Abstract
Bats and moths have been engaged in aerial warfare for nearly 65 Myr. This arms race has produced a suite of counter-adaptations in moths, including bat-detecting ears. One set of defensive strategies involves the active production of sound; tiger moths' ultrasonic replies to bat attack have been shown to startle bats, warn the predators of bad taste and jam their biosonar. Here, we report that hawkmoths in the Choerocampina produce entirely ultrasonic sounds in response to tactile stimulation and the playback of biosonar attack sequences. Males do so by grating modified scraper scales on the outer surface of the genital valves against the inner margin of the last abdominal tergum. Preliminary data indicate that females also produce ultrasound to touch and playback of echolocation attack, but they do so with an entirely different mechanism. The anti-bat function of these sounds is unknown but might include startling, cross-family acoustic mimicry, warning of unprofitability or physical defence and/or jamming of echolocation. Hawkmoths present a novel and tractable system to study both the function and evolution of anti-bat defences.
Keywords: arms race, bat, echolocation, Sphingidae, sphinx moth
1. Introduction
Aerial warfare between echolocating bats and their insect prey has escalated for nearly 65 Myr [1]. Ultrasonic ‘bat-detecting’ ears have independently evolved a minimum of 19 times in five insect orders [1]. Most insect ears are connected directly to neuronal circuits that steer the animals away from bats at low biosonar call intensities and trigger aerobatic evasive behaviours (loops, spirals and dives) at high call intensities [2]. Tiger moths (Erebidae: Arctiinae) have accelerated this arms race with an additional set of acoustic strategies. These moths have evolved paired metathoracic tymbals to create ultrasonic clicks to answer attacking bats. Empirical work supports three non-mutually exclusive functions for these acoustic signals. Sounds can startle naive bats [3], warn of unpalatability [4,5] and jam biosonar [6].
Roeder et al. [7] demonstrated almost a half century ago that choerocampine hawkmoths (Sphingidae) have bat-detecting ears. Hawkmoths are in a different superfamily from tiger moths, and the two groups are distantly related [8]. However, sphingids are also reported to produce sounds in the audible spectrum [9–12], though the function of sound production in sphingids is unknown. Here, we provide the first evidence that some hawkmoths produce ultrasound to playback of echolocation attack. Males of at least three choerocampine species produce entirely ultrasonic defensive sounds by stridulating modified scales on the genital valves against the inner margin of the last abdominal tergum. Preliminary evidence from two of the same hawkmoth species indicates that females also produce ultrasound, but with an entirely different mechanism. Both males and females produce ultrasound in response to both tactile stimulation and to playback of bat attacks.
2. Material and methods
(a). Data collection
We sampled hawkmoths with 175 W mercury vapour lights in Malaysia (peninsular and Borneo) during March 2012 and focused on three choerocampine species, Cechenena lineosa, Theretra boisduvalii and Theretra nessus. We tethered moths in free flight at the end of a 5 mm diameter hollow plastic rod; a monofilament line was tied between the thorax and abdomen, threaded through the rod and held securely while the flying moth was queried for acoustic response to tactile stimulation and playback of bat attacks. We examined acoustic responses in several tethering scenarios and elected to use the above method, as it did not interfere with sound production. To record moth sounds, we used Avisoft UltraSoundGate 116 Hn hardware (sampling at 375 kHz onto a laptop computer running Avisoft Recorder software) and a CM16 condenser microphone (±3 dB, 20–140 kHz) positioned 10 cm from the posterior end of the moth's abdomen (location of the sound-producing structures). Using the same computer and software, we presented moths with three echolocation attack sequences played back via an Avisoft UltraSoundGate Player BL Pro Speaker/Amplifier (±6 dB, 20–110 kHz, playback sampling rate 250 kHz) placed 10 cm from the moth's head (location of ultrasound-sensitive ears): in order of playback, Lasiurus borealis, Eptesicus fuscus, and a synthetic bat attack designed to approximate the echolocation behaviour of several bat species (see the electronic supplementary material, figure S1, for details). We obtained recordings of Eptesicus and Lasiurus biosonar attacks in a flight room as bats attacked mealworms tethered 10 cm from the same microphone as above. While these bat genera are not found in Malaysia, the family (Vespertilionidae) is well represented at our sample sites. The final second of biosonar attacks is similar across bats [12] and as hawkmoths cannot discriminate frequency [13,14], the temporal and amplitude dimensions of the attack are the relevant parameters. On a subsequent expedition (April 2013), we queried three C. lineosa moths with a ‘Malaysian synthetic attack sequence’ created from the echolocation attack of Kerivoula papillosa (characteristics taken from fig. 1 in [15]) to further address issues of the ecological relevance of our playback stimuli. Each attack was approximately 700–900 ms in duration and had a peak equivalent sound pressure level (SPL) of 116 ± 2 dB, as measured by a B&K ¼″ microphone (grid off) at 10 cm (±0.5 dB, 1–100 kHz). Four seconds of silence separated the playback of each attack. Voucher specimens are stored in the collection of the FLMNH, University of Florida; acoustic files are archived at the Cornell Laboratory of Ornithology Macaulay Library (accession no. ACC3006).
