Abstract
A recent study demonstrated that the embryos of soft-shelled turtles can reposition themselves within their eggs to exploit locally warm conditions. In this paper, we ask whether turtle embryos actively seek out optimal thermal environments for their development, as do post-hatching individuals. Specifically, (i) do reptile embryos move away from dangerously high temperatures as well as towards warm temperatures? and (ii) is such embryonic movement due to active thermoregulation, or (more simply) to passive embryonic repositioning caused by local heat-induced changes in viscosity of fluids within the egg? Our experiments with an emydid turtle (Chinemys reevesii) show that embryos avoid dangerously high temperatures by moving to cooler regions of the egg. The repositioning of embryos is an active rather than passive process: live embryos move towards a heat source, whereas dead ones do not. Overall, our results suggest that behavioural thermoregulation by turtle embryos is genuinely analogous to the thermoregulatory behaviour exhibited by post-hatching ectotherms.
Keywords: behavioural thermoregulation, ectotherm, embryo, oviparity
1. Introduction
Thermoregulatory behaviour plays an important role in the biology of ectotherms, allowing them to maintain relatively constant body temperatures even when ambient temperatures fluctuate strongly. For example, sun-basking can enable an ectotherm to increase its body temperature quickly in the morning, and shuttling between sun and shade may allow that animal to regulate its body temperature precisely at midday [1,2]. This thermoregulatory ability can enhance fitness-relevant behavioural and physiological processes, such as locomotor capacity, food assimilation rate and reproductive output [3,4]. Behavioural thermoregulation is widespread in the post-hatching stages of ectotherms, including insects, fishes, amphibians and reptiles, but until recently it was assumed not to occur in the embryonic phase of the life cycle [1,2]. A recent study challenged this assumption by demonstrating that embryos of the Chinese soft-shelled turtle (Pelodiscus sinensis) can move within the egg to exploit warmer regions (those that are closer to the sun-heated ground surface) [5].
Potential fitness benefits of behavioural thermoregulation by turtle embryos are clear (because developmental temperatures can substantially affect developmental rates and hatchling phenotypes), but whether this surprising behaviour in embryos is genuinely analogous to that in adults remains unclear. To assess the potential significance of thermoregulatory behaviour in embryonic development of oviparous reptiles, we need to answer two fundamental questions. First, do embryos reposition themselves not only to gain heat, but also to avoid thermal extremes (as do adult reptiles [1,2]). Unless embryos can move away from as well as towards ‘hot-spots’ within the egg, the analogy between embryonic repositioning and an adult reptile's behavioural thermoregulation is weak. Second, is there a simpler physics-based explanation for embryonic repositioning, perhaps due to thermal influences on egg-fluid viscosity rather than to active movement by embryos? The lack of overt locomotor structures in embryos suggests that embryos might simply drift towards a hot-spot because local temperatures affect the viscosity of fluids (e.g. the yolk or the amniotic fluid), causing the embryo to move passively towards regions of higher temperature. We conducted experiments on the embryos of an emydid turtle (Chinemys reevesii) to explore these two issues.
2. Material and methods
(a). Embryonic movement under different thermal regimes
We incubated eggs of the Chinese three-keeled pond turtle (C. reevesii) in various thermal environments to investigate embryonic movements, at temperatures ranging from 26°C to 33°C. This species is an aquatic emydid found in central and southern China and southeastern Asia; clutches average approximately six eggs [6]. Incubation temperatures affect developmental rate of embryos and body size, sex and locomotor performance of hatchlings in C. reevesii [7]. Most natural nests are likely to be cooler than 33°C (see the electronic supplementary material, figure S1), and eggs that are chronically exposed to temperatures above 32°C have low hatching success [7].
