Abstract
A recent study proposed that incubation behaviour (i.e. type of parental care) in theropod dinosaurs can be inferred from an allometric analysis of clutch volume in extant birds. However, the study in question failed to account for factors known to affect egg and clutch size in living bird species. A new scaling analysis of avian clutch mass demonstrates that type of parental care cannot be distinguished by conventional allometry because of the confounding effects of phylogeny and hatchling maturity. Precociality of young but not paternal care in the theropod ancestors of birds is consistent with the available data.
Keywords: parental care, birds, dinosaur, incubation, scaling
1. Introduction
Varricchio et al. [1] proposed that paternal incubation is the plesiomorphic condition for type of parental care in extant birds and was inherited from non-avian theropod dinosaurs. Their conclusion was based largely on an allometric analysis of avian clutch volume (CV) against female body mass (FBM) for each type of parental care behaviour. Varricchio et al.'s allometric analysis was recently used to infer the sex of the parent providing parental care in the sauropodomorph dinosaur Massospondylus [2]. However, Varricchio et al. [1] did not consider numerous well-known factors associated with variation in egg and clutch size in extant birds, which prompted us to re-evaluate their allometric model using our own data.
The basic logic used by Varricchio et al. was that parental care in the groups of theropod dinosaurs that are most closely related to birds, including troodontids and oviraptorids, could be inferred through an extant phylogenetic bracket using Crocodylia and Aves. By examining the relationship between CV and FBM by type of parental care, theropod parental care behaviour could then be extrapolated [1]. Therefore, two implicit assumptions of their analysis were: (i) rejection of a specific CV–FBM relationship in non-avian dinosaurs and (ii) phylogenetic relatedness and developmental maturity do not introduce any major bias in considerations of CV measurements in Aves. However, it is known that phylogeny does affect many allometric relationships in bird reproduction, including egg composition [3] and energetics [4]. Lack of consideration of possible confounding factors in Varricchio et al.'s study justifies a reanalysis, particularly because the application of their technique is apparently going to see wider use [2].
Here, we first present an analysis of the relationship between avian clutch mass and FBM using a dataset that includes more than three times the number of species used previously [1]. We then examine whether developmental maturity and phylogenetic variation confound the analysis. Finally, we use widely available behavioural pattern data to estimate the ancestral conditions for parental incubation behaviour in extant birds.
2. Material and methods
Clutch mass (CM) and FBM data were taken from a compilation of bird egg data (1254 species; 28 orders; see the electronic supplementary material, table S1). For species inclusion, we established three criteria: (i) known type of parental care classified according to the parent or parents participating in egg incubation (paternal, maternal, bi-parental); (ii) type of developmental maturity at hatching (altricial, precocial); and (iii) reliable attribution of a species to an order. We treated ratites, including tinamous, as a single order in regression analyses.
Our dataset differed in some respects from that used by Varricchio et al. [1]. First, the ostrich Struthio camelus exhibits bi-parental care [5]. Second, megapodes (Galliformes) were not included because their eggs are laid within a mound over the entire breeding season [6] and so cannot be regarded as forming a clutch in the same sense as in other birds. Third, because maximum clutch size can be biased by extreme cases of intraspecific brood parasitism [7], we used average rather than maximum values for clutch size. Fourth, Varricchio et al.'s data for dinosaur egg volume were converted to mass using egg density [8].
Data were first analysed by visual inspection of plots of log10 transformed values. We then used type 1 ordinary least-squares regression analysis and ANCOVA to evaluate sources of variation. If ANOVA found the interaction term (CM × FBM) to be significant (different slopes), then ANCOVA was not used to evaluate the treatment effect (difference in intercepts; NCSS statistical package [9]). If ANCOVA showed a significant factor effect, then the Tukey–Kramer test (p < 0.05) was used to examine pairwise differences. We analysed the effects of developmental maturity first and then the effect of taxonomic order. We note that some findings in the developmental maturity analysis are probably explained by results of the analysis of the effects of order. Estimates of evolutionary relationships for parental care conditions at the root of an order-level bird phylogeny [10] were obtained in Mesquite using Fitch parsimony (all changes among states are possible and count equally) and maximum likelihood under the Markov k-state single parameter (Mk1) model, suitable for discrete data [11].