(b). Analysis
We measured moth signals using Avisoft SASLab Pro and defined each cycle of the stridulatory apparatus as a modulation cycle. All signal parameters were computed from three modulation cycles per individual. We used responses from tactile trials to characterize signals to prevent corruption by overlapping bat sounds in the echolocation playback trials. To determine whether moth sounds were spectrally or temporally different when produced during tactile or playback trials, we examined several modulation cycles, and found no differences. We measured temporal parameters from the oscillogram and spectral and intensity values from power spectra (FFT 1024, 50% overlap). We converted relative intensity to peak equivalent SPL (dB peSPL re. 2 × 10−5 μPa) using a reference tone of a known intensity [16] and adjusted for the frequency response of the microphone. To calculate duty cycle of the moth sounds, we counted the number of clicks that occurred in 100 ms, multiplied this by the average click duration of the modulation cycle (both measured using the Pulse Train Analysis tool in SASLab Pro), and divided this value by 100. We used this approach to allow a direct comparison with tiger moth acoustic analyses [6,17].
3. Results and discussion
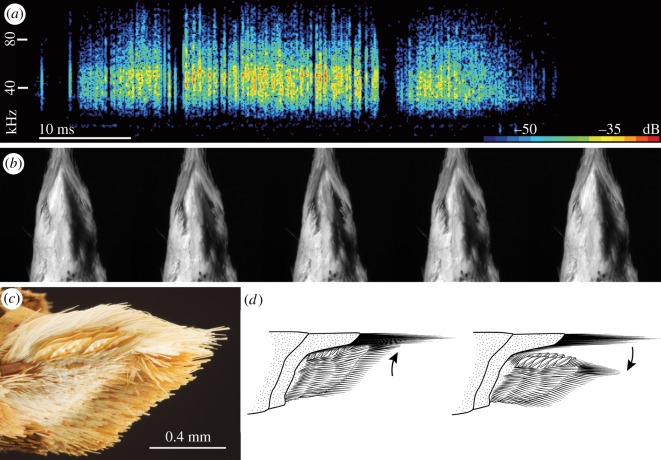
Three closely related choerocampine species [18] produce entirely ultrasonic signals when touched and when stimulated with bat echolocation playback (table 1). This discovery brings the number of insect groups that respond acoustically to bat biosonar up to three, including tiger moths [19] and tiger beetles [20]. Male hawkmoths that we studied produce ultrasonic clicks by stridulating a patch of large, marquise-shaped scales on the genital valve against the inner margin of the last abdominal tergum (figure 1; also see the electronic supplementary material, movie S1). A burst of ultrasound is created as the valves are moved dorsally and a second burst as they are moved ventrally. The second burst is often shorter in duration, perhaps because the scales are oriented dorsally. Data suggest that females also produce ultrasound to touch and to playback of echolocation attack, but with an entirely different, still genitally based, sound production mechanism (see the electronic supplementary material, figure S2, table S1 and movie S2). That hawkmoths produce sound with modified genital structures indicates the sounds might be used in mating behaviour. A single anecdote describes two male Psilogramma menephron stridulating while flying near females [21]. An acoustic sense mediated by the mouthparts (labral pilifers and labial palps) has been confirmed in the Choerocampina [7] and via a related but different mechanism in the Acherontiini [14]. An intriguing possibility is that the ultrasonic ears in hawkmoths might have first evolved for mates, not bats.
Table 1.
