Abstract
Tropical forests are experiencing structural changes that may reduce carbon storage potential. The recent increase in liana abundance and biomass is one such potential change. Lianas account for approximately 25 per cent of woody stems and may have a strong impact on tree dynamics because severe liana infestation reduces tree growth and increases tree mortality. Based on forest inventory data from 0.1 ha plots, we evaluated the association between above-ground carbon stocks and liana abundance in 145 tropical forests worldwide. Liana abundance was negatively associated with carbon stocks of large trees (greater than 10 cm diameter), while it was not related to small trees (10 cm diameter or less). Results suggest that liana abundance may have pervasive effects on carbon stocks in tropical forests, as large trees store about 90 per cent of total forest carbon. We stress the need to include liana abundance in carbon stocks estimates, as this can enhance the accuracy of predictions of global changes in tropical forests.
Keywords: carbon storage, global change, liana abundance, tree size, tropical forests
1. Introduction
Tropical forests contain over 30 per cent of global carbon stocks [1] and hence, significant modifications in them may have a major impact on the global carbon cycle. The recent increase in liana abundance and biomass is a remarkable structural change in tropical forests [2]. Increasing atmospheric CO2, evapotranspirative demand and forest fragmentation and turnover are among the main drivers of liana increase [2,3]. Lianas are a key structural component of tropical forests; they constitute approximately 25 per cent of woody stems and species diversity [3]. Lianas are structural parasites of trees and, as a result, are often detrimental to their host trees, competing for above- and below-ground resources and decreasing tree reproduction [3–5].
An increase in liana density could have a disproportionate impact on carbon dynamics [2]. Heavy liana infestations increase the probability of mortality of tree species in mature forests, reduce tree growth and limit tree regeneration [4,6,7]. Infestation rates by lianas increase with trunk diameter, with large trees (greater than 10 cm diameter), which usually store over 90 per cent of carbon [8], showing higher infestation rates than small trees (10 cm diameter or less; [9]). Thus, lianas may reduce the amount of carbon that is sequestered in tree biomass. The loss in tree biomass is hardly compensated for by the increase in liana biomass. Liana stems generally constitute less than 10 per cent of the above-ground biomass (AGB) in mature tropical forests [8] due to their relatively slender stems and low wood density [3].
The role of lianas in forest carbon sequestration has been discussed earlier [2,3]. Nonetheless, lianas have been neglected in studies evaluating variation in above-ground carbon (AGC) stocks in tropical forests [1,10]. We examined the relationship between AGC storage and liana abundance for small (10 cm diameter or less) and large trees (greater than 10 cm diameter) across 145 tropical forests worldwide. We hypothesized that AGC would be lower in plots with higher abundance of lianas, and that lianas would have a stronger effect on AGC of large trees. We also assessed the influence of climatic factors on carbon storage to weight their importance against lianas. Furthermore, we evaluated whether dominance of the tree community varies with liana abundance given that a few species may account for approximately 80 per cent of total carbon stocks in mature tropical forests [11].
2. Material and methods
(a). Study sites
We used a pantropical database collected by Gentry consisting of all lianas, trees and shrubs of diameter 2.5 cm or more at breast height (DBH) in 0.1 ha plots. Each plot encompasses 10 transects of 2 × 50 m distributed in zig-zag across mature forest, avoiding edges and successional habitats [12]. Mean annual precipitation (MAP) and mean annual temperature (MAT) were estimated for each location using the WorldClim database [13]. Dry season length was defined as the number of consecutive months with rainfall less than 100 mm [14]. The final dataset included 145 plots of tropical forests from five continents (see the electronic supplementary material, appendix S1): 46 dry forests, 49 moist forests and 50 wet forests (figure 1; categories follow Chave et al. [15]).
Figure 1.
Spatial distribution of Gentry's forest plots (0.1 ha) used in this study (n = 145). Forests were classified following Chave et al. [15].
(b). Carbon stocks
AGB of small trees was estimated using an allometric equation based on DBH (see the electronic supplementary material, appendix S2; [16]). To calculate the AGB of large trees, we used a pantropical equation described in Chave et al. [15], which uses DBH and wood density for each tree species for each forest type (see the electronic supplementary material, appendix S2). Wood density data were derived from the literature [17]. When wood densities were not available at species level, we used genus level (for 45% of species), family level (for 9% of species) or an overall tropical mean of 0.6 g cm−3 (1% of species). Special cases were solved as in Chave et al. [17]. For individuals with multiple stems, we calculated AGB of each stem and summed them. AGB of lianas was estimated using an allometric equation based on basal area [18]. We estimated AGC (Mg C ha−1) per plot as AGC = AGB × 0.47 per plot area [16]. Since liana infestation may covary with wood density [4], we also estimated AGC for large trees using an allometric equation based on DBH only [19]. Analyses for large trees using equations with and without wood density provided similar results (see the electronic supplementary material, appendices S2 and S3).
