Abstract
Most marine mammal strandings coincident with naval sonar exercises have involved Cuvier's beaked whales (Ziphius cavirostris). We recorded animal movement and acoustic data on two tagged Ziphius and obtained the first direct measurements of behavioural responses of this species to mid-frequency active (MFA) sonar signals. Each recording included a 30-min playback (one 1.6-s simulated MFA sonar signal repeated every 25 s); one whale was also incidentally exposed to MFA sonar from distant naval exercises. Whales responded strongly to playbacks at low received levels (RLs; 89–127 dB re 1 µPa): after ceasing normal fluking and echolocation, they swam rapidly, silently away, extending both dive duration and subsequent non-foraging interval. Distant sonar exercises (78–106 dB re 1 µPa) did not elicit such responses, suggesting that context may moderate reactions. The observed responses to playback occurred at RLs well below current regulatory thresholds; equivalent responses to operational sonars could elevate stranding risk and reduce foraging efficiency.
Keywords: acoustic disturbance, avoidance response, anthropogenic noise, mid-frequency active sonar, military, Ziphius cavirostris
1. Introduction
Unusual mass strandings of cetaceans, especially beaked whales, have been associated with the operation of military mid-frequency active (MFA) sonars; these sometimes fatal events have raised serious concern about impacts of sonar and other anthropogenic sounds on whales [1–3]. Behavioural responses to MFA sonar are thought to play an important role in the series of events that leads to such strandings [1]. An on-going series of controlled exposure experiments (CEEs) and opportunistic studies have begun to provide data on behavioural responses to MFA sonar by species including Blainville's beaked whales (Mesoplodon densirostris; [4]). Cuvier's beaked whales (Ziphius cavirostris Cuvier) make up the majority of fatalities in MFA-associated strandings [5], and Ziphius abundance along the US west coast is apparently declining [6], but until now, direct measurements of Ziphius behavioural responses to MFA sonar have not been described.
2. Material and methods
We present results from two Cuvier's beaked whales that were exposed to simulated MFA sonar during the Southern California Behavioural Response Study [7] in 2010–2011. The 2011 whale was also incidentally exposed to MFA sonar from a distant naval exercise. The whales were tagged with DTAGs ([8]; table 1), which recorded acoustic data (stereo, 16 bits, greater than or equal to 192 kHz sampling rate) and animal movement data (greater than or equal to 50 Hz sampling rate from tri-axial accelerometers and magnetometers and a pressure sensor, down-sampled to 5 Hz for analysis). To characterize baseline Ziphius behaviour, we used DTAG data from 13 whales in the Mediterranean Sea [9,10] and time-depth recorder data (mk9, Wildlife Computers, Redmond, WA, USA) from two whales in Hawai‘i ([11]; table 1). For each dive exceeding 50 m, we calculated: dive duration, maximum depth, duration and rate of descent and ascent, and time from surfacing until the next dive. For DTAG data only, we also calculated: duration of echolocation click production, time from surfacing until next echolocation click, average fluke-stroke rate [10], average overall dynamic body acceleration (ODBA; [12]), circular variance in heading [13] and source-whale range during exposures (figures 1 and 2; electronic supplementary material; data deposited in Dryad repository [14]).
Table 1.
Details of tag deployments on Ziphius cavirostris.
