Abstract
Extreme climatic events can substantially affect organismal performance and Darwinian fitness. In April 2011, a strong heat wave struck extensive geographical areas of the world, including Western Europe. At that time, we happened to resume and extend a long-term time series of seasonal genetic data in the widespread fly Drosophila subobscura, which provided a unique opportunity to quantify the intensity of the genetic perturbation caused by the heat wave. We show that the spring 2011 genetic constitution of the populations transiently shifted to summer-like frequencies, and that the magnitude of the genetic anomaly quantitatively matched the temperature anomaly. The results provide compelling evidence that direct effects of rising temperature are driving adaptive evolutionary shifts, and also suggest a strong genetic resilience in this species.
Keywords: climate change, adaptation, evolution, chromosomal inversions
1. Introduction
Extreme climatic events are predicted to increase in frequency and intensity due to climate change [1]. Climatic extremes have long been considered as major triggers of evolution [2,3]. We do not yet know, however, to what extent standing genetic variation responds to or eventually recovers from these acute environmental challenges, perhaps because of the swiftness and rarity of such large perturbations relative to the time frame of standard evolutionary genetic studies. Here, we take advantage of the long-term seasonal monitoring (starting in 1976) of chromosomal inversion polymorphisms in Drosophila subobscura [4], and the fact that in April 2011, an extreme summer-like heat wave event struck Western Europe [5] right at the time when we resumed and extended the seasonal studies.
Drosophila subobscura is native to the Palaearctic region, where it is geographically widespread from North Africa to Scandinavia [6]. In the late 1970s and early 1980s, the species successfully invaded South and North America after accidental introduction in both continents [7]. It harbours a rich chromosomal inversion polymorphism on its five major acrocentric chromosomes, with most inversions showing worldwide parallel latitudinal clines as well as putative long-term trends attributed to global warming that are congruent with the geographical patterns [8,9]. Strong seasonal variation for some inversions had been reported in one population (Mount Pedroso; 42°53′7.5″ N, 8°34′10.04″ W) in northwestern Spain [4]. The seasonal monitoring of inversion frequencies focused on one chromosome (chromosome O) sampled yearly in four seasons (spring, early summer, late summer and autumn). In agreement with the clinal patterns, ‘warm-climate’ inversions on this chromosome increased in frequency during summer and decreased during winter. This cycling pattern might hamper inferences from long-term records based on single-year data points [4,10,11]. Nevertheless, the generality of the seasonal cycles was questioned because the data were limited to one population and one chromosome [12,13].
To determine the genetic and geographical scope of the cyclic seasonal changes in the inversion frequencies of D. subobscura, we resumed the yearly seasonal population sampling in 2011. The study was extended (i) to all polymorphic inversions on the five acrocentric chromosomes in the original Mount Pedroso natural population, and (ii) to an additional Spanish population (Berbikiz; 43°11′20.31″ N, 3°5′23.74″ W) located approximately 600 km east (far enough to rule out homogenizing effects of gene flow at the study time scale). Here, we report how the April 2011 heat wave affected the chromosomal inversion polymorphism of D. subobscura, and also the rapid recovery of inversion frequencies to their normal seasonal range.
2. Material and methods
The two Spanish locations were sampled over two years: 2011 and 2012. This study is a follow-up of a long-term seasonal monitoring at Mount Pedroso and covers the periods 1976–1980, 1988–1991 and the present. Berbikiz was sampled here for the first time. Flies were caught between mid-afternoon and twilight by net-sweeping over banana baits fermented with baker's yeast. Chromosome gene arrangements were determined by crossing wild males to virgin females from the ch-cu marker strain as previously described [4].
Weather data were provided by the Spanish National Agency of Meteorology (AEMET) from two meteorological stations at each locality: Santiago de Compostela-EOAS (climate index (CI): 1475A; 240 m a.s.l; 1 km apart from the sampling site) and Lavacolla airport (CI: 1428; 370 m; 10 km) for Mount Pedroso; Güeñes (CI: 1078I; 208 m; 1 km) and Sondika airport (CI: 1082; 42 m; 17 km) for Berbikiz. We used the more comprehensive temperature data from the airport meteorological stations after correcting for average differences in temperature to better reflect the actual thermal environment at the sampling localities (Lavacolla is 1°C colder than Santiago de Compostela-EOAS; Sondika is 1.5°C warmer than Güeñes). (The correction was only applied in figure 2; namely, we added 1°C to Mount Pedroso and subtracted 1.5°C from Berbikiz.)
