Abstract
Bird song is hypothesized to be a reliable indicator of cognition because it depends on brain structure and function. Song features have been found to correlate positively with measures of cognition, but the relationship between song and cognition is complicated because not all cognitive abilities are themselves positively correlated. If cognition is not a unitary trait, developmental constraints on brain growth could generate trade-offs between some aspects of cognition and song. To further clarify the relationship between song and cognition in song sparrows (Melospiza melodia), we examined repertoire size and performance on a spatial task. We found an inverse relationship between repertoire size and speed of spatial learning and suggest that a developmental trade-off between the hippocampus and song control nuclei could be responsible for this relationship. By attending to male song, females may learn about a suite of cognitive abilities; this study suggests that females may glean information about a male's cognitive weaknesses as well as his strengths.
Keywords: song, spatial learning, trade-off
1. Introduction
Sexual ornaments can be honest signals of male quality if they are linked to traits that enhance female reproductive success [1]. Bird song is a sexual ornament that depends principally upon brain structure and function, which reflect response to developmental stressors [2,3]. Thus, song may reflect lifelong neural capacity, which should be tied to cognitive ability [3–8].
The relationship between cognition and song is complicated, however, because different cognitive abilities are not always positively correlated within individuals, and song predicts performance on some but not all cognitive tasks [8,9]. This dissociation of cognitive measures from one another and from song may reflect trade-offs between aspects of brain development or function [10]. Spatial ability has particular potential to be traded-off with song because these traits are mediated by independent brain regions [11]. In many songbirds, including song sparrows (Melospiza melodia), larger song repertoires are preferred by females [12,13] and are positively correlated with brain structure, specifically the volume of the song control nucleus HVC [14]. Similarly, spatial memory is positively correlated with the volume of the hippocampus [15]. Because the hippocampus and song control nuclei are structurally and functionally distinct [11,16], the two behavioural traits should be independently controlled and could be inversely related. Therefore, we compared performance on a spatial task and song repertoire size in adult male song sparrows.
2. Material and methods
We measured the song repertoire sizes of 16 male song sparrows from a colour-banded population in Durham, NC, USA. We recorded at least 300 songs from each individual to ensure that we captured complete repertoires (Marantz PMD660 recorder, Sennheiser ME62 microphone, Sony PBR-330 parabolic reflector; [17]). We determined repertoire size by visual inspection of spectrograms (Syrinx, v. 2.6h).
After recording repertoires in the field (7–28 May 2010), we captured males, took morphological measurements and conducted cognitive tests in the laboratory after a four week acclimation period. We assayed the males for neophobia, following Roth et al. [18], and for spatial performance, following an adaptation of Pravosudov et al. [16]. All behavioural assays were performed in the males' home cages (46 × 23 × 23 cm), with birds food deprived for 6 h prior to each trial.
The neophobia assay measured the time (s) elapsed before the bird ate from a novel food cup relative to the time elapsed before the bird ate from its familiar cup in a subsequent trial 5 min later. The results of this assay were used to control for individual differences in motivation or behavioural strategy (measured as latency to approach novel objects).
The spatial assay used a foraging grid (13.5 × 9 × 2.5 cm) containing 12 wells, six of which were covered with identically coloured plastic lids. Before testing for spatial ability, we trained males to remove the lids from the grid following Boogert et al. [9] (two birds failed this stage, yielding a sample size of 14).
During spatial trials, one of the six covered wells contained a freshly killed mealworm. The orientation of the grid within the cage, the locations of lids and the location of the baited well remained constant across all trials for a single male, with each male assigned a different lid distribution and baited well location. During the initial training trial, males had an opportunity to learn that only a single well in a particular location was baited with food (all males removed all six lids during the training trial). After a 10 min interval, we ran the first test trial, with the same well baited. We ran seven subsequent test trials, each lasting 10 min, one trial per day, for a total of eight test trials over eight consecutive days. We conducted an unbaited probe trial on the 9th day to ensure that males were not using odour cues to perform the task. We measured latency to eat from a familiar food cup after each trial to assess hunger. We counted the number of errors (unbaited lids removed) that males made before removing the lid from the baited well for each trial. For each bird, we summed the number of errors across the eight test trials, a measure that captured speed of improvement in the spatial task (see §3).