| tag ID | date and local time | tagging location (° N, ° E) | region | acoustic exposure | tag recording duration (h) | no. dives |
|---|---|---|---|---|---|---|
| zc03_260a | 17 Sep 2003 17.48.03 | 44.1238, 8.8520 | Mediterranean Sea | none | 2.8 | 5 |
| zc03_263a | 20 Sep 2003 15.24.28 | 44.0960, 8.5897 | Mediterranean Sea | none | 15.6 | 19 |
| zc04_160a | 8 June 2004 16.44.23 | 44.0354, 8.7671 | Mediterranean Sea | none | 5.6 | 8 |
| zc04_161a | 9 June 2004 12.58.05 | 44.1107, 8.5819 | Mediterranean Sea | none | 8.9 | 18 |
| zc04_161b | 9 June 2004 14.07.37 | 44.0853, 8.5622 | Mediterranean Sea | none | 15.7 | 32 |
| zc04_175a | 23 June 2004 16.28.52 | 44.1117, 8.6856 | Mediterranean Sea | none | 7.5 | 6 |
| zc04_179a | 23 June 2004 16.28.52 | 44.1576, 8.7039 | Mediterranean Sea | none | 3.8 | 4 |
| zc05_167a | 16 June 2005 16.12.27 | 44.1568, 8.8253 | Mediterranean Sea | none | 7.6 | 15 |
| zc05_170a | 19 June 2005 15.24.13 | 44.1638, 8.7316 | Mediterranean Sea | none | 11.9 | 17 |
| zc06_204a | 23 July 2006 12.21.27 | 43.8190, 8.7255 | Mediterranean Sea | none | 6.2 | 6 |
| zc06_205a | 24 July 2006 13.57.43 | 43.7680, 8.7460 | Mediterranean Sea | none | 13.5 | 34 |
| zc08_164a | 12 June 2008 18.13.48 | 35.9321, −3.2773 | Mediterranean Sea | none | 16.2 | 25 |
| zc10_272a | 29 Sep 2010 09.49.43 | 32.8066, −119.0153 | Southern California Bight | MFA | 18.3 | 33 |
| zc11_267a | 24 Sep 2011 08.49.56 | 33.5105, −119.2806 | Southern California Bight | MFA | 21.3 | 39 |
| zc12_169a | 17 June 2012 17.49.40 | 43.7383, −8.4742 | Mediterranean Sea | none | 14.3 | 16 |
| Nov. 2004 (mk9) | 28 Nov 2004 13.02 | 19.32219, −156.04654 | West of Hawai‘i | none | 9.8 | 12 |
| Nov. 2006 (mk9) | 30 Nov 2006 09.41 | 19.44436, −156.05162 | West of Hawai‘i | none | 34.1 | 38 |
| totals | ||||||
| control (DTAG) | 129.6 | 205 | ||||
| control (mk9) | 43.9 | 50 | ||||
| exposed (DTAG) | 39.6 | 72 | ||||
| all | 213.1 | 327 | ||||
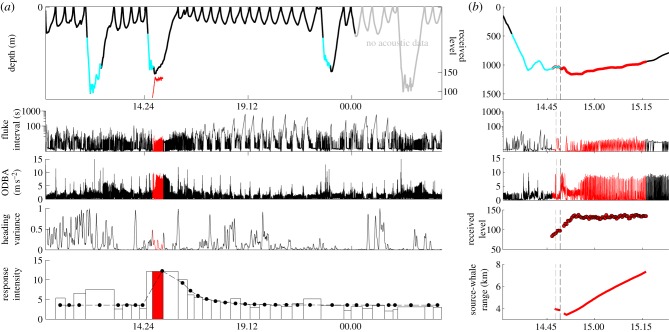
Figure 1.
DTAG data from the Ziphius tagged in 2010, which underwent controlled exposure to simulated MFA sonar sounds. (a) Dive profile, with periods of echolocation clicking in cyan; time between fluke-strokes [10]; ODBA [12]; circular variance [13] of the animal's heading and the RI metric. For RI, boxes are observed data; dotted line with filled circles is fitted model output. (b) Zoomed view of the dive profile, fluke interval, ODBA, received MFA sonar level (dB re 1 µPa rms) and source-whale range. Grey vertical lines indicate the time of cessation of normal fluking in response to the sonar, and black lines the time when the strong avoidance response began. Throughout, pre- and post-exposure periods are in black, and controlled exposure periods in red.
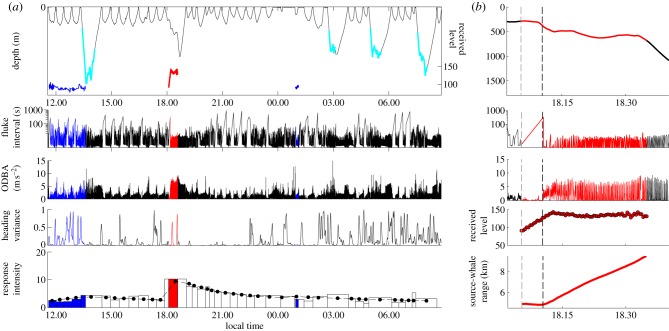
Figure 2.