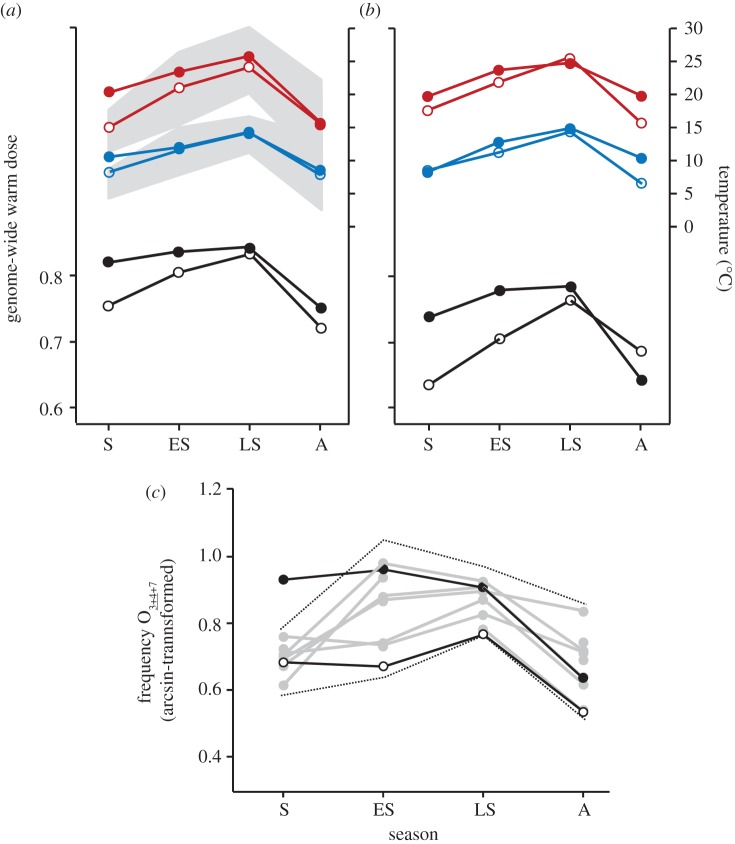
Figure 2.
Seasonal patterns of the genome-wide warm dose at (a) Mount Pedroso and (b) Berbikiz. Plotted are the maximum (red) and minimum (blue) temperatures in 2011 (solid dots) and 2012 (open dots) in the four seasonal collections: spring (S), early summer (ES), late summer (LS) and autumn (A). The temperature values refer to the averages during the 30 day period prior each sampling date. The grey bands plot the normal range (within ± 3σ around the average) of maximum and minimum temperatures for the historical records at Mount Pedroso. Black dots plot the genome-wide warm dose of chromosome inversion polymorphism in 2011, and white dots in 2012. Spearman's rank correlation coefficients between the genome-wide warm dose and Tmax were rS = 0.857 (p = 0.006) at Mount Pedroso and rS = 0.762 (p = 0.028) at Berbikiz; and between the genome-wide warm dose and Tmin were rS = 0.929 (p = 0.001) at Mount Pedroso and rS = 0.714 (p = 0.046) at Berbikiz. (c) Genetic anomaly of the warm-climate chromosome inversion O3+_4+7 at Mount Pedroso in 2011 (black dots) and 2012 (white dots). The background grey lines plot the historical time series of frequency data (1976–1980; 1988–1991) that show a marked seasonal pattern; and the dotted lines their range within ± 2σ around the seasonal average. To estimate ± 2σ, we used the variance within each season because of heteroscedasticity (Levene's F3,31 = 4.17, p = 0.014; the year 2011 was not included). The frequency of O3+_4+7 in spring 2011 deviates + 5.4σ from the historical spring's average and + 3.5σ from the previous maximum frequency recorded at that season.
The heat wave began on 1 April, approximately three weeks before the collection dates in spring 2011 (see the electronic supplementary material, table S1). Maximum (Tmax) and minimum (Tmin) temperatures were within the average range on 22 April when flies were collected at Mount Pedroso. Therefore, we can rule out potential sampling effects related to flies' thermoregulatory behaviours [14] that could have biased upwards our estimate of some warm-climate gene arrangement frequencies.