(c). Statistical analyses
To evaluate the effect of lianas on carbon stocks, we used simple and multiple regressions. First, we conducted simple linear regressions to evaluate the independent effects of lianas and each climatic factor on AGC. We then conducted a multiple regression including only those variables that had significant effects in simple regressions. To examine the relative importance of each parameter in the full model, we partitioned the total variation in the response variable. We evaluated the effect of lianas on the dominance of the tree community with simple linear regression using the Simpson's dominance index, which was calculated using abundance as tree density (no. trees ha−1) and basal area (m2 ha−1).Variables were square-root transformed to meet regression assumptions. We tested normality of residuals using the Shapiro–Wilk test and plotted fitted values against residuals to test for non-constant variance.
3. Results
The 145 pantropical sites spanned a considerable range in MAP (494–5175 mm), MAT (12–28°C) and dry season length (0–10 months; electronic supplementary material, appendix S1). Small trees accounted for less than 10 per cent of carbon stocks, whereas large trees stored over 90 per cent of total forest carbon (table 1). Neither liana abundance nor climate variables were related to carbon stocks of small trees. However, carbon stocks of large trees were negatively associated with liana abundance at plot level (figure 2). The linear fit showed that stored carbon in forest plots where lianas were highly abundant was less than one-half of that in plots with low liana density (figure 2). Liana abundance alone explained as much variation in carbon stocks as MAT (R2 = 0.11; table 2). After controlling for variation in climate variables across sites, this effect was still significant, and liana abundance explained more variation in carbon stocks (R2 = 0.10) than MAT and MAP together (R2 = 0.06 and R2 = 0.03, respectively; table 2). Lianas contributed 5 per cent of forest AGC (see table 1 and electronic supplementary material, appendix S1). Liana abundance did not affect dominance of the tree community as indicated by the Simpson index using tree density (t = −0.31, p = 0.75) or tree basal area (t = −1.35, p = 0.17).
Table 1.
AGC storage (Mg C ha−1) and density (no. individuals per 0.1 ha) of small (≤10 cm diameter), large trees (>10 cm) and lianas (≥2.5 cm) in tropical forests (n = 145). s.e., standard error; CI, confidence interval.
| mean | s.e. | 95% CI | |
|---|---|---|---|
| AGC | |||
| small trees | 4.90 | 0.13 | 4.65–5.16 |
| large trees | 146.50 | 6.04 | 134.5–158.4 |
| lianas | 5.58 | 0.47 | 4.77–6.64 |
| density | |||
| small trees | 202.47 | 5.40 | 191.7–213.2 |
| large trees | 92.70 | 3.45 | 85.8–99.5 |
| lianas | 62.60 | 2.72 | 57.3–68.0 |
Figure 2.
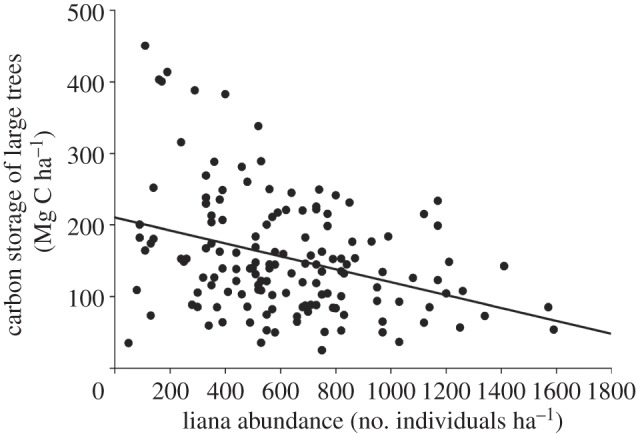
Relationship between liana abundance and above-ground carbon storage of large trees (>10 cm diameter) in tropical forests (n = 145). Carbon storage decreases with liana abundance (p-value < 0.001; R2 = 0.11). Carbon storage was estimated using allometric equations based on DBH and wood density for each forest type following Chave et al. [15].
Table 2.