Response characteristics of three choerocampine hawkmoth species to tactile stimulation and bat echolocation playback. Time of onset to bat attack, referenced to the last biosonar cry of the playback, is included for three echolocation sequences. We measured temporal response from the end of the recordings due to higher signal-to-noise ratios. Sample sizes (n) listed for onset timing note subsets of moths out of the total number that responded to one or more playbacks. Values are mean ± s.d. Asterisk (*) indicates data collected during a separate expedition.
| species | n | no. and percentage (in brackets) of response to playback of bat attack | no. and percentage (in brackets) of response to tactile stimulation | duty cycle, % (n) | onset from end of bat attack, ms (n) | onset from end of bat attack 2, ms (n) | onset from end of bat attack 3, ms (n) | onset from end of Malaysian bat attack, ms (n) |
|---|---|---|---|---|---|---|---|---|
| Cechenena lineosa | 26 | 24 (92%) | 26 (100%) | 20.2 ± 5.8 (18) | 421 ± 137 (19) | 333 ± 254 (18) | 302 ± 174 (15) | 420 ± 61 (3*) |
| Theretra boisduvalii | 13 | 12 (92%) | 13 (100%) | 19.2 ± 6.9 (5) | 360 ± 119 (8) | 196 ± 151 (7) | 244 ± 93 (8) | n.a. |
| Theretra nessus | 12 | 12 (100%) | 11 (92%) | 21.8 ± 5.9 (8) | 391 ± 111 (8) | 273 ± 304 (7) | 268 ± 175 (7) | n.a. |
Figure 1.
(a) A spectrogram of the anti-bat sound produced by Cechenena lineosa is depicted above a series of high-speed video frames (b) of the stridulatory apparatus completing a modulation cycle of the valves. (c) Lateral view of genital valve shows enlarged scales for ultrasound production. (d) A line drawing depicts the motion of the valve as it moves dorso-proximally when pulled inward and ventro-distally as it moves outward.
Hawkmoths responded similarly to playback of all echolocation attacks, with sound onset occurring approximately 200–400 ms before the end of the biosonar sequence (table 1 and figure 2). Sound intensities of 68–64 dB (peSPL; table 2) indicate that these signals could operate at greater distances but, as in tiger moths, anti-bat sound production is used as a secondary defence deployed late in the attack [17]. The peak frequency of these moth sounds was 53–57 kHz with a ±15 dB bandwidth ranging from 26–29 kHz up to 86–105 kHz. This wide bandwidth is audible to sympatric bats [22] and more broadly to rodents, shrews, cats and primates, among others [23]. It seems likely that bats are the primary intended receivers for these ultrasonic sounds. Many bats are known to prey on hawkmoths [24], and previous work has shown that choerocampines display evasive behaviour when stimulated with ultrasound [7].
Figure 2.
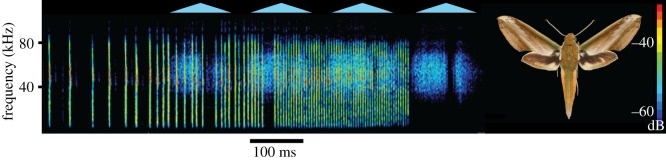
Theretra nessus responding to playback of a bat echolocation attack. Triangles indicate the timing of modulation cycles.
Table 2.
Acoustic attributes of three hawkmoth anti-bat sounds. Values are mean ± s.d.
| species | n | duration of the entire modulation cycle (ms) | duration of the first burst of clicks (ms) | duration of silent interval (ms) | duration of the second burst of clicks (ms) | individual click duration (ms) | dominant frequency (kHz) | intensity (dB peSPL) | +15 dB bandwidth (kHz) | −15 dB bandwidth (kHz) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cechenena lineosa | 18 | 111.2 ± 27.5 | 59.6 ± 17.8 | 6.6 ± 2.6 | 45.4 ± 12.0 | 0.15 ± 0.02 | 54.8 ± 3.3 | 68 ± 4 | 90.2 ± 17.4 | 29.5 ± 3.0 |
| Theretra boisduvalii | 5 | 100.2 ± 27.7 | 52.5 ± 16.8 | 6.7 ± 2.0 | 41.4 ± 13.7 | 0.18 ± 0.05 | 56.5 ± 3.3 | 64 ± 3 | 104.9 ± 28.9 | 25.8 ± 6.8 |
| Theretra nessus | 8 | 105.3 ± 27.2 | 60.8 ± 12.0 | 4.9 ± 2.2 | 39.3 ± 17.3 | 0.15 ± 0.02 | 53.3 ± 3.8 | 68 ± 3 | 85.8 ± 10.7 | 27.1 ± 1.4 |
The specific function of anti-bat ultrasound production in hawkmoths remains unknown, but it might play a similar role as in tiger moths—to startle, warn of chemical defense or jam biosonar. It is unlikely that sphingids are warning bats of bad taste, as they do not appear to sequester host plant toxins as adults [25,26]. However, larvae of some species can sequester defensive chemicals and regurgitate gut contents containing chemical compounds in the direction of predators [27], and this might be why some sphingid caterpillars are aposematically coloured [26]. Hawkmoth adults might benefit through Batesian mimicry, as has been shown in tiger moths [5]. Sphingids, which include species that can fly up to 5 ms−1 [28], could also be signalling unprofitable characteristics such as flight speed and difficulty of capture, or their pronounced tibial spines [29]. Jamming is also a viable function for hawkmoth sounds. Duty cycle, or sound production per unit time, is theoretically and empirically related to jamming efficacy. Two tiger moth species have been pitted against naive bats to experimentally test jamming and only one, Bertholdia trigona, has been shown to jam biosonar: the duty cycle of its signal is approximately 44 per cent [6]. Euchaetes egle did not successfully avoid naive bats and has a duty cycle of approximately 3 per cent [4]. The three hawkmoth species that we tested are intermediate between these two with a duty cycle of approximately 20 per cent. Clearly, experiments are needed to further elucidate the function of hawkmoth ultrasound.