3. Results and discussion
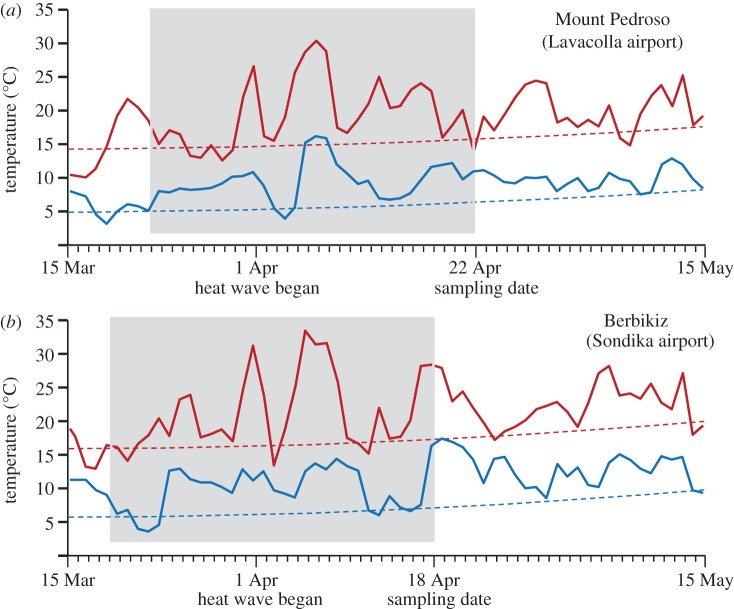
April 2011 was the warmest April on record across Spain and the UK [5]. To gauge the intensity of the heat wave, we estimated the ‘normal’ range of interannual variability in average surface air temperature during the 30 day period prior each sampling date (figure 1); normal temperatures were defined as those within three standard deviations (± 3σ) around the average for each sampling season [15]. Spring 2011 is the only data point that deviates from the normal thermal environment; the average D. subobscura fly should have experienced in the corresponding season: Tmax = + 5.7σ (6.0°C above the mean) and Tmin = + 5.4σ (+ 4.2°C) at Mount Pedroso; Tmax = + 4.3σ (+ 5.4°C) and Tmin = + 2.8σ (+ 2.9°C) at Berbikiz.
Figure 1.
Daily temperatures in spring 2011 at (a) Lavacolla and (b) Sondika airports. Daily maximum (red solid line) and minimum (blue solid line) temperatures for the 15 Mar–15 May period in 2011 at Mount Pedroso and Berbikiz. Dotted lines show the average maximum (red) and minimum (blue) temperatures for the 1971–2000 base period. The heat wave began on 1 Apr, approximately three weeks before the dates of collection. Boxed in grey is the 30 day period prior to the sampling dates used to calculate the temperature anomalies in spring 2011.
The genetic response to the April 2011 heat wave was studied using a genome-wide index that combines the frequencies of warm-climate inversions on all chromosomes into a warm-climate inversion dose (see the electronic supplementary material for an alternative analysis using the genome-wide chromosome index as in Balanyà et al. [9]). A remarkably consistent pattern between populations, between years and among seasons was observed (figure 2a,b). The warm dose was higher at the warmer Mount Pedroso locality (p = 0.006; one-tailed Wilcoxon matched-pairs signed-ranks test), and also higher in the warmer 2011 year compared with 2012 (p = 0.035). It peaked from early summer to late summer and dropped in autumn and spring (Friedman's ANOVA: 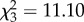 , p = 0.011; Kendall's coefficient of concordance: W = 0.925); a regular pattern already observed in the historical records of warm-climate inversions on chromosome O [4]. The apparent genetic footprint that the April 2011 heat wave left in the chromosomal inversion polymorphism, with a 2011 spring warm dose more typical of the summer season, should also be detected in the time series of chromosome O inversion data from Mount Pedroso assuming temperature is the causal factor.
, p = 0.011; Kendall's coefficient of concordance: W = 0.925); a regular pattern already observed in the historical records of warm-climate inversions on chromosome O [4]. The apparent genetic footprint that the April 2011 heat wave left in the chromosomal inversion polymorphism, with a 2011 spring warm dose more typical of the summer season, should also be detected in the time series of chromosome O inversion data from Mount Pedroso assuming temperature is the causal factor.