A total of 125 recently laid C. reevesii eggs (mean mass = 10.2 g, mean egg length = 35 mm) from a turtle farm in Zhejiang were incubated individually in 80 ml jars containing moist vermiculite (−220 kPa), in an incubator (FPQ incubator, Ningbo Life Science and Technology Ltd, China) set at 26°C, and randomly assigned among five thermal treatments: (i) constant temperature of 26°C; (ii) dorsal heating to 29°C on the upper surface of the egg; (iii–v) lateral heating to 29°C, 30°C and 33°C, directed towards the pointed ends of eggs. The eggs were heated by 75 watt electronic heating mats (500 × 450 mm), with the distance between the jars and the heating mats adjusted to 220, 154 or 41 mm to obtain the desired egg-surface temperatures of 29°C, 30°C or 33°C (at the end of the egg that was closest to the heat source). We monitored temperatures at the ends of eggs closest to and furthest from the heat source at 30 s intervals, using 40 gauge thermocouples (TCTTT140, Temperature Controls Pty Ltd) connected to a data-taker (DT-80, Datataker Pty Ltd) to measure thermal gradients within a single egg. The thermocouples (±0.01°C) were calibrated to a standard thermometer prior to the experiment. The thermal difference between the hot and cold ends of an egg averaged 1.0°C, 1.2°C or 1.6°C for 29°C, 30°C or 33°C treatments, respectively. At the beginning of each experiment, we used candling to quantify the position of the embryo's midpoint (defined as the point where the neck met the carapace, an obvious morphological feature in turtle embryos; figure 1), which was normally close to the midline of eggs, and marked it on the egg surface with a pencil. One week later, we quantified the position of the embryo's midpoint again to determine the distance (±0.01 mm) that embryos had shifted from their original position along the long axis of the egg. This measurement is highly repeatable (mean disparity between repeated measures = 1.26 mm, 95% CL = 0.94–1.59 mm; see the electronic supplementary material).
Figure 1.
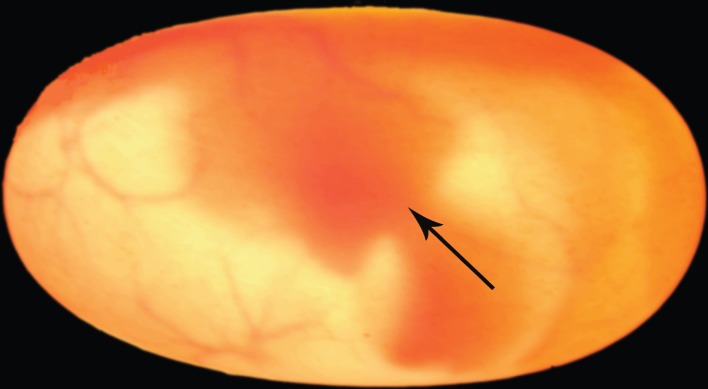
The position of embryonic Chinese pond turtles (C. reevesii) inside eggs, as shown by candling. The arrow indicates the site that we used to score embryonic position within the egg: the point where the neck joins the carapace. (Online version in colour.)
(b). Movement of live versus dead embryos
Eggs of C. reevesii were incubated individually in 80 ml jars containing moist vermiculite (−220 kPa), exposed to lateral heating at a temperature of 28°C (optimal for embryonic development in this species [7]). After 10 days (for the turtle, the developmental stage of embryos = 18 [8]) of incubation, we injected half of the eggs (randomly selected) with urethane to euthanize the embryos. We measured the position of embryos (as above) before injecting, and one week later by candling. Changes in the embryo's location within its egg were calculated as the distance shift of the embryo's body in the week after treatment, along the long axis of the egg.
(c). Data analysis
We used Kruskal–Wallis ANOVA to analyse the effect of the direction of heat source on embryonic positions in turtle eggs. The post hoc Nemenyi test was used to identify significant differences in embryonic movement among treatments. The Mann–Whitney U test was used to compare the extent of repositioning of live versus dead embryos in response to a lateral heat source. In the text, data are presented as mean ± s.e. The raw data used in the analyses may be found in the Dryad data depository (http://dx.doi.org/10.5061/dryad.d35q5).
3. Results
(a). Embryonic movement under different thermal regimes
Thermal treatments affected embryonic positions in the emydid turtle C. reevesii (H4,124 = 15.4, p < 0.01). Under constant temperature or dorsally directed heating, embryos remained near the midpoints of their eggs. Under lateral heating, the embryos moved towards a heat source generating egg-surface temperatures of 29°C and 30°C, but moved away from a more intense heat source that raised egg-surface temperatures to deleteriously high levels (33°C; figure 2). Multiple comparisons among treatments indicated that embryonic displacement at lateral heating of 30°C differed significantly from that at constant temperature incubation at 26°C (x2 = 10.25, p = 0.01), dorsal heating of 29°C (x2 = 6.86, p = 0.03) and lateral heating at 33°C (x2 = 10.82, p < 0.001).
Figure 2.