DTAG data from the Ziphius tagged in 2011, which underwent controlled exposure to simulated MFA sonar sounds and incidental exposure to naval MFA sonar. Figure layout and colour- and symbol-coding are the same as figure 1, but with blue traces for incidental exposure periods.
CEE methods are described elsewhere [7]. After a 4.9 h (2010) or 9.3 h (2011) pre-exposure period, each whale experienced a 30-min controlled exposure from a stationary sound source 3.4–9.5 km away. During this period, the source transmitted a 1.6 s simulated MFA sonar signal every 25 s. The initial source level of 160 dB re 1 μPa-m was increased (‘ramped up’) by 3 dB per transmission to a maximum of 210 dB re 1 µPa-m. Incidental MFA sonar from a distant naval exercise was detected on the tag before and after the 2011 CEE. Based on US Navy logs, the ships were approximately 118 km away. RLs of MFA sonar signals from controlled and incidental exposures (calculated as in [4]) were 84–144 and 78–106 dB re 1 μPa root mean squared (rms), respectively (figures 1 and 2). (All RLs reported hereafter are in dB re 1 μPa rms)
Whale responses to MFA sonar sounds were scored according to a qualitative response severity scale [15]. To quantify overall response intensity (RI), we calculated a Mahalanobis distance-based RI metric that summarizes all DTAG dive parameters and quantifies how much each dive differs from the average baseline shallow or foraging dive. We modelled RI as a function of RL, source-whale range (2011 only, since 2010 had only one exposure dive), and time since sonar exposure (figures 1 and 2). We compared the full models with a nested set of models with fewer covariates using Akaike's information criterion (AIC; see the electronic supplemental materials for statistical details).
3. Results
Both whales showed clear responses to the CEEs, escalating from initial moderate orientation changes (corresponding to a score of 2 on the severity scale [15]) to a strong avoidance response sustained beyond the end of the exposure (severity score 8 [15]); however, the 2011 whale did not respond similarly to incidental naval sonar exposures (figures 1 and 2). When CEEs began, both whales stopped fluking, perhaps to monitor the sound and prepare to respond (2010: three 15–23 s pauses at RL 89–97 dB; 2011: 320 s pause at RL 90 dB; figures 1 and 2). In 2010, when RL reached 98 dB, the whale ceased its echolocation click production, interrupting foraging. It initiated an avoidance response that included energetic fluking, swimming away from the source at 2.6 m s−1, extended dive duration with an unusually slow ascent and a long (6.6 h) post-exposure inter-deep-dive interval (figure 1). The whale continued this strong and sustained avoidance (with rapid fluking, high ODBA and minimal heading variance) until about 1.6 h post-exposure (figure 1). In the 2011 CEE, when RL reached 127 dB, the whale initiated a similar avoidance response, including energetic fluking, swimming away from the source at 3.1 m s−1 and a long (7.6 h) post-exposure inter-deep-dive interval (figure 2; electronic supplementary material). This avoidance response lasted at least 1.7 h post-exposure (figure 2). The 2011 whale did not echolocate during the CEE dive, making the dive difficult to classify. It matches foraging dives in duration and depth but not dive shape and is unusually long and deep for a silent dive (figure 2; electronic supplementary material). By contrast, shallow dives coinciding with the distant naval exercise were similar to control shallow dives (figures 1 and 2; electronic supplementary material).
Statistical modelling of RI showed full models to be far superior to all reduced models (ΔAIC ≥ 28.5), suggesting that both RL and source-whale range influence response. RI remained elevated after exposure for 2.7 and 3.8 h in 2010 and 2011, respectively (figures 1 and 2).