To obtain comparative data, we focused on the most common warm-climate chromosome gene arrangement O3+_4+7, which has an average annual frequency of 47.7 per cent at Mount Pedroso. Unlike other inversions on the same chromosome, this arrangement shows no detectable long-term trend in annual average frequencies (r2 = 0.002, p = 0.905) underlying its pronounced seasonal cycling [4,16] and can be used as a baseline for comparisons. The frequency of O3+_4+7 in spring 2011 was the highest ever recorded in the population at that season, deviating + 5.4σ from the average spring frequency and basically the same deviation as that observed for the temperature anomaly in Tmax and Tmin at Mount Pedroso (see the electronic supplementary material for estimates of the high selection coefficients involved). Nevertheless, the inversion recovered its typical seasonal frequencies swiftly after the heat wave (figure 2c).
This is the first study, to our knowledge, that provides unequivocal evidence that natural populations are genetically responding in situ to higher temperature alone [17]. Studies of long-term trends of chromosomal inversion polymorphisms tracking global warming [9,18,19] cannot rule out a progressive expanding of low latitude genotypes poleward [9,20]. Furthermore, some reported evolutionary responses to climate change can be better explained as adaptive shifts in the timing of seasonal events rather than as a direct effect of increasing temperature [21]. A wide variety of temperate animals and plants use photoperiodic cues to time their seasonal activities [22], but D. subobscura seems to be idiosyncratic in this regard because it combines a short generation time with an absence of strong photoperiodism [23] and an ecological generalism [24]. The marked seasonal cycling of its inversion polymorphism is not restricted to one chromosome but happens genome-wide at various localities, and the pattern of this cycling neatly matches the seasonal variation in ambient temperature (figure 2a,b). Moreover, there seem to be no time lag for evolutionary response to a heat wave in this population: thermal selection is apparent one generation after a heat wave event. Evolution of chromosome inversion polymorphisms in D. subobscura is fast and reversible, and the environmental cue is fluctuating temperature. However, we caution that our findings cannot be extrapolated to tropical species living in more constant thermal regimes as they seem to have far less genetic flexibility [25] than temperate D. subobscura.
Acknowledgements
We appreciate all the reviewers’ constructive comments on our manuscript. The Spanish Meteorological Agency (AEMET) provided climatic records. V. Pérez and J. Taboada from MeteoGalicia gave advice on weather information. Funded by grant nos CGL2010–15395 from the Ministerio de Ciencia e Innovación, Spain; 2009SGR 636 from Generalitat de Catalunya and the ICREA Acadèmia programme.
References
- 1.Hegerl GC, Hanlon H, Beierkuhnlein C. 2011. Climate science: elusive extremes. Nat. Geosci. 4, 142–143 10.1038/ngeo1090 (doi:10.1038/ngeo1090) [DOI] [Google Scholar]
- 2.Darwin C. 1859. On the origin of species by means of natural selection. London, UK: John Murray [Google Scholar]
- 3.Grant BR, Grant PR. 1993. Evolution of Darwin's finches caused by a rare climatic event. Proc. R. Soc. Lond. B 251, 111–117 10.1098/rspb.1993.0016 (doi:10.1098/rspb.1993.0016) [DOI] [Google Scholar]
- 4.Rodríguez-Trelles F, Alvarez G, Zapata C. 1996. Time-series analysis of seasonal changes of the O inversion polymorphism of Drosophila subobscura. Genetics 142, 179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NOAA 2011. National Climatic Data Center, State of the Climate: Global analysis for April 2011, published online May 2011 (retrieved on 9 January 2013). See http://www.ncdc.noaa.gov/sotc/global/2011/4
- 6.Krimbas CB. 1993. Drosophila subobscura: biology, genetics and inversion polymorphism. Hamburg, Germany: Verlag Dr. Kovac [Google Scholar]
- 7.Ayala FJ, Serra L, Prevosti A. 1989. A grand experiment in evolution: the Drosophila subobscura colonization of the Americas. Genome 31, 246–255 10.1139/g89-042 (doi:10.1139/g89-042) [DOI] [Google Scholar]