3. Results
Maternal and paternal parental care types show the same relationship between CM and FBM by incubation type in birds (figure 1a and table 1; CM × FBM: F1,667 = 0.10, p = 0.79; ANCOVA: F1,668 = 0.84, p = 0.36). Paternal and maternal care had a common slope that was different from that of bi-parental care (CM × FBM: F2,1248 = 87.19, p < 0.001).
Figure 1.
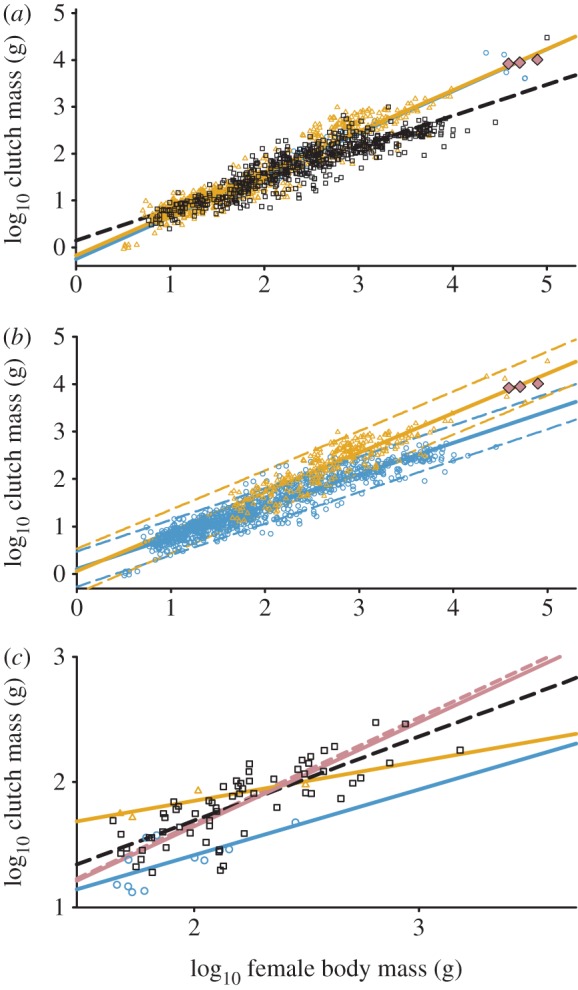
Log clutch mass as a function of Log female body mass in birds. (a) Comparison of (blue circles) paternal, (yellow triangles) maternal (same relationship, lines are superimposed) and (white squares) bi-parental care with (pink diamonds) dinosaurs. (b) Comparison of (blue circles) altricial and (yellow triangles) precocial clutch masses. Dashed lines are prediction intervals. (c) Comparison of types of parental care in the Charadriiformes (symbols as in (a)). Vermillion solid line is for paternal care from 1A, dashed line is for ratites.
Table 1.
Allometric regression analyses of clutch in mass in birds for linear fits presented in figure 1. N is number of species, and CI is confidence interval. Italicized terms are significantly different than others with groups p < 0.05. See text.
| N | N orders | intercept | 95% CI | slope | 95% CI | r2 | p | |
|---|---|---|---|---|---|---|---|---|
| all | ||||||||
| paternal | 29 | 2 | −0.2502 | −0.5051–0.0046 | 0.8956 | 0.8064–0.9848 | 0.94 | <0.00001 |
| maternal | 642 | 11 | −0.1735 | −0.2170–−0.1300 | 0.8816 | 0.8597–0.9034 | 0.91 | <0.00001 |
| bi-parental | 583 | 23 | 0.1497 | 0.0868–0.2126 | 0.6641 | 0.6394–0.6889 | 0.83 | <0.00001 |
| precocial | 270 | 6 | 0.0888 | −0.0392–0.2169 | 0.8219 | 0.7760–0.8679 | 0.82 | <0.00001 |
| altricial | 984 | 18 | 0.1036 | 0.0731–0.1341 | 0.6640 | 0.6497–0.6784 | 0.89 | <0.00001 |
| maternal | ||||||||
| precocial | 150 | 4 | 0.6298a | 0.4365–0.8232 | 0.6672 | 0.6014–0.7330 | 0.73 | <0.00001 |
| altricial | 492 | 7 | 0.1126 | 0.0710–0.1541 | 0.6539 | 0.6278–0.6800 | 0.83 | <0.00001 |
| bi-parental | ||||||||
| precocial | 91 | 6 | 0.2705 | 0.0705–0.4706 | 0.7038 | 0.6236–0.7841 | 0.77 | <0.00001 |
| altricial | 492 | 18 | 0.1201 | 0.0596–0.1806 | 0.6598 | 0.6360–0.6835 | 0.86 | <0.00001 |
| paternal | ||||||||
| precocial | 29 | 2 | −0.2502 | −0.5051–0.0046 | 0.8956b | 0.8064–0.9848 | 0.94 | <0.00001 |
| ratites | 17 | 1 | 0.0813 | −0.3630–0.5257 | 0.8128 | 0.6807–0.9450 | 0.92 | <0.00001 |
| Charadriiformes | 12 | 1 | 0.3711 | −0.4030–1.1451 | 0.5237 | 0.1220–0.9253 | 0.46 | 0.0157 |
aDifferent from bi-parental precocial.