4. Discussion
We observed intense, consistent, long-lasting responses by two Ziphius to simulated MFA sonar at short ranges. However, MFA sonar from a distant naval exercise did not elicit a similar response, even at comparable RLs. Source-whale range may therefore moderate a level-driven response to sonar, as may other factors (e.g. ramp-up; behavioural/environmental context). We note that Ziphius resident in the study area probably have prior experience with MFA sonar, as naval operations routinely occur in the region [16]. This complexity should be considered carefully in interpreting these results and planning future studies.
Our results extend previous findings on beaked whale noise responses [4,9,17], addressing in detail the key case of Ziphius and MFA sonar. Response of one Ziphius to ship noise (136 dB maximum received level) included cessation of echolocation, as in our MFA sonar CEEs, but in contrast, dive duration and inter-deep-dive interval were short (see the electronic supplementary material; [9]). Acoustic recordings of Blainville's beaked whales during ship noise exposure were consistent with directed travel [17]. Early cessation of clicking and horizontal avoidance are also components of MFA sonar response by Blainville's beaked whales [4]. Cessation of echolocation clicks was thus a common response to acoustic disturbance in beaked whales studied to date, but sonar avoidance responses were stronger and more prolonged than responses to vessel noise.
The silent, underwater avoidance reaction of Ziphius to MFA sonar is consistent with their cryptic behaviour: they spend minimal time at the surface, rarely produce sounds other than echolocation clicks and click only at depths exceeding 200 m [10]. The observed responses included vigorous swimming and extended time without echolocation-based foraging, imposing a net energetic cost that (if repeated) could reduce individual fitness. Rapid, directed swimming could increase stranding risk, particularly if it occurs near shore [5]. Our results are inconsistent with the hypothesis that unusually rapid ascents cause emboli found in beaked whales from sonar-related strandings [3]. However, the dive profile and swim-speed alterations we observed might still affect dive metabolism, perhaps reducing capacity to control perfusion of tissues with inert gases, and increasing the risk of gas-bubble lesions during decompression [18].
Our results represent the first empirical demonstration of behavioural responses to MFA sonar by Z. cavirostris, the species that accounts for 69 per cent of recorded cetacean strandings associated with MFA sonar [5]. Although we observed strong responses to nearby, controlled exposures to simulated MFA sonar, we did not detect similar responses to distant, incidental exposure to naval sonar exercises at comparable RLs, which is an important consideration in the application of these data and should be explored in future experiments. However, we particularly emphasize that Ziphius initiated intense, sustained responses to controlled exposures at RLs of 89–127 dB. Current US management practices assume that significant behaviour disruption almost never occurs at exposure levels this low [19,20]. This study provides a much-needed scientific basis to inform decisions and reduce adverse effects of MFA sonar on beaked whales.
Acknowledgements
The authors gratefully acknowledge the work of the R.V. Truth crew and the SOCAL10–11 science parties, especially Annie Douglas and Todd Pusser; Catriona Harris and Sascha Hooker for helpful comments; Mark Johnson and Leigh Hickmott for providing Mediterranean datasets and Robin Baird for Hawai‘i datasets. This work was supported by the US Navy Chief of Naval Operations, Environmental Readiness Program; the Office of Naval Research and the MASTS pooling initiative (Marine Alliance for Science and Technology for Scotland; supported by the Scottish Funding Council (grant reference HR09011) and contributing institutions). Experiments were performed under NMFS permit no. 14534 (B. Southall, principal investigator). Data available from Dryad repository, doi:10.5061/dryad.n77k3.