- 8.Balanyà J, Serra L, Gilchrist GW, Huey RB, Pascual M, Mestres F, Solé E. 2003. Evolutionary pace of chromosomal polymorphism in colonizing populations of Drosophila subobscura: an evolutionary time series. Evolution 57, 1837–1845 10.1111/j.0014-3820.2003.tb00591.x (doi:10.1111/j.0014-3820.2003.tb00591.x) [DOI] [PubMed] [Google Scholar]
- 9.Balanyà J, Oller JM, Huey RB, Gilchrist GW, Serra L. 2006. Global genetic change tracks global climate warming in Drosophila subobscura. Science 313, 1773–1775 10.1126/science.1131002 (doi:10.1126/science.1131002) [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Trelles F, Rodríguez MA. 2007. Comment on ‘Global genetic change tracks global climate warming in Drosophila subobscura’. Science 315, 1497. 10.1126/science.1136298 (doi:10.1126/science.1136298) [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Trelles F, Rodríguez MA. 2010. Measuring evolutionary responses to global warming: cautionary lessons from Drosophila. Insect .Conserv. Div. 3, 44–50 10.1111/j.1752-4598.2009.00071.x (doi:10.1111/j.1752-4598.2009.00071.x) [DOI] [Google Scholar]
- 12.Solé E, Balanyà J, Sperlich D, Serra L. 2002. Long-term changes in the chromosomal inversion polymorphism of Drosophila subobscura. I. Mediterranean populations from southwestern Europe. Evolution 56, 830–835 10.1111/j.0014-3820.2002.tb01393.x (doi:10.1111/j.0014-3820.2002.tb01393.x) [DOI] [PubMed] [Google Scholar]
- 13.Balanyà J, Oller JM, Huey RB, Gilchrist GW, Serra L. 2007. Response to comment on ‘Global genetic change tracks global climate warming in Drosophila subobscura’. Science 315, 1497. 10.1126/science.1138090 (doi:10.1126/science.1138090) [DOI] [PubMed] [Google Scholar]
- 14.Dolgova O, Rego C, Calabria G, Balanyà J, Pascual M, Rezende EL, Santos M. 2010. Genetic constraints for thermal coadaptation in Drosophila subobscura. BMC Evol. Biol. 10, 363. 10.1186/1471-2148-10-363 (doi:10.1186/1471-2148-10-363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen J, Sato M, Ruedy R. 2012. Perception of climate change. Proc. Natl Acad. Sci. USA 109, E2415–E2423 10.1073/pnas.1205276109 (doi:10.1073/pnas.1205276109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Trelles F, Rodríguez MA. 1998. Rapid microevolution and loss of chromosomal diversity in Drosophila in response to climate warming. Evol. Ecol. 12, 829–838 10.1023/A:1006546616462 (doi:10.1023/A:1006546616462) [DOI] [Google Scholar]
- 17.Merilä J. 2012. Evolution in response to climate change: in pursuit of the missing evidence. Bioessays 34, 811–818 10.1002/bies.201200054 (doi:10.1002/bies.201200054) [DOI] [PubMed] [Google Scholar]
- 18.Levitan M, Etges WJ. 2005. Climate change and recent genetic flux in populations of Drosophila robusta. BMC Evol. Biol. 5, 4. 10.1186/1471-2148-5-4 (doi:10.1186/1471-2148-5-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umina PA, Weeks AR, Kearney MR, McKechnie SW, Hoffmann AA. 2005. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 308, 691–693 10.1126/science.1109523 (doi:10.1126/science.1109523) [DOI] [PubMed] [Google Scholar]
- 20.Rezende EL, Balanya J, Rodríguez-Trelles F, Rego C, Fragata I, Matos M, Serra L, Santos M. 2010. Climate change and chromosomal inversions in Drosophila subobscura. Clim. Res. 43, 103–114 10.3354/cr00869 (doi:10.3354/cr00869) [DOI] [Google Scholar]
- 21.Bradshaw WE, Holzapfel CM. 2006. Evolutionary response to rapid climate change. Science 312, 1477–1478 10.1126/science.1127000 (doi:10.1126/science.1127000) [DOI] [PubMed] [Google Scholar]
- 22.Bradshaw WE, Holzapfel CM. 2010. Insects at not so low temperatures: climate change in the temperate zone and its biotic consequences. In Low temperature biology of insects (eds Denlinger DL, Lee RE.), pp. 242–275 Cambridge, UK: Cambridge University Press [Google Scholar]
- 23.Lankinen P. 1993. North-south differences in circadian eclosion rhythm in European populations of Drosophila suboobscura. Heredity 71, 210–218 10.1038/hdy.1993.126 (doi:10.1038/hdy.1993.126) [DOI] [Google Scholar]
- 24.Begon M, Shorrocks B. 1978. The feeding- and breeding-sites of Drosophila obscura Fallén and D. subobscura Collin. J. Nat. Hist. 12, 137–151 10.1080/00222937800770031 (doi:10.1080/00222937800770031) [DOI] [Google Scholar]
- 25.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246 10.1126/science.1175443 (doi:10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]