Abstract
White-nose syndrome is devastating North American bat populations but we lack basic information on disease mechanisms. Altered blood physiology owing to epidermal invasion by the fungal pathogen Geomyces destructans (Gd) has been hypothesized as a cause of disrupted torpor patterns of affected hibernating bats, leading to mortality. Here, we present data on blood electrolyte concentration, haematology and acid–base balance of hibernating little brown bats, Myotis lucifugus, following experimental inoculation with Gd. Compared with controls, infected bats showed electrolyte depletion (i.e. lower plasma sodium), changes in haematology (i.e. increased haematocrit and decreased glucose) and disrupted acid–base balance (i.e. lower CO2 partial pressure and bicarbonate). These findings indicate hypotonic dehydration, hypovolaemia and metabolic acidosis. We propose a mechanistic model linking tissue damage to altered homeostasis and morbidity/mortality.
Keywords: dehydration, plasma electrolytes, Geomyces destructans, hibernation, hypovolemia
1. Introduction
Infectious fungal diseases are causing unprecedented wildlife die-offs [1]. Amphibian chytridiomycosis has caused the most severe disease-related loss of biodiversity ever observed [2], and bat white-nose syndrome (WNS) in North America has caused the fastest decline of wild mammals in history, threatening common species with extinction [3]. Both diseases are caused by fungal skin infections. In amphibians, disrupted cutaneous function appears to cause a cascade of pathophysiological changes leading to mortality [4]. In bats, the WNS pathogen, Geomyces destructans (Gd), affects the skin of the nose, muzzle and ears of bats but most severely damages the wings [5,6], which play a crucial role in thermoregulation, water economy, gas exchange and immune function [7,8]. Altered water and electrolyte balances due to wing lesions have been hypothesized as important in WNS pathogenicity [9–11], while mortality from WNS is preceded by a progressive increase in the frequency of periodic arousals from torpor during hibernation and premature fat depletion [12]. Therefore, interactions among cutaneous infection, hibernation physiology and mortality are likely, but mechanisms linking these processes are not understood.
Skin lesions that occur with Gd infection superficially resemble burn injuries and could lead to similar complications, including fluid loss [9,10,13]. This has led to the ‘hypotonic dehydration hypothesis’ that affected bats exhibit increased water and solute loss across wing lesions, replenish body water by drinking inside hibernacula but eventually suffer electrolyte depletion because they have no access to food [10]. Measurements of sodium and chloride from wild and experimentally infected, WNS-positive little brown bats (Myotis lucifugus) are consistent with this hypothesis [10]. However, pronounced fluid loss and hypotonic dehydration predict other changes in blood physiology such as hypovolaemia and metabolic acidosis [14]. In addition to shedding light on the pathophysiology of WNS, understanding how these factors influence morbidity and mortality could be valuable for rehabilitation of infected bats and potentially disease mitigation.
We studied blood physiology of M. lucifugus experimentally inoculated with Gd by Warnecke et al. [12] to test the hypotonic dehydration hypothesis [10], identify other consequences of Gd infection and help understand WNS mortality. Specifically, we tested for changes in electrolytes, haematology and acid–base balance. We predicted that infected bats would exhibit: (i) altered extracellular electrolyte concentrations (e.g. reduced sodium) owing to fluid loss over the epidermis from wing lesions [10]; (ii) haematological evidence of dehydration and starvation (e.g. elevated haematocrit owing to fluid loss [9] and decreased glucose because of depleted fat reserves [12]); and (iii) evidence of disrupted acid–base balance associated with hypovolaemia (e.g. reduced pH with reduction of bicarbonate and respiratory compensation resulting in decreased carbon dioxide). We also predicted that the magnitude of these changes would be associated with severity of wing tissue necrosis.
2. Material and methods
We collected 54 M. lucifugus from a WNS-negative cave and inoculated them with either a North American isolate of Gd, a European isolate or sham-inoculate (n = 18 each) as described in Warnecke et al. [12] (see the electronic supplementary material). All inoculated bats, but no controls, contracted WNS based on histopathology [12]. Bats were housed at 7°C and greater than 97% relative humidity with ad libitum water. After four months, we increased enclosure temperature to 25°C for approximately 1 h to assist rewarming from torpor, anaesthetized bats and collected blood (see the electronic supplementary material). Sampling from infected bats was complicated by high blood viscosity, but we obtained samples from eight inoculated individuals and all controls. Pathogenicity of both Gd isolates was similar [12] so we pooled results for inoculated bats. Using a blood analyser (i-STAT1, CG8+ cartridge, Abaxis, Union City, CA) we measured, (i) electrolytes: concentrations of sodium ([Na+], mmol l−1) and potassium ([K+], mmol l−1); (ii) haematology: haematocrit (Hct, % packed cell volume) and glucose concentration ([Glu], mg dl−1); (iii) acid–base: carbon dioxide partial pressure (pCO2, mmHg) and pH. The analyser calculated bicarbonate concentration ([HCO3−], mmol l−1) based on pH and pCO2.
