Abstract
Although it is well known that spatial learning can be important in the biology of predators that actively move around in search for food, comparatively little is known about ways in which spatial learning might function in the strategies of sit-and-wait predators. In this study, Cyclosa octotuberculata, an orb-web spider that uses its legs to contract radial threads of its web to increase thread tension, was trained to capture prey in limited web sectors. After training, spiders that had captured prey in horizontal web sectors applied more tension on radial threads connected to horizontal sectors than spiders that had captured prey in vertical sectors. This result suggests that the effect of experience on C. octotuberculata's behaviour is not expressed in the way the trained spider responds to prey-derived stimuli and is instead expressed in behaviour by which the spider anticipates the likely direction from which prey will arrive in the future. This illustrates that learning can be important even when the predator remains in one location during foraging bouts.
Keywords: foraging strategy, vibration signals from prey, tactile sense, thread tension
1. Introduction
‘Learning’ is the ability of animals to adaptively modify their behaviour as a consequence of their experience [1]. For animals that actively move about in search of food, it is easy to envisage how well-developed capacity for spatial learning (i.e. learning the spatial structure of the environment) would be advantageous [2]. Less attention has been given to the ways in which spatial learning might be advantageous for predators that adopt sit-and-wait strategies.
The orb-web spider is a predator that uses a sit-and-wait strategy. Its prey capture is strictly limited to the web area that is covered with sticky spiral threads. A spider typically rests at the centre of its web, the hub, where non-sticky radial threads typically run in 360° around the hub in a two-dimensional web plane. When prey collide with the web and get entangled in sticky spirals, vibrations emitted from the struggling prey travel through the radial threads. For a spider to sense the prey entangled in the web, the vibration signals from prey must reach the hub. The spider then starts locating and eventually runs to and feeds on the prey.
Thread tension affects vibration transmission capacity through radial threads [3], thereby affecting the spider's prey detection efficiency. Spiders are more sensitive to prey on the web when thread tension is increased [4,5]. Recent studies have provided evidences for the hypothesis that some orb-web spiders actively regulate thread tension to enhance the transmission capacity of vibration signals from prey [4,5]. Cyclosa octotuberculata is an example; the spider pulls the radial threads of the web and hence increases thread tension when it waits for prey, and thread pulling is stronger in vertical than in horizontal web sectors [4]. These findings provide a way to investigate spatial learning in orb-web spiders.
This study aims to examine whether a spider makes decisions to change web sectors to ‘monitor’ for prey arrival by using its legs to apply tension to the radial threads in these particular sectors. Moreover, I propose that these decisions are an expression of spatial learning in a manner that is particularly appropriate for a sit-and-wait predator. For this purpose, spiders were trained to capture prey in limited web sectors. I predict that spiders that capture their prey only in horizontal web sectors will shift to ‘monitor’ horizontal sectors by pulling threads connected to horizontal sectors more strongly than those that capture their prey only in vertical web sectors.