There are anecdotal reports of audible sounds from only a few genera of hawkmoths, but these genera are widely distributed across all three subfamilies of the Sphingidae [9–12,18], arguing that sound production is deeply rooted within the history of the group. To fully understand the evolution of hawkmoth anti-bat sounds, many moth species must be tested for response to bat echolocation attack and the data correlated with phylogeny.
Acknowledgements
We thank J. Breinholt, P. Houlihan, A. Keener, I. Kitching, B. Leavell, C. McClure, P. Padron, J. Yack and W. Conner. Support was provided by NSF IOS 1121739, 1121807 and the American Philosophical Society.
References
- 1.Yager DD. 1999. Structure, development, and evolution of insect auditory systems. Microsc. Res. Tech. 47, 380–400 (doi:10.1002/(SICI)1097-0029(19991215)47:6<380::AID-JEMT3>3.0.CO;2-P) [DOI] [PubMed] [Google Scholar]
- 2.Miller LA, Surlykke A. 2001. How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. Bioscience 51, 570–581 (doi:10.1641/0006-3568(2001)051[0570:HSIDAA]2.0.CO;2) [Google Scholar]
- 3.Bates DL, Fenton MB. 1990. Aposematism or startle? Predators learn their responses to the defenses of prey. Can. J. Zool. 68, 49–52 (doi:10.1139/z90-009) [Google Scholar]
- 4.Hristov IH, Conner WE. 2005. Sound strategy: acoustic aposematism in the bat–tiger moth arms race. Naturwissenschaften 92, 164–169 (doi:10.1007/s00114-005-0611-7) [DOI] [PubMed] [Google Scholar]
- 5.Barber JR, Conner WE. 2007. Acoustic mimicry in a predator–prey interaction. Proc. Natl Acad. Sci. USA 104, 9331–9334 (doi:10.1073/pnas.0703627104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corcoran AJ, Barber JR, Conner WE. 2009. Tiger moth jams bat sonar. Science 325, 325–327 (doi:10.1126/science.1174096) [DOI] [PubMed] [Google Scholar]
- 7.Roeder KD, Treat AE, Vandeberg JS. 1968. Auditory sense in certain sphingid moths. Science 159, 331–333 (doi:10.1126/science.159.3812.331) [DOI] [PubMed] [Google Scholar]
- 8.Regier JC, et al. 2013. A large-scale, higher-level, molecular phylogenetic study of the insect order Lepidoptera (moths and butterflies). PLoS ONE 8, e58568 (doi:10.1371/journal.pone.0058568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaber WH. 1886. Stridulation of Sphinx convolvuli. Entomologist 19, 179–180 [Google Scholar]
- 10.van Doesburg PH., Jr 1966. Über valväre Stridulation bei Schwärmer (Lepidoptera Sphingidae). Zool. Meded. Leiden 41, 161–170 [Google Scholar]
- 11.Nässig WA, Lüttgen M. 1988. Notes on genital stridulation in male hawkmoths in South East Asia (Lepidot., Sphingidae). Heterocera Sumatrana 2, 75–77 [Google Scholar]
- 12.Nässig WA, Oberprieler RG, Duke NJ. 1992. Preliminary observations of sound production in South African hawk moths (Lepidoptera; Sphingidae). Ent. Soc. S. Afr. 55, 277–279 [Google Scholar]
- 13.Schnitzler H, Kalko EKV. 2001. Echolocation by insect-eating bats. Bioscience 51, 557–569 (doi:10.1641/0006-3568(2001)051[0557:EBIEB]2.0.CO;2) [Google Scholar]
- 14.Göpfert MC, Wasserthal LT. 1999. Hearing with mouthparts: behavioural responses and the structural basis of ultrasound perception in acherontiine hawkmoths. J. Exp. Biol. 202, 909–918 [DOI] [PubMed] [Google Scholar]
