agricultural and Food Chemistry. 2008;56:1928–1935. doi: 10.1021/jf0734727. [DOI] [PubMed] [Google Scholar]
- Ponglux D., Wong S., Phadungcharoen T., Ruangrungsri N., Likhitwitayawuid K. Victory Power Point Corp. Ltd.; Bangkok, Thailand: 1987. Medicinal Plants. [Google Scholar]
- Prabhu M.S., Patel K., Saraawathi G., Srinivasan K. Effect of orally administered betel leaf (Piper betle leaf Linn.) on digestive enzymes of pancreas and intestinal mucosa and on bile production in rats. Indian Journal of Experimental Biology. 1995;33:752–756. [PubMed] [Google Scholar]
- Rahman M., Tondel M., Ahmad S.A. Diabetes mellitus associated with arsenic exposure in Bangladesh. American Journal of Epidemiology. 1998;148:198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- Ramesh B., Satakopan V.N. Antioxidant activities of hydroalcoholic extract of Ocimum sanctum against cadmium induced toxicity in rats. Indian Journal of Clinical Biochemistry. 2010;25:307–310. doi: 10.1007/s12291-010-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji N., Iyer R., Chandrasekaran S. Phenolic antibacterials from piper betel in the prevention of halitosis. Journal of Ethanopharmacology. 2002;83:149–152. doi: 10.1016/s0378-8741(02)00194-0. [DOI] [PubMed] [Google Scholar]
- Rao K.S., Recknagel R.O. Early onset of lipid peroxidation in rat liver after carbon tetrachloride administration. Experimental and Molecular Pathology. 1968;9:271–278. doi: 10.1016/0014-4800(68)90041-5. [DOI] [PubMed] [Google Scholar]
- Rathee J.S., Patro B.S., Hula S., Gamre S., Chattopadhyay S. Antioxidant activity of Piper betle leaf extract and its constituents. Journal of Agricultural and Food Chemistry. 2006;54:9046–9054. doi: 10.1021/jf061679e. [DOI] [PubMed] [Google Scholar]
- Reed D.J. Glutathione, toxicological implications. Annual Review of Pharmacology and Toxicology. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- Rosalki S.B., Rao D., Lchman D., Prentice M. Determination of serum gamma-glutamyl transpeptitase activity and its clinical applications. Annals of Clinical Biochemistry. 1970;7:143–147. [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.C., Hoekstra W.G. Selenium: biochemical roles as a component of glutathione peroxidase. Science. 1973;9:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Santhanam G., Nagarajan S. Wound healing activity of Curcuma aromatica and Piper betle. Fitoterapia. 1990;61:458–459. [Google Scholar]
- Saravanan R., Rajendra Prasad N., Pugalendi K.V. Effect of Piper betle leaf extract on alcoholic toxicity in the rat brain. Journal of Medicinal Food. 2004;6:261–265. doi: 10.1089/10966200360716689. [DOI] [PubMed] [Google Scholar]
- Satarug S., Baskar J.R., Urbenjapol S., Haswell-Elkins M., Reilly P.E.B., Willians D.J., Moore M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicology Letters. 2003;137:65–83. doi: 10.1016/s0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound and non-protein sulphydryl groups in tissue with Ellman’s reagent. Annals of Biochemistry. 1958;24:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Selvarajan N., Hemalatha S., Ramesh Kumar T., Milton Prabu S. Antihepatotoxic effect of Vernonia cinerea against cadmium induced toxicity in rats. Biochemistry and Cell Biology. 2007;7:23–32. [Google Scholar]
- Sinha A.K. Colorimetric assay of catalase. Analytical Biochemistry. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Sirivasan V., Gillespie B.E., Lewis M.J., Nguyen L.T., Headrick S.I., Schukken Y.H., Oliver S.P. Phenotypic and genotypic antimicrobial resistance patterns of Escherichia coli isolated from dairy cows with mastitis. Veterinary Microbiology. 2007;124:319–328. doi: 10.1016/j.vetmic.2007.04.040. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radicals in Biology and Medicine. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D., Hassoun E., Bagchi M.M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. Journal of Environmental Pathology, Toxicology and Oncology. 2000;19:201–213. [PubMed] [Google Scholar]
- Vinoth Kumar P., Amala Bricey A., Veerathamari Selvi V., Sudheer Kumar C., Ramesh N. Antioxidant effect of green tea extract in cadmium chloride intoxicated rats. Advances in Applied Science Research. 2010;1:9–13. [Google Scholar]
- Watkin R.D., Nawrot T., Potts R.J., Hart B.A. Mechanisms regulating the cadmium-mediated suppression of special transcription factor activity in alveolar epithelial cells. Toxicology. 2003;18:157–178. doi: 10.1016/s0300-483x(02)00577-2. [DOI] [PubMed] [Google Scholar]
- Wealth of India, 1992. The dictionary of Indian raw materials and industrial products. Raw Material, CSIR.
- Weil C.S. Tables for convenient calculation of median effective dose (LD50 and ED50) and instructions in their use. Biometrics. 1952;8:249–265. [Google Scholar]
- Weisberg M., Joseph P., Hale B., Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis: a review. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Williams F., Robertson R., Roworth M. SCEIH; Glasgow: 1999. Detailed Profile of 25 Major Organic and Inorganic Substances. pp. 12–19. [Google Scholar]
- Williamson E.M., Okpako D.T., Evans F.J. John Wiley; England: 1996. Selection, Preparation and Pharmacological Evaluation of Plant Material. p. 1. [Google Scholar]
- Zimmerman, H.J., Seef, L.B., 1970. The functions and tests of liver. In: Diagnostic Enzymology, Philadelphia, Pergamon Press, pp. 1–4.
- Zitkevicius V., Smalinskiene A., Savickiene N., Savickar A., Ryselis S., Sadauskiene I., Ivanov L., Lesauskaite L. Assessment of the effect of Echinacea purpurea extract on the accumulation of Cadmium in liver and kidney; apoptotic-mitotic activity of liver cells. Journal of Medicinal Plants Research. 2011;5:743–750. [Google Scholar]