bDifferent from maternal and bi-parental precocial.
Developmental maturity was a confounding factor for the relationship between CM and FBM (figure 1b and table 1; CM × FBM: F1,1251 = 712.6, p < 0.0001). CM for precocial species was generally greater than that for altricial species, and the distribution of altricial and precocial species in the maternal and bi-parental care groups was FBM dependent. Altricial species generally had a lower FBM than precocial species (see the electronic supplementary material, table S2), although the relationship for altricial species was not affected by type of parental care (ANCOVA: F1,981 = 1.59, p = 0.21). For precocial species maternal CM is greater than bi-parental CM (ANCOVA: F1,238 = 87.59 p < 0.0001). Data for theropod dinosaurs were within the prediction interval for precocial bird species but outside that for bi-parental species (figure 1b).
The analysis of CM in relation to parental care was repeated using just precocial species. Paternal care had a significantly different slope than other care types (CM × FBM: F2,264 = 10.97, p < 0.0001). The effect of order in the precocial species was significant (F5,258 = 8.596, p < 0.0001). To investigate parental care effects separate from order-level effects, we examined the Charadriiformes, the only clade exhibiting all three parental care types (figure 1c). Here, species with paternal care had smaller clutch masses than other care types (ANCOVA: F2,74 = 14.32, p < 0.0001).
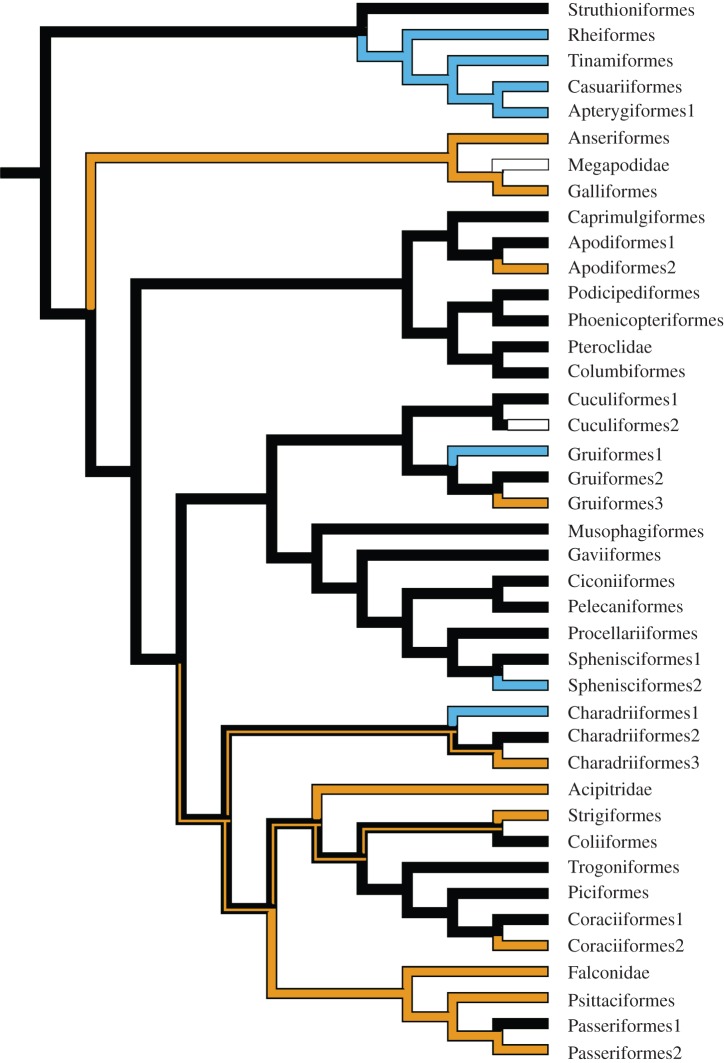
At the root of an order-level avian phylogeny, bi-parental incubation is the ancestral condition under Fitch parsimony (figure 2), and also the one with the highest proportional likelihood of all four parental care types (paternal proportional likelihood = 0.18641621; bi-parental proportional likelihood = 0.54888465; maternal proportional likelihood = 0.19824909; ‘none’ proportional likelihood = 0.06645005).