To quantify severity of infection, we processed, using standard histological techniques and periodic acid-Schiff stain, a total of 15–20 cm of 5 µm-thick sections of skin from the entire left wing of each bat and evaluated them using light microscopy [6]. We scored necrosis based on the percentage of skin surface with fungal hyphae present that was necrotic (i.e. loss of epidermis and disruption of underlying connective tissue): 0, no necrotic tissue; 1, less than 1 per cent; 2, 1–10%; 3, 10–30%; 4, 30–50%; 5, greater than 50 per cent. We used t-tests to assess the effect of infection on blood parameters, and regression to test for relationships between necrosis and blood parameters using StatistiXL v. 1.9 (see the electronic supplementary material). We adjusted alpha levels (α = 0.1 due to our small sample size) using the false discovery rate procedure suggested by Narum [15]: t-tests: α = 0.07, regressions: α = 0.04 (see the electronic supplementary material for details).
3. Results
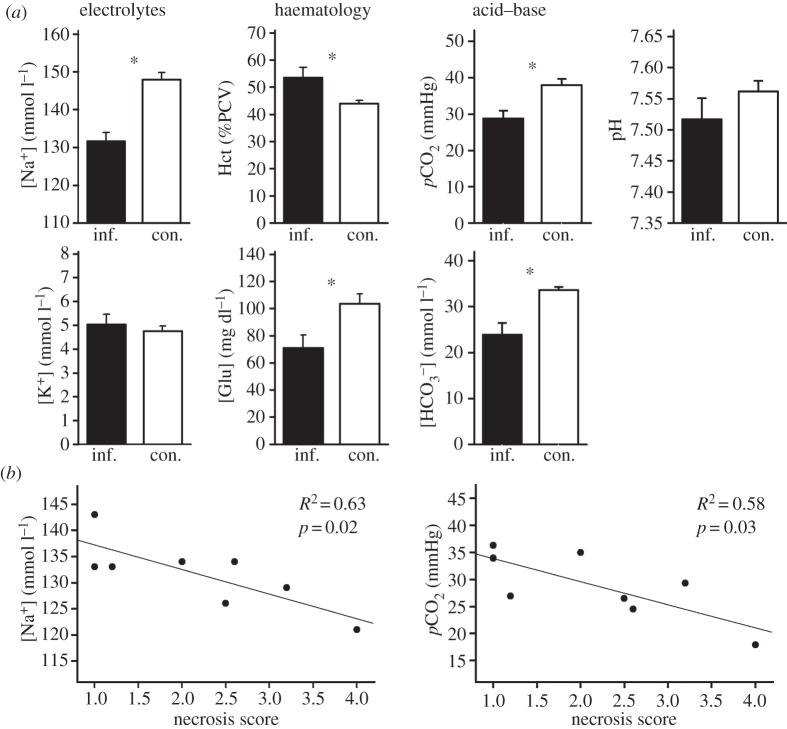
Blood parameters differed between infected and control bats (table 1). In terms of electrolytes, sodium concentration was lower for infected bats than controls but potassium did not differ (figure 1a and table 1). Haematology of infected bats differed from controls, with increased haematocrit and decreased glucose concentration (figure 1a and table 1). With regards to acid–base status, we found reduced pCO2 and bicarbonate concentrations, but no change in pH (figure 1a and table 1).
Table 1.