Simple and multiple regressions evaluating the relationship between liana abundance and climate (MAP, MAT and DSL) and AGC storage (Mg C ha−1) in tropical forests (n = 145). β denotes the standardized regression coefficients, and s.e. their standard error (MAP, mean annual precipitation; MAT, mean annual temperature; DSL, dry season length).
| AGC trees ≤10 cm |
AGC trees >10 cm |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | s.e. | t-value | p-value | R2 | β | s.e. | t-value | p-value | R2 | |
| simple regression | ||||||||||
| liana abundance | −0.02 | 0.01 | −1.19 | 0.235 | — | −0.16 | 0.03 | −4.22 | <0.001 | 0.11 |
| MAP | 0.002 | 0.003 | 0.81 | 0.418 | — | 0.05 | 0.02 | 2.26 | 0.02 | 0.03 |
| MAT | 0.12 | 0.08 | 1.38 | 0.17 | — | −2.65 | 0.63 | −4.19 | <0.001 | 0.11 |
| DSL | −0.006 | 0.03 | −0.18 | 0.850 | — | −0.27 | 0.27 | −0.99 | 0.32 | — |
| multiple regression | ||||||||||
| liana abundance | −0.10 | 0.03 | −2.58 | 0.01 | 0.10 | |||||
| MAP | 0.05 | 0.02 | 2.36 | 0.02 | 0.03 | |||||
| MAT | −2.20 | 0.65 | −3.37 | <0.001 | 0.06 | |||||
| full model | F3,141 = 11.43 | <0.001 | 0.19 | |||||||
4. Discussion
This is the first comprehensive evaluation of the relationship between liana abundance and AGC stocks across tropical forests. Results indicate that lianas could reduce AGC by up to 50 per cent in large trees, which account for 90 per cent of total carbon stored in tropical forests. A study in an Amazonian forest reported that liana-free trees contained 25 per cent less carbon per unit basal area than liana-infested trees [9], thus suggesting a positive association between lianas and carbon. However, this one-site study included areas with regular disturbance (seasonal flooding), and thus it cannot be considered representative of tropical mature forests. We found that dominance of the tree community did not vary with liana abundance across forest plots. Although we lack data on individual tree infestation, this suggests that lianas reduce carbon stocks via increased proportion of infested trees and/or more severe infestations rather than by removing high-biomass, dominant trees. The proportion of infested trees per hectare may be as high as 50 per cent in some tropical forests [6,9]. Reduced C stocks in trees were not driven by changes in wood density as a consequence of compositional shifts. This was verified by the fact that results were the same when we estimated AGB with and without considering wood density, thus suggesting that the negative relationship between lianas and C stocks is mainly explained by lianas' impact on tree diameter.
Empirical studies provide possible mechanisms underlying the negative effects of lianas on carbon stocks. The high leaf–stem ratio allows lianas to aggressively compete with trees by deploying leaves on the canopy and covering tree crowns. By competing with trees, lianas are able to reduce annual increments of tree biomass by 10 per cent, equivalent to 0.25 Mg C ha−1 yr−1 [4], and may reduce tree growth and reproduction even at low abundance [4,5]. Large lianas, which represent approximately 6 per cent of woody stems of 10 cm or more, are able to remove 30 per cent of tree basal area in mature forests [20]. Moreover, the probability of mortality for liana-infested trees is two to three times greater than for liana-free trees [6,20]. Over time, this liana-driven reduction in tree biomass may affect the ability of tropical forests to store carbon and might alter global climate by releasing some of the carbon currently stored [1].
Notwithstanding the compelling arguments and evidence presented here, alternative explanations for the liana–carbon relationship may be explored. Liana abundance is correlated negatively with rainfall and positively with seasonality across the tropics [14,21]. Low-carbon forests in this study include seasonal and deciduous forests with long dry seasons. Lianas may be particularly successful in these forests due to their efficient vascular system and their extended leaf longevity [3,21]. Future research should address these alternative explanations quantitatively. This will lead to a better understanding of the impact of lianas on carbon stocks, thus improving predictions of global change in tropical forests.
Acknowledgements
We thank the Missouri Botanical Garden for making available Alwyn Gentry's dataset. S. J. Wright, anonymous reviewers and members of the Functional Ecology Lab (Universidad de la Serena) provided valuable comments.