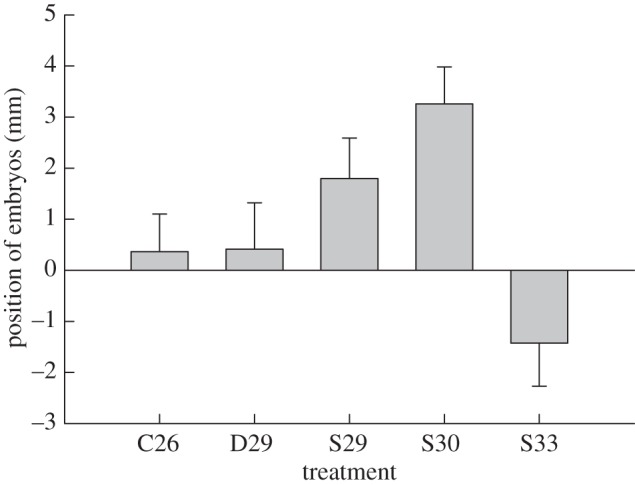
Shifts in the position of embryonic turtles (C. reevesii) inside eggs that were incubated at constant temperatures, or were heated from above or from the side of the egg. Changes in the embryo's location within its egg are shown by the distance shift of the embryo's body position from the mid-point of its egg along the long axis. Embryos moved towards the heat source when ‘hot-spot’ temperatures were 29°C and 30°C, but moved away from the heat source when the ‘hot-spot’ temperature increased to 33°C. Data are presented as mean ± s.e. Sample sizes are 29, 19, 25, 30 and 22 for constant temperature of 26°C (C26), dorsal heating of 29°C (D29), left-side heating of 29°C (S29), 30°C (S30) and 33°C (S33), respectively.
(b). Movement of live versus dead embryos
When provided with a lateral heat source, live embryos moved towards the heat source, whereas dead embryos did not (2.31 ± 0.38 mm (n = 21) versus 0.28 ± 0.38 mm (n = 20), Z = 3.52, p < 0.001, n = 41).
4. Discussion
Similar to those of a previously studied soft-shell turtle species [5], the embryos of an emydid turtle (C. reevesii) repositioned themselves within the egg in response to thermal gradients. Importantly, our study shows that the embryos not only moved towards warmer areas, but also moved away from dangerously high temperatures. Our results also clarify the mechanistic basis for repositioning of embryos within their eggs. We have interpreted these movements as behavioural thermoregulation and invoked an adaptive advantage to embryonic control over incubation regimes [5]. Alternatively, one could posit that the repositioning of embryos is driven by simple physics, perhaps related to thermally induced heterogeneity in fluid viscosity within the egg. Such a behaviour might still enhance offspring fitness (via changes to thermal conditions), but would be only weakly analogous to thermoregulatory behaviour as seen in post-hatching reptiles. To test between these two interpretations, we examined responses of embryos to lethally high temperatures; and compared repositioning of live versus dead embryos. If the movement of embryos was a simple physical response to thermal heterogeneity, we would expect that embryos would move towards any hot area (even if it was above optimal temperature), and that dead embryos would move in similar ways to live embryos. Our results falsified both of these predictions. First, turtle embryos moved towards ‘warm’ conditions, but away from ‘hot’ conditions (figure 2). Thus, behavioural thermoregulation in embryos, as in adult reptiles, involves avoidance of conditions that are too hot as well as those that are too cold. Second, live but not dead embryos moved within their eggs when lateral heating was applied.
Much remains to be learnt about this phenomenon. For example, control of fluid flow by a live embryo would provide a potential mechanism by which these tiny embryos might influence their own positions. The most parsimonious interpretation of our data is that turtle embryos are capable of behavioural thermoregulation that is broadly similar to the behaviours seen in adult conspecifics. Importantly, the turtle (C. reevesii) has temperature-dependent sex determination (TSD) with clutches consisting entirely of males or of females unless the eggs are incubated in a narrow intermediate range [7,9]. The magnitude of thermal differentials within an egg ranged from 1.0°C to 1.6°C in the experimental treatments of this study. Thermal differentials were even larger in simulated nests of this species (up to 2.9°C within an egg; electronic supplementary material, figure S2), and in field nests of an Australian freshwater turtle, Emydura macquarii (up to 5.9°C from upper to lower eggs) [10]. Thus, thermal differentials within the eggs of C. reevesii (and presumably other turtle species) may well encompass the range from male-producing to female-producing temperatures (1–2°C, [7,9]), and allow embryos to influence their own sex, as well as other phenotypic traits at hatching. Current hypotheses for the evolution of TSD all assume that hatchling sex is a consequence of maternal nest-site choice, rather than being under embryonic control [11,12]. The possibility that embryos can influence their own sex also bears upon speculations that TSD species will be vulnerable to climate change, via shifts in nest temperatures and thus sex ratio bias [13]. Such effects may be buffered not only by flexibility in maternal nest-site choice [14], but also by active behavioural thermoregulation by embryos.