Statistics for the effects of Geomyces destructans infection on blood parameters, and regression analyses describing the relationship between necrosis score and blood parameters.
| category | variable |
t-test (infected versus control) |
regression (effect of necrosis score) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | d.f. | p | F | d.f. | p | intercept | slope coefficient | ±95% CI | ||
| electrolytes | [Na+] | 5.06 | 24 | <0.001a | 10.28 | 1,6 | 0.018a | 141.9 | −4.714 | −8.311/−1.117 |
| [K+] | −0.52 | 24 | 0.608b | 3.67 | 1,6 | 0.104 | 3.5 | 0.697 | −0.194/1.588 | |
| haematology | Hct | −2.44 | 8.44 | 0.040a | 2.41 | 1,6 | 0.172 | 42.3 | 5.120 | −2.956/13.196 |
| [Glu] | 2.60 | 24 | 0.016a | 0.13 | 1,6 | 0.730 | 78.7 | −3.593 | −27.938/20.752 | |
| acid–base | pCO2 | 3.03 | 24 | 0.006a | 8.18 | 1,6 | 0.029a | 38.1 | −4.272 | −7.928/−0.617 |
| pH | 1.32 | 24 | 0.200 | 2.71 | 1,6 | 0.150 | 7.6 | −0.049 | −0.121/0.024 | |
| [HCO3−] | 3.65 | 8.24 | 0.006a | 15.93 | 1,6 | 0.007a | 36.1 | −5.577 | −8.997/−2.158 | |
aRepresents p < 0.07 (t-tests) and p < 0.04 (regression).
bData log-transformed.
Figure 1.
(a) Blood parameters of bats infected with Geomyces destructans (inf., black bars, n = 8) versus controls (con., white bars, n = 18) illustrating electrolyte concentrations, haematology and acid–base balance. Error bars indicate standard error, and asterisk represents p < 0.07 (table 1). (b) Relationship between wing necrosis score and sodium concentration and carbon dioxide partial pressure.
Mean necrosis score of infected bats was 2.2 ± 0.39 (range 1.0–4.0). Sodium concentration, as well as pCO2 and bicarbonate concentrations, were negatively related to the severity of necrosis but other variables were not (figure 1b and table 1).
4. Discussion
Bats inoculated with Gd showed pronounced changes in blood physiology. Specifically, we observed (i) extracellular electrolyte depletion (reduced sodium), (ii) changes in haematology indicative of dehydration (increased haematocrit) and possibly starvation (decreased glucose) and (iii) evidence consistent with disrupted acid–base balance (reduced pCO2 and bicarbonate). Sodium, pCO2 and bicarbonate were negatively associated with wing necrosis score suggesting a mechanistic link between wing damage and pathophysiology. This supports the hypothesis that hypotonic dehydration associated with fungal infection contributes to WNS mortality, and implies that water and electrolyte imbalance, reduced blood supply to tissues and altered acid–base balance play a role in disease-related morbidity.
The decreased sodium concentrations we observed are consistent with severe fluid loss. Sodium concentrations measured previously for naturally and experimentally infected M. lucifugus, as well as healthy controls [10], were nearly identical to the values we observed. Cryan et al. [10] also found reduced chloride (not measured in our study) but ruled out renal failure based on normal urine specific gravity values [10], and histological examination showed no evidence of kidney damage in their study, or ours [12]. Hypotonic dehydration can be caused by severe burns [16], and burn victims may suffer hypovolaemia and increased evaporative water loss (EWL; [13]). In addition to sodium depletion, we found elevated haematocrit, which could be explained by erythrocyte swelling in hypotonic plasma but is also consistent with hypovolaemia and dehydration [14] and, given the critical water economy of hibernating bats in general [17], a dehydrated state is highly plausible. Taken together, these results support the hypothesis that electrolyte imbalance resulted from fluid loss across the compromised epidermis.
Based on these results, and the emaciated state of infected bats [12], we propose a mechanistic model connecting wing lesions caused by Gd with a cascade of pathophysiological responses, disrupted homeostasis and morbidity/mortality (figure 2). Wing damage in our model has two direct consequences: damage to the epidermis enables fluid loss and depletion of sodium (and presumably chloride [10]), while damage to underlying connective tissue increases vascular permeability, further accelerating fluid loss [9,10]. Both contribute to reduced plasma volume (i.e. hypovolaemia), causing elevated haematocrit and increased blood viscosity [14]. Hypovolaemia reduces blood pressure and inhibits capillary refill, causing local hypoxia. Hypovolaemia stimulates thirst [18] and could trigger bats to arouse from torpor to drink. EWL also influences torpor bout duration in hibernators even at high relative humidity [17]. Thus, fluid loss over the compromised epidermis could explain the increased frequency of arousals from torpor and premature fat depletion we reported previously [12]. Results here suggest that increased arousals reflected a response to elevated EWL, hypovolaemia and thirst.