References
- 1.Keith H, Mackey BG, Lindenmayer DB. 2009. Re-evaluation of forest biomass carbon stocks and lessons from the world's most carbon-dense forests. Proc. Natl Acad. Sci. USA 106, 11 625–11 640 10.1073/pnas.0901970106 (doi:10.1073/pnas.0901970106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips OL, et al. 2002. Increasing dominance of large lianas in Amazonian forests. Nature 418, 770–774 10.1038/nature00926 (doi:10.1038/nature00926) [DOI] [PubMed] [Google Scholar]
- 3.Schnitzer SA, Bongers F. 2011. Increasing liana abundance and biomass in tropical forests: emerging patterns and putative mechanisms. Ecol. Lett. 14, 397–406 10.1111/j.1461-0248.2011.01590.x (doi:10.1111/j.1461-0248.2011.01590.x) [DOI] [PubMed] [Google Scholar]
- 4.van der Heijden GMF, Phillips OL. 2009. Liana infestation impacts tree growth in a lowland tropical moist forest. Biogeosciences 6, 2217–2226 10.5194/bg-6-2217-2009 (doi:10.5194/bg-6-2217-2009) [DOI] [Google Scholar]
- 5.Wright SJ, Jaramillo MA, Pavon J, Condit R, Hubbell SP, Foster RB. 2005. Reproductive size thresholds in tropical trees: variation among individuals, species and forests. J. Trop. Ecol. 21, 307–315 10.1017/S0266467405002294 (doi:10.1017/S0266467405002294) [DOI] [Google Scholar]
- 6.Ingwell LL, Wright SJ, Becklund KK, Hubbell SP, Schnitzer SA. 2010. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. J. Ecol. 98, 879–887 10.1111/j.1365-2745.2010.01676.x (doi:10.1111/j.1365-2745.2010.01676.x) [DOI] [Google Scholar]
- 7.Schnitzer SA, Carson WP. 2010. Lianas suppress tree regeneration and diversity in treefall gaps. Ecol. Lett. 13, 849–857 10.1111/j.1461-0248.2010.01480.x (doi:10.1111/j.1461-0248.2010.01480.x) [DOI] [PubMed] [Google Scholar]
- 8.DeWalt SJ, Chave J. 2004. Structure and biomass of four lowland Neotropical forests. Biotropica 36, 7–19 10.1111/j.1744-7429.2004.tb00291.x (doi:10.1111/j.1744-7429.2004.tb00291.x) [DOI] [Google Scholar]
- 9.van der Heijden GMF, Healey JR, Phillips OL. 2008. Infestation of trees by lianas in a tropical forest in Amazonian Peru. J. Veg. Sci. 19, 747–756 10.3170/2008-8-1845 (doi:10.3170/2008-8-1845) [DOI] [Google Scholar]
- 10.Stegen JC, Swenson NG, Enquist BJ, White EP, Phillips OL, Jørgensen PM, Weiser MD, Monteagudo Mendoza A, Núñez Vargas P. 2011. Variation in above-ground forest biomass across broad climatic gradients. Glob. Ecol. Biogeogr. 20, 744–754 10.1111/j.1466-8238.2010.00645.x (doi:10.1111/j.1466-8238.2010.00645.x) [DOI] [Google Scholar]
- 11.Balvanera P, Kremen C, Martínez-Ramos M. 2005. Applying community structure analysis to ecosystem function: examples from pollination and carbon storage. Ecol. Appl. 15, 360–375 10.1890/03-5192 (doi:10.1890/03-5192) [DOI] [Google Scholar]
- 12.Phillips OL, Miller JS. 2002. Global patterns of plant diversity: Alwyn H. Gentry’s forest transect dataset. St Louis, MO: Missouri Botanical Garden Press [Google Scholar]
- 13.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 10.1002/joc.1276 (doi:10.1002/joc.1276) [DOI] [Google Scholar]
- 14.DeWalt SJ, et al. 2010. Annual rainfall and seasonality predict pan-tropical patterns of liana density and basal area. Biotropica 42, 309–317 10.1111/j.1744-7429.2009.00589.x (doi:10.1111/j.1744-7429.2009.00589.x) [DOI] [Google Scholar]
- 15.Chave J, et al. 2005. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 10.1007/s00442-005-0100-x (doi:10.1007/s00442-005-0100-x) [DOI] [PubMed] [Google Scholar]
- 16.Hughes RF, Kauffman JB, Jaramillo VJ. 1999. Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of Mexico. Ecology 80, 1892–1907 10.2307/176667 (doi:10.2307/176667) [DOI] [Google Scholar]
- 17.Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 10.1111/j.1461-0248.2009.01285.x (doi:10.1111/j.1461-0248.2009.01285.x) [DOI] [PubMed] [Google Scholar]
- 18.Putz FE. 1983. Liana biomass and leaf area of a ‘tierra firme’ forest in the Rio Negro Basin, Venezuela. Biotropica 15, 185–189 10.2307/2387827 (doi:10.2307/2387827) [DOI] [Google Scholar]
- 19.Brown S. 1997. Estimating biomass and biomass change of tropical forests: a primer. FAO Forestry Paper No. 134, Rome, Italy
- 20.Phillips OL, Martínez RV, Mendoza AM, Baker TR, Vargas PN. 2005. Large lianas as hyperdynamic elements of the tropical forest canopy. Ecology 86, 1250–1258 10.1890/04-1446 (doi:10.1890/04-1446) [DOI] [Google Scholar]
- 21.Schnitzer SA. 2005. A mechanistic explanation for global patterns of liana abundance and distribution. Am. Nat. 166, 262–276 10.1086/431250 (doi:10.1086/431250) [DOI] [PubMed] [Google Scholar]