2. Material and methods
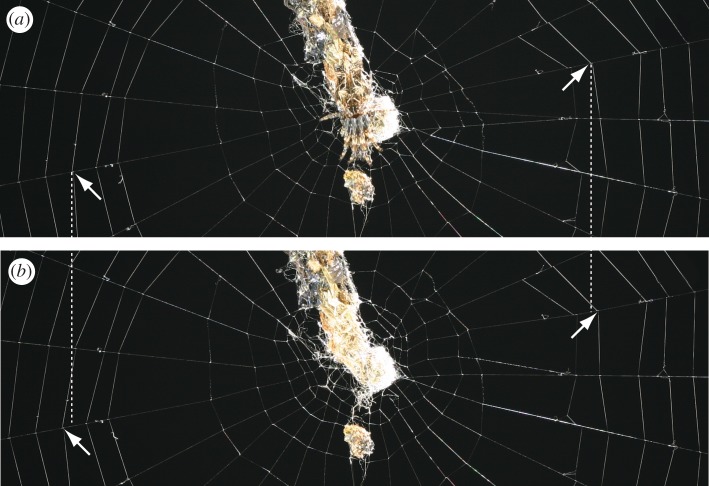
Cyclosa octotuberculata is a diurnal spider that builds vertical orb-webs with debris decoration on which the spider rests waiting for prey (figure 1a). In debris decoration, prey remnants and spider's own moulted exoskeletons are arranged in a vertical line [4]. Adult females with debris decorations were collected at Mt. Inasa Park, Nagasaki, Japan, in late May in 2011 (n = 26) and 2012 (n = 22). They were taken to the laboratory where each spider, together with its debris decoration, was individually housed in a transparent acrylic frame (45 × 45 × 6 cm) covered with two transparent acrylic sheets (50 × 50 × 0.3 cm). Frames with spiders were located near the window. They were maintained under natural dark–light conditions: during the experiment, the sun rose at 4 : 42–4 : 46 and the length of daylight was about 14 h 30 min. After sunset, no illumination was provided. Room temperature was not controlled, and the mean temperature ranged approximately from 20°C to 25°C. Spiders built their webs inside the frames within several days after the collection. Some spiders hung their debris decoration on their first webs and others did not. For the latter, debris decorations were attached to the first webs, and spiders incorporated them into their webs in subsequent web building. This initial difference in the spider's handling of debris decoration did not affect the subsequent experimental procedure and results. Each spider was assigned to one of two feeding groups (vertical or horizontal) immediately after building its first web. During the training period, each individual spider was supplied with one prey item (a field-collected syrphid fly) on its web per day. For the vertical feeding group, the prey was placed in either the north or south web sector. For the horizontal feeding group, the prey was placed in either the east or west sector. For each spider, the web sector in which the prey was first supplied was determined randomly, and thereafter, the prey supply sector was alternated at each feeding. When spiders had rebuilt their webs after four training feedings, measurements were initiated. Cyclosa octotuberculata typically rebuilds its web daily in nature, but in the laboratory, it sometimes used the same web for more than one day (to avoid unnecessary disturbance, webs were not destroyed). When a spider did not rebuild its web on the day after the fourth feeding, feeding was continued until rebuilding. Thus, the number of feedings during training ranged from four to seven. A single measurement consisted of taking three photographs. The first photograph pictured the entire web with the spider resting on the hub. The second photograph was a close-up of the web centre, including the hub with the spider pulling on radii and the innermost spiral turns. The spider was then removed from its web without damaging the web threads. After removal of spiders that had pulled the web thread to the centre, the web expanded outward (figure 1). The third photograph was then taken to show the web centre without the spider from the same camera position and magnification. From the second and third photographs, the displacements of the junctions of each radial thread with an arbitrarily chosen spiral turn were measured. The maximum displacements in the north and south sectors were then summed. The sum was used as an indicator of the force of the spider's thread pulling in the vertical direction. I did not use the north and south values separately because they were not independent: a spider, to keep its position, must apply forces simultaneously in opposite directions. Similarly, the sum of the values in the east and west sectors was used for the horizontal direction (for more details, refer to Nakata [4]). From the first photograph, the length of the debris decoration was measured. After the third photograph was taken, the spider was returned to its web and supplied with prey according to its feeding regime. Measurements were repeated five times for each individual in different webs built on different days. Some spiders died before the five measurements were completed and were dropped from the analysis. In total, 20 (10 in 2011 and 10 in 2012) and 16 (seven in 2011 and nine in 2012) spiders were used, and 100 and 80 webs were measured for the vertical and horizontal feeding groups, respectively. The effect of feeding sectors on the spider's pulling distance in horizontal directions was analysed by longitudinal regression with feeding regimes and experimental year as independent variables and pulling distance in vertical directions as a covariate in the analysis of pulling distance. The Wald test was used to examine whether the regression coefficient for feeding regime was significantly different from zero. Prey supply order within each feeding group (whether the first prey was supplied in the north or south sectors in vertical feeding group or in the west or east sectors in horizontal feeding group), the number of prey received during training and the length of debris decoration were not incorporated in the analysis because preliminary analysis revealed no significant effect of these factors.
Figure 1.
The centre of a web (a) with a spider and (b) without a spider. Debris decorations are also seen. Pairs of arrows indicate the same junctions of a radial thread with a spiral turn. Dotted lines indicate the horizontal position of junctions with the spider. Without the spider, junctions were displaced away from the centre. (Online version in colour.)