Figure 2.
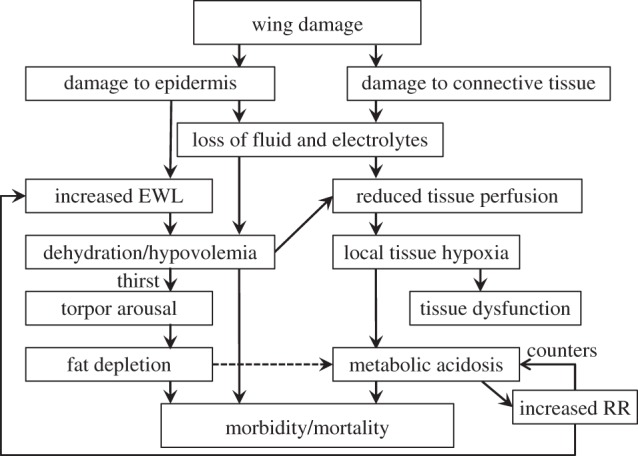
Theoretical model connecting damage to bat wings caused by Geomyces destructans with disruption of physiological processes which maintain homeostasis during winter hibernation, thus ultimately leading to mortality. Dashed line indicates alternative possible relationship. EWL, evaporative water loss; RR, respiration rate.
Our model explains the disruption in acid–base balance suggested by our results. Dehydration and hypovolaemia can cause metabolic acidosis due to anaerobic lactic acid production in tissues with reduced blood flow. Plasma pH was not significantly reduced with infection probably owing to buffering by bicarbonate and the quick response by peripheral chemoreceptors to acidosis triggering an increase in respiration rate to off-load CO2 [19]. This is consistent with the decreased pCO2 we observed. Increased respiration would further increase EWL and therefore arousal frequency [17]. Thus, the model accounts for altered torpor patterns as a combined result of hypotonic dehydration and respiratory compensation for metabolic acidosis. Starvation, hypovolaemia, metabolic acidosis or some combination of the three could all be the final cause of death.
Our model is hypothetical due to lack of data on the normal physiology of healthy, hibernating bats and on other pathophysiological effects of Gd. There are alternative explanations for the changes we observed. Hibernating ground squirrels (Ictidomys tridecemlineatus) preferentially rely on fatty acids and fat-derived ketones to fuel metabolism and, although healthy individuals are not acidotic, ketosis can cause metabolic acidosis in starvation [20]. Therefore, evidence for metabolic acidosis, combined with the reduced glucose we observed, could reflect increased energy expenditure with keto-acidosis and/or hypoglycaemia as potential causes of death. Owing to small blood volumes collected, we could not measure all relevant parameters. We suggest that future studies quantify serum/plasma protein and albumin concentrations to confirm hypovolaemia; plasma chloride, sodium, potassium and bicarbonate to calculate anion gap and test for metabolic acidosis; and lactate and ketone concentrations to differentiate lactic from keto-acidosis. Our model provides testable hypotheses that can be targeted by studies of particular pathways.
Epidermal invasion by Gd and subsequent pathophysiological changes are reminiscent of amphibian chytridiomycosis [9]. Both pathogens directly affect only skin but cause fatal disruptions of homeostasis. Although underlying mechanisms appear to differ (i.e. in amphibians, electrolyte imbalance leads to cardiac arrest [4]), WNS is superficially similar. Given the scale of wildlife population declines caused by fungal pathogens [1], understanding the pathophysiology underlying these diseases is important for developing strategies to hopefully mitigate mortality of affected species.
Acknowledgements
Methods were approved by the University Committee on Animal Care and Supply of the University of Saskatchewan.
We thank ACU and CCWHC staff in Saskatoon. K. Castle, C. Cooper, L. McGuire, J. Voyles, N.R. Willis, P. Withers, M. Wojciechowski and two reviewers gave outstanding feedback. Financially supported by US Fish and Wildlife Service, Natural Sciences and Engineering Research Council (Canada), Canada Foundation for Innovation, Manitoba Research and Innovation Fund, Government of Canada Post-doctoral Fellowship and German Academic Exchange Service (DAAD). Any use of trade products or firm names is for description only and does not imply endorsement by the US Government.