Figure 2.
Pattern of evolutionary relationships for avian incubation. Paternal (blue lines), bi-parental (black lines), maternal (yellow lines) and ‘none’ (white lines). Branches with one colour indicate no ambiguity in the pattern of distribution of alternative states. Two colours indicate equally possible alternative conditions under Fitch parsimony.
4. Discussion
The analysis of all CM data failed to confirm the results of Varricchio et al. [1]. Maternal and paternal care show the same relationship and association with troodontid and oviraptorid dinosaur data. Comparison of our data with those of Varricchio et al. [1] indicated it was the improved representation of the Anseriformes in our dataset that was most influential in altering the relationship between maternal and paternal care in the present analysis. The Anseriformes (prominent in the maternal care data) and the ratites (dominating the paternal care data) are both precocial and contain a significant number of species exhibiting intraspecific brood parasitism [7]. Therefore, intraspecific brood parasitism may account in part for the similarity in the functions for maternal and paternal care in this study. This finding was consistent with our hypothesis that phylogeny confounded the earlier analysis.
Developmental maturity at hatching is correlated with a range of different reproductive characteristics in birds, e.g. [3,4]. It is clear this now includes CM. The mixing of altricial and precocial species has marked effects on the analysis. The differences in body mass distributions correlated with type of developmental maturity results in an increased slope and decreased intercept for the maternal and bi-parental care relationships. Thus, mixing of species with different degrees of developmental maturity at hatching accounts for some of the differences observed in the relationships between parental care groups in the analysis by Varricchio et al. [1] and in figure 1a.
The implications of developmental maturity on avian reproductive effort should also be considered. The relationship between the altricial and precocial functions for CM suggests a convergence in reproductive effort at low FBM. However, a factor that is not accounted for in this and in other recent analyses [4] is the variation in egg energy density [12]. Precocial species have a significantly higher egg energy density. Thus, precocial species will generally devote a greater amount of energy to reproduction in the form of eggs for any given FBM than altricial species.
The analysis of developmental maturity on CM also indicates that troodontid and oviraptorid dinosaurs produced precocial young. This adds evidence to the debate on developmental maturity across dinosaur clades [13,14]. Although this particular analysis appears robust, we would be cautious in applying it to larger dinosaurs as the Aves have never achieved the truly giant sizes observed in many dinosaur clades [15], and there is some debate about what constitutes clutch size in some large dinosaurs [16].
All paternal care data are regarded as precocial, so a reanalysis using just precocial species was appropriate. However, comparisons of paternal care with other care types was not possible because of a difference in slopes (see below).
Phylogenetic effects on avian reproductive traits are well known (see [3,4] for refs). Typically, the approach to analysing for an effect independent of phylogeny is to choose a single phylogenetic unit (in this case order) and analyse for the source of variation (parental care) within it. For the Charadriiformes, the results were inconsistent with the analyses derived from the use of multiple orders of birds. CM for paternal care was less than that for other forms of care (figure 1c). This suggests that the effects being observed between care types for precocial species may be the result of phylogenetic differences. In examining our paternal care data, we note they are comprising values from only two orders, the ratites and Charadriiformes (Varricchio et al. [1] also included the megapodes from the Galliformes). The Charadriiformes data are all at the lower end of the data range and exhibit a shallower slope than the ratite data. The ratite and paternal care relationships are basically one and the same (figure 1c). Differences in paternal care slopes observed across several of these analyses are explained by a phylogenetic influence and not related to type of parental care. We conclude that, for avian CM, developmental maturity and phylogeny are the dominant effects and the effect of parental care is quite small or non-existent. At present, our data indicate bi-parental care as the presumed ancestral parental care type of extant birds (figure 2).