References
- 1.Cox TM, et al. 2006. Understanding the impacts of anthropogenic sound on beaked whales. J. Cetacean Res. Manag. 7, 177–187 [Google Scholar]
- 2.Simmonds MP, Lopez-Jurado LF. 1991. Whales and the military. Nature 337, 448 (doi:10.1038/351448a0) [Google Scholar]
- 3.Jepson PD, et al. 2003. Gas-bubble lesions in stranded cetaceans. Nature 425, 575–576 (doi:10.1038/425575a) [DOI] [PubMed] [Google Scholar]
- 4.Tyack PL, et al. 2011. Beaked whales respond to simulated and actual navy sonar. PLoS ONE 6, e17009 (doi:10.1371/journal.pone.0017009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Amico A, Gisiner RC, Ketten DR, Hammock JA, Johnson C, Tyack PL, Mead J. 2009. Beaked whale strandings and naval exercises. Aquat. Mamm. 35, 452–472 (doi:10.1578/AM.35.4.2009.452) [Google Scholar]
- 6.Moore JE, Barlow JP. 2013. Declining abundance of beaked whales (Family Ziphiidae) in the California current large marine ecosystem. PLoS ONE 8, e52770 (doi:10.1371/journal.pone.0052770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Southall BL, Moretti D, Abraham B, Calambokidis J, DeRuiter SL, Tyack PL. 2012. Marine mammal behavioral response studies in southern California: advances in technology and experimental methods. Mar. Technol. Soc. J. 46, 48–59 (doi:10.4031/MTSJ.46.4.1) [Google Scholar]
- 8.Johnson MP, Tyack PL. 2003. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean. Eng. 28, 3–12 (doi:10.1109/JOE.2002.808212) [Google Scholar]
- 9.Aguilar Soto N, Johnson MP, Madsen PT, Tyack PL, Bocconcelli A, Borsani JF. 2006. Does intense ship noise disrupt foraging in deep-diving Cuvier's beaked whales (Ziphius cavirostris)? Mar. Mamm. Sci. 22, 690–699 (doi:10.1111/j.1748-7692.2006.00044.x) [Google Scholar]
- 10.Tyack PL, Johnson MP, Soto NA, Sturlese A, Madsen PT. 2006. Extreme diving of beaked whales. J. Exp. Biol. 209, 4238–4253 (doi:10.1242/jeb.02505) [DOI] [PubMed] [Google Scholar]
- 11.Baird RW, Webster DL, Schorr GS, McSweeney DJ, Barlow J. 2008. Diel variation in beaked whale diving behavior. Mar. Mamm. Sci. 24, 630–642 (doi:10.1111/j.1748-7692.2008.00211.x) [Google Scholar]
- 12.Qasem L, Cardew A, Wilson A, Griffiths I, Halsey LG, Shepard ELC, Gleiss AC, Wilson R. 2012. Tri-axial dynamic acceleration as a proxy for animal energy expenditure; should we be summing values or calculating the vector? PLoS ONE 7, e31187 (doi:10.1371/journal.pone.0031187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zar JH. 2010. Biostatistical analysis, 5th edn Upper Saddle River, NJ: Pearson Prentice-Hall [Google Scholar]
- 14.DeRuiter SL, et al. 2013. Data from: First direct measurements of behavioural responses by Cuvier's beaked whales to mid-frequency active (MFA) sonar. Dryad Digital Repository. (doi:10.5061/dryad.n77k3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Southall BL, et al. 2007. Marine mammal noise exposure criteria: initial scientific recommendations. Aquat. Mamm. 33, 411–521 (doi:10.1578/AM.33.4.2007.411) [Google Scholar]
- 16.Falcone EA, Schorr GS, Douglas AB, Calambokidis J, Henderson E, McKenna MF, Hildebrand J, Moretti D. 2009. Sighting characteristics and photo-identification of Cuvier's beaked whales (Ziphius cavirostris) near San Clemente Island, California: a key area for beaked whales and the military? Mar. Biol. 156, 2631–2640 (doi:10.1007/s00227-009-1289-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirotta E, Milor R, Quick N, Moretti D, Di Marzio N, Tyack P, Boyd I, Hastie G. 2012. Vessel noise affects beaked whale behavior: results of a dedicated acoustic response study. PLoS ONE 7, e42535 (doi:10.1371/journal.pone.0042535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooker SK, et al. 2012. Deadly diving? Physiological and behavioural management of decompression stress in diving mammals. Proc. R. Soc. B 279, 1041–1050 (doi:10.1098/rspb.2011.2088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonymous 2009. Taking and importing marine mammals; navy training activities conducted within the northwest training range complex; proposed rule. Fed. Regist. 74, 33 828–33 900 [Google Scholar]
- 20.Anonymous 2010. Taking and importing marine mammals; navy training activities conducted within the northwest training range complex; final rule. Fed. Regist. 75, 69 296–69 326 [Google Scholar]