References
- 1.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194 10.1038/nature10947 (doi:10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wake DB, Vredenburg VT. 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA 105, 11 466–11 473 10.1073/pnas.0801921105 (doi:10.1073/pnas.0801921105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, Butchkoski CM, Kunz TH. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682 10.1126/science.1188594 (doi:10.1126/science.1188594) [DOI] [PubMed] [Google Scholar]
- 4.Voyles J, et al. 2009. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326, 582–585 10.1126/science.1176765 (doi:10.1126/science.1176765) [DOI] [PubMed] [Google Scholar]
- 5.Blehert DS, Hicks AC, Behr M. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 227. 10.1126/science.1163874 (doi:10.1126/science.1163874) [DOI] [PubMed] [Google Scholar]
- 6.Meteyer CU, Buckles EL, Blehert DS, Hicks AC, Green DE, Shearn-Bochsler V, Thomas NJ, Gargas A, Behr MJ. 2009. Histopathologic criteria to confirm white-nose syndrome in bats. J. Vet. Diagn. Invest. 21, 411–414 10.1177/104063870902100401 (doi:10.1177/104063870902100401) [DOI] [PubMed] [Google Scholar]
- 7.Herreid CF, Bretz WL, Schmidt-Nielsen K. 1968. Cutaneous gas exchange in bats. Am. J. Physiol. 215, 506–508 [DOI] [PubMed] [Google Scholar]
- 8.Dongaonkar RM, Stewart RH, Laine GA, Davis MJ, Zawieja DC, Quick CM. 2009. Venomotion modulates lymphatic pumping in the bat wing. Am. J. Physiol. Heart Circul. Physiol. 296, H2015–H2021 10.1152/ajpheart.00418.2008 (doi:10.1152/ajpheart.00418.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. 2010. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 8, 135. 10.1186/1741-7007-8-135 (doi:10.1186/1741-7007-8-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cryan PM, et al. 2013. Electrolyte depletion in white-nose syndrome bats. J. Wildl. Dis. 49, 398–402 10.7589/2012-04-121 (doi:10.7589/2012-04-121) [DOI] [PubMed] [Google Scholar]
- 11.Willis CKR, Menzies AK, Boyles JG, Wojciechowski MS. 2011. Evaporative water loss is a plausible explanation for mortality of bats from white-nose syndrome. Integr. Comp. Biol. 51, 364–373 10.1093/icb/icr076 (doi:10.1093/icb/icr076) [DOI] [PubMed] [Google Scholar]
- 12.Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR. 2012. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Nat. Acad. Sci. USA 109, 6999–7003 10.1073/pnas.1200374109 (doi:10.1073/pnas.1200374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keck M, Herndon DH, Kamolz LP, Frey M, Jeschke MG. 2009. Pathophysiology of burns. Wien. Med. Wochenschr. 159, 327–336 10.1007/s10354-009-0651-2 (doi:10.1007/s10354-009-0651-2) [DOI] [PubMed] [Google Scholar]
- 14.Beck N. 2009. Blood hematology. London, UK: Springer [Google Scholar]
- 15.Narum SR. 2006. Beyond Bonferroni: less conservative analyses for conservation genetics. Conserv. Genet. 7, 783–787 10.1007/s10592-005-9056-y (doi:10.1007/s10592-005-9056-y) [DOI] [Google Scholar]
- 16.Gangopadhyay H. 2008. Clinical hyponatremia and hypernatremia. In Renal failure and replacement therapies (ed. Blakeley S.), pp. 77–80 London, UK: Springer [Google Scholar]
- 17.Thomas DW, Geiser F. 1997. Periodic arousals in hibernating mammals: is evaporative water loss involved? Funct. Ecol. 11, 585–591 10.1046/j.1365-2435.1997.00129.x (doi:10.1046/j.1365-2435.1997.00129.x) [DOI] [Google Scholar]
- 18.Stricker EM. 1968. Some physiological and motivational properties of the hypovolemic stimulus for thirst. Physiol. Behav. 3, 379–385 10.1016/0031-9384(68)90066-8 (doi:10.1016/0031-9384(68)90066-8) [DOI] [Google Scholar]
- 19.Nestler JR. 1990. Relationships between respiratory quotient and metabolic rate during entry to and arousal from daily torpor in deer mice (Peromyscus maniculatus). Physiol. Zool. 63, 504–515 [Google Scholar]
- 20.Andrews MT, Russeth KP, Drewes LR, Henry P-G. 2009. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am. J. Physiol. 296 R383–R393 10.1152/ajpregu.90795.2008 (doi:10.1152/ajpregu.90795.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]