3. Results
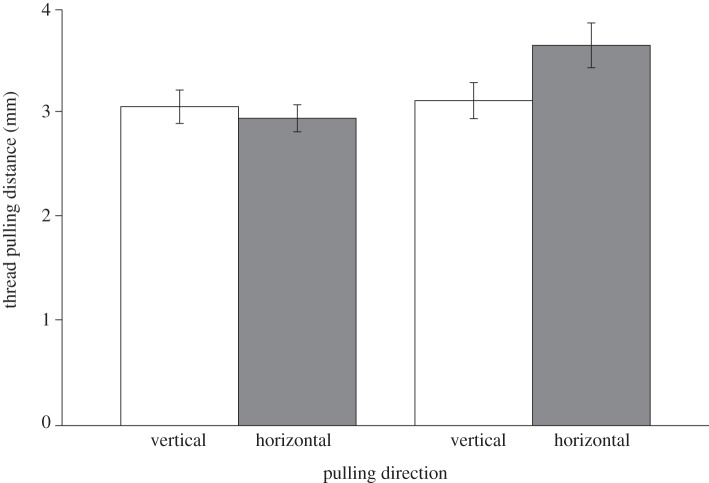
Spiders in the vertical feeding group pulled horizontal radii less strongly than vertical radii, and spiders in the horizontal feeding group pulled horizontal radii more strongly than vertical radii (figure 2). The Wald test after longitudinal regression revealed a significant effect of the feeding regime on the thread-length change with which spiders pulled horizontal radii (F1,33 = 6.37, p = 0.017).
Figure 2.
The length of thread pulled in horizontal and vertical directions in spiders that captured their prey in vertical web sectors and those that captured their prey in horizontal web sectors. Error bars indicate standard error.
4. Discussion
Experimental manipulation of the location of prey captured in a web revealed that C. octotuberculata pulled radial threads more strongly in the direction in which it had repeatedly experienced prey capture. This finding indicates that the spider learns particular web sectors in which prey capture is more commonly experienced and prepares future prey arrivals by enhancing the transmission capacity of vibration signals from prey in those sectors. In nature, the prey interception rate may not always be uniform among different web sectors [6]. Thus, spatially uneven prey interception appears to render the ability of spatial learning beneficial in orb-web spiders, because learning to prepare for future prey arrivals would be expected to shorten the mean time for spiders to respond to prey intercepted on webs and increase prey capture success.
In C. octotuberculata, as a sit-and-wait forager, spatial learning resulted in modification of the spider's sensory environment, that is, it altered how information from the prey is delivered through the web. This mechanism is very different from that in known examples of spatial learning in animals that actively move around. In these active animals, the consequence of spatial learning typically appears as a change in movement pattern, e.g. during homing or searching for previously stored food [2]. Instead, spatial learning of C. octotuberculata seemed to result in the anticipation of the direction of future prey arrival, and the spider proactively concentrated its prey detection ability on this direction by enhancing transmission capacity of vibration signal from prey in this direction. Some animals have the ability to allocate their limited cognitive resources in advance of expected events [7,8]. Such selective attention focusing resembles the thread pulling of C. octotuberculata observed in this and a previous study [4]. The spider may show attentional ability in a very basic form [9].
Cyclosa octotuberculata actively pulls web threads in different directions, presumably to enhance information transmission in the direction of strong thread pulling. Control of information transmission by adjusting thread tension has been found in another spider species. Satiated Octonoba sybotides builds its web with lower thread tension, thereby lowering its sensitivity to small prey that would provide little fitness gain to the satiated spider [5,10]. Ability to filter out less important information may be crucial for small animals. In orb-web spiders, filtering may occur at least partly outside the spider's body and via a structure built by the animal, the web. Nesting, foraging, communication and tools are the functions of animal-built structures described to date [11]. This study suggests that control of information acquisition and transmission should be added to the list as another function of animal-built structures.
The main function of a spider web is capturing prey, and silk properties affect an orb-web's performance when prey collide with the web [12]. Spider foraging efficiency is affected by thread arrangement as well as by silk properties [13,14]. This study suggests that spider behaviour when it waits for its prey may also be important for foraging via control of properties of the web as a sensory organ outside the spider's body.