We conclude, furthermore, that scaling of clutch mass or volume against body mass does not represent a viable technique for inferring the type of parental care in birds or dinosaurs. Our data support the hypothesis of precocial young in the theropod ancestors of birds and would suggest the possibility of inferring developmental maturity in other similarly sized dinosaurs.
Acknowledgements
We wish to thank the reviewers and editors for constructive comments that improved this manuscript.
References
- 1.Varricchio DJ, Moore JR, Erickson GM, Norell MA, Jackson FD, Borkowski JJ. 2008. Avian paternal care had dinosaur origin. Science 322, 1826–1828 10.1126/science.1163245 (doi:10.1126/science.1163245) [DOI] [PubMed] [Google Scholar]
- 2.Reisz RR, Evans DC, Roberts EM, Sues H-D, Yates AM. 2012. Oldest known dinosaurian nesting site and reproductive biology of the Early Jurassic sauropodomorph Massospondylus . Proc. Natl Acad. Sci. USA 109, 2428–2433 10.1073/pnas.1109385109 (doi:10.1073/pnas.1109385109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeming DC. 2007. Allometry of mass and composition in bird eggs: effects of phylogeny and hatchling maturity. Avian Poult. Biol. Rev. 18, 71–86 10.3184/147020607X289022 (doi:10.3184/147020607X289022) [DOI] [Google Scholar]
- 4.Sibly RM, Witt CC, Wright NA, Venditti C, Jetze W, Brown JH. 2012. Energetics, lifestyle, and reproduction in birds. Proc. Natl Acad. Sci. USA 109, 10 937–10 941 10.1073/pnas.1206512109 (doi:10.1073/pnas.1206512109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertram BCR. 1992. The ostrich communal nesting system. Princeton, NJ: Princeton University Press [Google Scholar]
- 6.Booth DT, Jones DN. 2002. Underground nesting in the megapodes. In Avian incubation: behaviour, environment and evolution (ed. Deeming DC.), pp. 192–206 Oxford, UK: Oxford University Press [Google Scholar]
- 7.Yom-Tov Y. 2001. An updated list and some comments on the occurrence of instraspecific nest parasitism in birds. Ibis 143, 133–143 10.1111/j.1474-919X.2001.tb04177.x (doi:10.1111/j.1474-919X.2001.tb04177.x) [DOI] [Google Scholar]
- 8.Dickison MH. 2007. Allometry of giant flightless birds. PhD dissertation 114 pp Duke University, Durham, NC [Google Scholar]
- 9.Hintze J. 2012. NCSS 8. Kaysville, UT: NCSS, LLC; See http://www.ncss.com [Google Scholar]
- 10.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 10.1126/science.1157704 (doi:10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 11.Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis, version 2.75 See http://mesquiteproject.org [Google Scholar]
- 12.Sotherland PR, Rahn H. 1987. On the composition of bird eggs. Condor 89, 48–65 See http://www.jstor.org/stable/1368759 [Google Scholar]
- 13.Isles TE. 2009. The socio-sexual behaviour of extant archosaurs: implications for understanding dinosaur behaviour. Hist. Biol. 21, 139–214 10.1080/08912960903450505 (doi:10.1080/08912960903450505) [DOI] [Google Scholar]
- 14.Horner JR. 2000. Dinosaur reproduction and parenting. Ann. Rev. Earth Planet. Sci. 28, 19–45 10.1146/annurev.earth.28.1.19 (doi:10.1146/annurev.earth.28.1.19) [DOI] [Google Scholar]
- 15.Deeming DC, Birchard GF. 2008. Why were extinct gigantic birds so small? Avian Biol. Res. 1, 187–194 10.3184/175815508X402482 (doi:10.3184/175815508X402482) [DOI] [Google Scholar]
- 16.Werner J, Griebeler EM. 2011. Reproductive biology and its impact on body size: comparative analysis of mammalian, avian and dinosaurian reproduction. PLoS ONE 6, e28442. 10.1371/journal.pone.0028442 (doi:10.1371/journal.pone.0028442) [DOI] [PMC free article] [PubMed] [Google Scholar]