Acknowledgements
Ethical approval was given by Animal Ethics Committees at the Institute of Zoology, Chinese Academy of Sciences.
We thank Y. Wang and Y. Chen for their help in the laboratory and three anonymous reviewers for their constructive suggestions. This work was supported by grants from the Natural Science Foundation of China (30970362), Program for New Century Excellent Talents in University (NCET-10-0911) and ‘Hundred Talents Program’ of the Chinese Academy of Sciences, and by the Australian Research Council. Data deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.d35q5.
References
- 1.May ML. 1979. Insect thermoregulation. Annu. Rev. Entomol. 24, 313–349 [Google Scholar]
- 2.Huey RB. 1982. Temperature, physiology, and the ecology of reptiles. In Biology of the reptilia (eds Gans C, Pough FH.), pp. 25–91 New York, NY: Academic Press [Google Scholar]
- 3.Du WG, Yan SJ, Ji X. 2000. Selected body temperature, thermal tolerance and thermal dependence of food assimilation and locomotor performance in adult blue-tailed skinks, Eumeces elegans. J. Therm. Biol. 25, 197–202 10.1016/S0306-4565(99)00022-4 (doi:10.1016/S0306-4565(99)00022-4) [DOI] [Google Scholar]
- 4.Madsen T, Shine R. 1999. Life history consequences of nest-site variation in tropical pythons (Liasis fuscus). Ecology 80, 989–997 10.2307/177032 (doi:10.2307/177032) [DOI] [Google Scholar]
- 5.Du WG, Zhao B, Chen Y, Shine R. 2011. Behavioral thermoregulation by turtle embryos. Proc. Natl Acad. Sci. USA 108, 9513–9515 10.1073/pnas.1102965108 (doi:10.1073/pnas.1102965108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao E, Adler K. 1993. Herpetology of China. Oxford, OH: SSAR [Google Scholar]
- 7.Du WG, Hu LJ, Lu JL, Zhu LJ. 2007. Effects of incubation temperature on embryonic development rate, sex ratio and post-hatching growth in the Chinese three-keeled pond turtle, Chinemys reevesii. Aquaculture 272, 747–753 10.1016/j.aquaculture.2007.09.009 (doi:10.1016/j.aquaculture.2007.09.009) [DOI] [Google Scholar]
- 8.Greenbaum E. 2002. A standardized series of embryonic stages for the emydid turtle Trachemys scripta. Can. J. Zool. 80, 1350–1370 10.1139/z02-111 (doi:10.1139/z02-111) [DOI] [Google Scholar]
- 9.Valenzuela N, Lance V. 2004. Temperature dependent sex determination in vertebrates. Washington, DC: Smithsonian Books [Google Scholar]
- 10.Thompson MB. 1988. Nest temperatures in the Pleurodiran turtle, Emydura macquarii. Copeia 1988, 996–1000 10.2307/1445723 (doi:10.2307/1445723) [DOI] [Google Scholar]
- 11.Warner DA, Shine R. 2008. Maternal nest-site choice in a lizard with temperature-dependent sex determination. Anim. Behav. 75, 861–870 10.1016/j.anbehav.2007.07.007 (doi:10.1016/j.anbehav.2007.07.007) [DOI] [Google Scholar]
- 12.Juliana JRS, Bowden RM, Janzen FJ. 2004. The impact of behavioral and physiological maternal effects on offspring sex ratio in the common snapping turtle, Chelydra serpentina. Behav. Ecol. Sociobiol. 56, 270–278 10.1007/s00265-004-0772-y (doi:10.1007/s00265-004-0772-y) [DOI] [Google Scholar]
- 13.Janzen FJ. 1994. Climate-change and temperature-dependent sex determination in reptiles. Proc. Natl Acad. Sci. USA 91, 7487–7490 10.1073/pnas.91.16.7487 (doi:10.1073/pnas.91.16.7487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doody JS, Guarino E, Georges A, Corey B, Murray G, Ewert M. 2006. Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination. Evol. Ecol. 20, 307–330 10.1007/s10682-006-0003-2 (doi:10.1007/s10682-006-0003-2) [DOI] [Google Scholar]