To determine if subjects improved performance over time in the spatial assay, indicating learning of the location of the baited well, we ran a generalized linear mixed model (GLMM) using the package nlme in R v. 2.7.2 software [19] with number of errors in a trial as the dependent variable and individual coded as the random intercept to specify a repeated measures design. We examined the relationship between song repertoire size and spatial performance using a Pearson's correlation. To ensure that a correlation between spatial performance and repertoire size was not explained by neophobia or body condition, we also ran generalized linear models (GLMs) with repertoire as the dependent variable, total spatial errors as the independent variable and neophobia or body condition (calculated as body mass regressed by tarsus length) as covariates.
3. Results
Performance on the spatial task improved significantly across trials (GLMM, effect of trial number, t = −2.985, p = 0.004; figure 1), and all males performed better than chance by the 8th trial, showing that the males learned the location of the baited well. Although males achieved equal performance in the task, they differed in total errors across trials (3–32), reflecting variation in the speed of spatial learning. Song repertoire size was inversely related to total spatial errors (Pearson's correlation, R = 0.539, p = 0.047; figure 2); males that learned faster (made fewer spatial errors) had smaller song repertoires. Neophobia did not explain the relationship between repertoire size and total spatial errors (GLM, effect of song repertoire size on spatial errors, t = 2.270, p = 0.044; effect of neophobia, t = 0.682, p = 0.509) nor did body condition (GLM, effect of song repertoire size on spatial errors, p = 0.034, effect of body condition, p = 0.309). Finally, speed of spatial learning was not correlated with hunger (Pearson's correlation, R = −0.008, p = 0.978).
Figure 1.
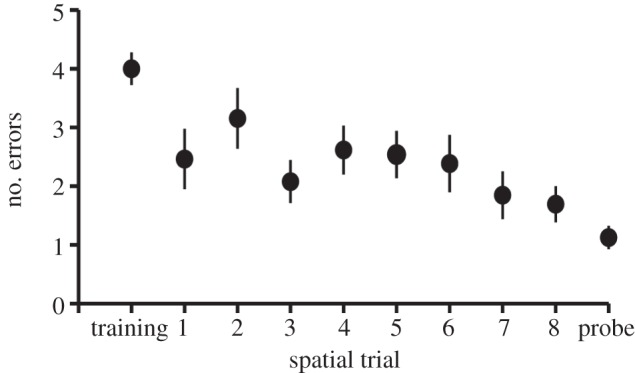
The mean (± s.e.) number of errors made by males before finding the baited well in a foraging grid during a training trial, eight daily test trials and an unbaited probe trail in the spatial assay.
Figure 2.
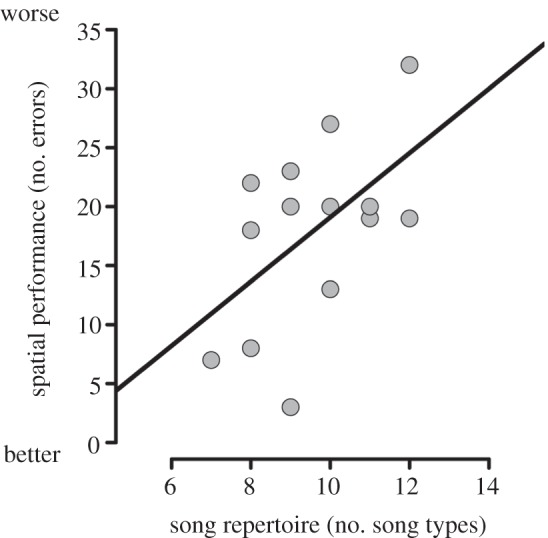
The relationship between song repertoire size and speed of spatial learning for 14 song sparrows.
4. Discussion
Unlike earlier studies reporting positive relationships between song and measures of cognition [8,9,20], we found an inverse relationship between song repertoire size and speed of spatial learning in song sparrows. Previous research has shown that the relationship between song and cognition is complex [9], but the present finding is, to our knowledge, the first to raise the hypothesis of a trade-off between sexual ornamentation and cognition.
Song has been suggested to serve as a reliable indicator of cognition, because song depends upon brain structure and function [5–7]. Developmental conditions can impact brain structure [4,16,21], song [3,22] and cognition [23], potentially linking these traits. However, the mechanisms that link sexual ornaments with other character traits also may be the basis for trade-offs between traits. Natural selection should ensure that traits important to survival receive maximal investment, potentially at the expense of non-essential traits including sexual ornaments, which facilitate but are not essential to reproductive success [24]. Thus, we might anticipate trade-offs between song and cognitive traits when: (i) resources available for investment in the two traits are limited and (ii) compromising the cognitive trait will improve the quality of song yet maintain equal chances of survival, or vice versa [10,24,25]. These conditions may be met by song and spatial abilities in song sparrows.