Acknowledgements
I thank Dr Yusuke Shigemiya for his help in collecting spiders. I also appreciate Prof. Robert Jackson and an anonymous reviewer for their helpful comments. This study was partly supported by a JSPS grant-in-aid for Scientific Research (C) (no. 23570037).
References
- 1.Dall SRX, Giraldeau LA, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 10.1016/j.tree.2005.01.010 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 2.Capaldi EA, Robinson GE, Fahrbach SE. 1999. Neuroethology of spatial learning: the birds and the bees. Annu. Rev. Psychol. 50, 651–682 10.1146/annurev.psych.50.1.651 (doi:10.1146/annurev.psych.50.1.651) [DOI] [PubMed] [Google Scholar]
- 3.Frohlich C, Buskirk RE. 1982. Transmission and attenuation of vibration in orb spider webs. J. Theor. Biol. 95, 13–36 10.1016/0022-5193(82)90284-3 (doi:10.1016/0022-5193(82)90284-3) [DOI] [Google Scholar]
- 4.Nakata K. 2010. Attention focusing in a sit-and-wait forager: a spider controls its prey-detection ability in different web sectors by adjusting thread tension. Proc. R. Soc. B 277, 29–33 10.1098/rspb.2009.1583 (doi:10.1098/rspb.2009.1583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe T. 2000. Web tuning of an orb-web spider, Octonoba sybotides, regulates prey-catching behaviour. Proc. R. Soc. Lond. B 267, 565–569 10.1098/rspb.2000.1038 (doi:10.1098/rspb.2000.1038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nentwig W. 1985. Top-bottom asymmetry in vertical orbwebs: a functional explanation and attendant complications. Oecologia 67, 111–112 10.1007/BF00378459 (doi:10.1007/BF00378459) [DOI] [PubMed] [Google Scholar]
- 7.Chan AAY-H, Blumstein DT. 2011. Attention, noise, and implications for wildlife conservation and management. Appl. Anim. Behav. Sci. 131, 1–7 10.1016/j.applanim.2011.01.007 (doi:10.1016/j.applanim.2011.01.007) [DOI] [Google Scholar]
- 8.Langley CM. 1996. Search images: selective attention to specific visual features of prey. J. Exp. Psychol. Anim. Behav. Process 22, 152–163 10.1037/0097-7403.22.2.152 (doi:10.1037/0097-7403.22.2.152) [DOI] [PubMed] [Google Scholar]
- 9.Jackson RR, Cross FR. 2011. Spider cognition. Adv. Insect Physiol. 41, 115. 10.1016/B978-0-12-415919-8.00003-3 (doi:10.1016/B978-0-12-415919-8.00003-3) [DOI] [Google Scholar]
- 10.Watanabe T. 1999. The influence of energetic state on the form of stabilimentum built by Octonoba sybotides (Araneae: Uloboridae). Ethology 105, 719–725 10.1046/j.1439-0310.1999.00451.x (doi:10.1046/j.1439-0310.1999.00451.x) [DOI] [Google Scholar]
- 11.Hansell M. 2005. Animal architecture. Oxford, UK: Oxford University Press [Google Scholar]
- 12.Cranford SW, Tarakanova A, Pugno NM, Buehler MJ. 2012. Nonlinear material behaviour of spider silk yields robust webs. Nature 482, 72–76 10.1038/nature10739 (doi:10.1038/nature10739) [DOI] [PubMed] [Google Scholar]
- 13.Blackledge TA, Kuntner M, Agnarsson I. 2011. The form and function of spider orb webs: evolution from silk to ecosystems. In Advances in Insect Physiology (ed. Casas J.), pp. 175–262 Burlington, VT: Academic Press [Google Scholar]
- 14.Harmer AMT, Blackledge TA, Madin JS, Herberstein ME. 2011. High-performance spider webs: integrating biomechanics, ecology and behaviour. J. R. Soc. Interface 8, 457–471 10.1098/rsif.2010.0454 (doi:10.1098/rsif.2010.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]