- 15.Schmieder DA, Kingston T, Hashim R, Siemers BM. 2010. Breaking the trade-off: rainforest bats maximize bandwidth and repetition rate of echolocation calls as they approach prey. Biol. Lett. 6, 604–609 (doi:10.1098/rsbl.2010.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stapells DR, Picton TW, Smith AD. 1982. Normal hearing threshold for clicks. J. Acoust. Soc. Am. 72, 74–79 (doi:10.1121/1.388026) [DOI] [PubMed] [Google Scholar]
- 17.Barber JR, Conner WE. 2006. Tiger moth responses to a simulated bat attack: timing and duty cycle. J. Exp. Biol. 209, 2637–2650 (doi:10.1242/jeb.02295) [DOI] [PubMed] [Google Scholar]
- 18.Kawahara AY, Mignault AA, Regier JC, Kitching IJ, Mitter C. 2009. Phylogeny and biogeography of hawkmoths (Lepidoptera: Sphingidae): evidence from five nuclear genes. PLoS ONE 4, e5719 (doi:10.1371/journal.pone.0005719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullard JH, Fenton MB. 1977. Acoustic behavioral analyses of the sounds produced by some species of Nearctic Arctiidae (Lepidoptera). Can. J. Zool. 55, 1213–1224 (doi:10.1139/z77-160) [Google Scholar]
- 20.Yager DD, Spangler HG. 1997. Behavioral response to ultrasound in the tiger beetle, Cicindela marutha Dow combines aerodynamic changes and sound production. J. Exp. Biol. 200, 649–659 [DOI] [PubMed] [Google Scholar]
- 21.Mell R. 1922. Beiträge zur Fauna Sinica 2 (Biologie und Systematic der sudchinesischen Sphingiden). Berlin, Germany: Friedländer [Google Scholar]
- 22.Kingston T, Francis CM, Zubaid A, Kunz TH. 2003. Species richness in an insectivorous bat assemblage from Malaysia. J. Trop. Ecol. 19, 67–79 (doi:10.1017/S0266467403003080) [Google Scholar]
- 23.Heffner RS, Heffner HE. 2010. Explaining high-frequency hearing. Anat. Rec. 293, 2080–2082 (doi:10.1002/ar.21292) [DOI] [PubMed] [Google Scholar]
- 24.Faure PA, Barclay RMR. 1992. The sensory basis of prey detection by the long-eared bat, Myotis evotis, and the consequences for prey selection. Anim. Behav. 44, 31–39 (doi:10.1016/S0003-3472(05)80751-1) [Google Scholar]
- 25.Wink M, Theile V. 2002. Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae). Chemoecology 12, 29–46 (doi:10.1007/s00049-002-8324-2) [Google Scholar]
- 26.Hundsdoerfer AK, Tshibangu JN, Wetterauer B, Wink M. 2005. Sequestration of phorbol esters by aposematic larvae of Hyles euphorbiae (Lepidoptera: Sphingidae)? Chemoecology 15, 261–267 (doi:10.1007/s00049-005-0321-9) [Google Scholar]
- 27.Bowers MD. 2003. Hostplant suitability and defensive chemistry of the Catalpa sphinx, Ceratomia catalpae. J. Chem. Ecol. 29, 2359–2367 (doi:10.1023/A:1026234716785) [DOI] [PubMed] [Google Scholar]
- 28.Willmott AP, Ellington CP. 1997. The mechanics of flight in the hawkmoth Manduca sexta 0.1. Kinematics of hovering and forward flight. J. Exp. Biol. 200, 2705–2722 [DOI] [PubMed] [Google Scholar]
- 29.Rothschild M, Reichstein T, von Euw J, Harman RRM. 1970. Toxic Lepidoptera. Toxicon 8, 293–299 (doi:10.1016/0041-0101(70)90006-1) [DOI] [PubMed] [Google Scholar]