Spatial cognition has not been extensively studied in song sparrows, but males in our study population do not engage in long-distance migration or food caching, two life-history traits known to select for specialized spatial memory [15,26]. Thus, it is possible that in these males spatial ability is of relatively low adaptive value as compared with other cognitive abilities. Further, resources essential to the growth of the brain regions responsible for repertoire size (HVC) and spatial learning (the hippocampus) can be limiting early in life [3,16,21,22], and subsequent growth cannot fully compensate for early deficits [3,16,23]. Thus, a developmental trade-off might exist between investment in the hippocampus (and spatial learning) and investment in the song control nuclei (and song repertoire size). It is important to note that males in this study differed in speed of spatial learning, not absolute success in the task and thus spatial memory, so future studies examining spatial memory in song sparrows and the timing of neural development are needed.
While our results suggest a trade-off between song repertoire size and speed of spatial learning in song sparrows, other cognitive measures are positively associated with repertoire size in this species [9]. Thus, females attending to song might learn about both a male's cognitive strengths [8,9,20] and his cognitive weaknesses.
Acknowledgements
The U.S. Fish and Wildlife Service (permit MB672712), the State of North Carolina (licence 10-SC00104), the Town of Durham, NC, USA and the Duke Institutional Animal Care and Use Committee (protocol A290-10-11) each granted permission to conduct the procedures decribed in this study.
We thank B. E. Wilson for assistance with fieldwork, R. C. Anderson for advice on assay design and W. A. Searcy for comments on the manuscript. NIH provided funding (grant no. HD056981 to K.B.S.).
References
- 1.Searcy WA, Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Catchpole CK. 1996. Song and female choice: good genes and big brains? Trends Ecol. Evol. 11, 358–360 10.1016/0169-5347(96)30042-6 (doi:10.1016/0169-5347(96)30042-6) [DOI] [PubMed] [Google Scholar]
- 3.Nowicki S, Peters S, Podos J. 1998. Song learning, early nutrition and sexual selection in songbirds. Amer. Zool. 38, 179–190 10.1093/icb/38.1.179 (doi:10.1093/icb/38.1.179) [DOI] [Google Scholar]
- 4.Nowicki S, Searcy WA, Peters S. 2002. Brain development, song learning and mate choice in birds: a review and experimental test of the ‘nutritional stress hypothesis’. J. Comp. Physiol. A 188, 1003–1014 10.1007/s00359-002-0361-3 (doi:10.1007/s00359-002-0361-3) [DOI] [PubMed] [Google Scholar]
- 5.Nowicki S, Searcy WA. 2011. Are better singers smarter? Behav. Ecol. 22, 10–11 10.1093/beheco/arq081 (doi:10.1093/beheco/arq081) [DOI] [Google Scholar]
- 6.Spencer KA, MacDougall-Shackleton SA. 2011. Indicators of development as sexually selected traits: the developmental stress hypothesis in context. Behav. Ecol. 22, 1– 9 10.1093/beheco/arq068 (doi:10.1093/beheco/arq068) [DOI] [Google Scholar]
- 7.Nowicki S, Hasselquist D, Bensch S, Peters S. 2000. Nestling growth and song repertoire size in great reed warblers: evidence for song learning as an indicator mechanism in mate choice. Proc. R. Soc. Lond. B 267, 2419–2424 10.1098/rspb.2000.1300 (doi:10.1098/rspb.2000.1300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boogert NJ, Giraldeau L-A, Lefebvre L. 2008. Song complexity correlates with learning ability in zebra finch males. Anim. Behav. 76, 1735–1741 10.1016/j.anbehav.2008.08.009 (doi:10.1016/j.anbehav.2008.08.009) [DOI] [Google Scholar]
- 9.Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S. 2011. Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim. Behav. 81, 1209–1216 10.1016/j.anbehav.2011.03.004 (doi:10.1016/j.anbehav.2011.03.004) [DOI] [Google Scholar]
- 10.Nowicki S, Searcy WA. 2005. Adaptive priorities in brain development: theoretical comment on Pravosudov et al. (2005). Behav. Neurosci. 119, 1415–1418. (doi:10.1037/0735-7044.119.5.1415) [DOI] [PubMed] [Google Scholar]
- 11.Bailey DJ, Wade J, Saldanha CJ. 2009. Hippocampal lesions impair spatial memory performance, but not song: a developmental study of independent memory systems in the zebra finch. Dev. Neurobiol. 69, 491–504 10.1002/dneu.20713 (doi:10.1002/dneu.20713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Searcy WA. 1984. Song repertoire size and female preferences in song sparrows. Behav. Ecol. Sociobiol. 14, 281–286 10.1007/BF00299499 (doi:10.1007/BF00299499) [DOI] [Google Scholar]
- 13.Nowicki S, Searcy WA. 2004. Song function and the evolution of female preferences: why birds sing, why brains matter. Ann. N. Y. Acad. Sci. 1016, 704–723 10.1196/annals.1298.012 (doi:10.1196/annals.1298.012) [DOI] [PubMed] [Google Scholar]
- 14.DeVoogd TJ, Krebs JR, Healy SD, Purvis A. 1993. Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc. Biol. Sci. 254, 75–82 10.1098/rspb.1993.0129 (doi:10.1098/rspb.1993.0129) [DOI] [PubMed] [Google Scholar]
- 15.Sherry DF, Jacobs LF, Gaulin SJ. 1992. Spatial memory and adaptive specialization of the hippocampus. Trends Neurosci. 15, 298–303 10.1016/0166-2236(92)90080-R (doi:10.1016/0166-2236(92)90080-R) [DOI] [PubMed] [Google Scholar]
- 16.Pravosudov VV, Lavenex P, Omanska A. 2005. Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behav. Neurosci. 119, 1368–1374 10.1037/0735-7044.119.5.1368 (doi:10.1037/0735-7044.119.5.1368) [DOI] [PubMed] [Google Scholar]
- 17.Hughes M, Nowicki S, Searcy WA, Peters S. 1998. Song-type sharing in song sparrows: implications for repertoire function and song learning. Behav. Ecol. Sociobiol. 42, 437–446 10.1007/s002650050458 (doi:10.1007/s002650050458) [DOI] [Google Scholar]
- 18.Roth TC, LaDage LD, Pravosudov VV. 2010. Learning capabilities enhanced in harsh environments: a common garden approach. Proc. R. Soc. B 277, 3187–3193 10.1098/rspb.2010.0630 (doi:10.1098/rspb.2010.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 20.Farrell TM, Weaver K, An Y-S, MacDougall-Shackleton SA. 2012. Song bout length is indicative of spatial learning in European starlings. Behav. Ecol. 23, 101–111 10.1093/beheco/arr162 (doi:10.1093/beheco/arr162) [DOI] [Google Scholar]
- 21.MacDonald IF, Kempster B, Zanette L, MacDougall-Shackleton SA. 2006. Early nutritional stress impairs development of a song-control brain region in both male and female juvenile song sparrows (Melospiza melodia) at the onset of song learning. Proc. R. Soc. B 273, 2559–2564 10.1098/rspb.2006.3547 (doi:10.1098/rspb.2006.3547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer KA, Buchanan KL, Leitner S, Goldsmith AR, Catchpole CK. 2005. Parasites affect song complexity and neural development in a songbird. Proc. R. Soc. B 272, 2037–2043 10.1098/rspb.2005.3188 (doi:10.1098/rspb.2005.3188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wainwright PE, Colombo J. 2006. Nutrition and the development of cognitive functions: interpretation of behavioral studies in animals and human infants. Am. J. Clin. Nutr. 84, 961–970 [DOI] [PubMed] [Google Scholar]
- 24.Andersson M. 1986. Evolution of condition-dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution 40, 804–816 10.2307/2408465 (doi:10.2307/2408465) [DOI] [PubMed] [Google Scholar]
- 25.Schew WA, Ricklefs RE. 1998. Developmental plasticity. In Avian growth and development: evolution within the altricial–precocial spectrum (eds Starck JM, Ricklefs RE.), pp. 288–304 New York, NY: Oxford University Press [Google Scholar]
- 26.Pravosudov VV, Kitaysky AS, Omanska A. 2006. The relationship between migratory behaviour, memory and the hippocampus: an intraspecific comparison. Proc. R. Soc. B 273, 2641–2649 10.1098/rspb.2006.3624 (doi:10.1098/rspb.2006.3624) [DOI] [PMC free article] [PubMed] [Google Scholar]


