Abstract
The extraoral presence of taste signal transduction proteins has recently been reported in rodents and humans. Here, we report for the first time the presence of these signal transduction proteins in the caecum of a non-human primate, the common marmoset. Quantitative RT-PCR data on the gene expression of taste signal transduction molecules (gustducin and TRPM5) in common marmosets suggested high expression in the caecum, which was not observed in other non-human primates. Immunohistochemical analysis confirmed the specific presence of gustducin and taste receptors in marmoset caecal cells. These results may relate to the specific feeding behaviour of marmosets, which consume plant exudates, primarily gums.
Keywords: common marmoset, New World monkey, Old World monkey
1. Introduction
Mammals can perceive and distinguish five basic taste qualities: sweet, bitter, sour, salty and umami (the taste of glutamate) [1]. Among these, the sweet and umami tastes are mediated by the TAS1R families, and bitterness is mediated by the TAS2R families, which consist of heptahelical G-protein-coupled receptors and can activate the G-protein gustducin [2]. Gustducin plays a pivotal role in activating downstream signal transduction cascades, including PLCβ2 and TRPM5, according to receptor activation [3]. Recently, these molecules were detected not only in human and rodent oral taste cells, but also in the gastrointestinal tract and other internal organs (for recent reviews, see [4–7]). However, some of these results in humans and rodents are partially inconsistent, and the expression of taste receptors and taste signal transduction molecules in the internal organs of non-human primates has yet to be investigated. To better understand the biological meaning of these taste-related molecules in the gastrointestinal tract, here we attempted to detect the expression of these molecules from the guts of non-human primates displaying specific feeding behaviours through quantitative RT-PCR and immunohistochemistry. The results showed the specific expression of these molecules in the caecum and colon of the common marmoset, suggesting a relationship to the specific feeding behaviours of the species.
2. Material and methods
Samples of monkey gut (four newborn and three adult common marmosets; two newborn and two adult Japanese macaques, one adult squirrel monkey, one adult hamadryas baboon) were acquired from animals culled for other purposes, as approved by the Animal Ethics Committee of Kyoto University's Primate Research Institute (nos. 2011-001, 2012-017). For gene expression analysis, small blocks of tissue were incubated at 4°C in RNAlater (Agilent Technology, CA, USA) overnight, and stored at −80°C until use. For analysis of the tongue, the circumvallate papillae of macaques and adult marmosets were sampled, while whole tongues were sampled in the case of newborn marmosets. For immunohistochemistry analysis, specimens were incubated in 4% paraformaldehyde at 4°C before use. Additional chemicals were purchased from commercial suppliers.
Total RNA was extracted from the organs using an RNeasy Plus mini kit (QIAGEN GmbH, Hilden, Germany) and its quality was checked with Bioanalyzer (Agilent). Genomic DNA removal and reverse transcription were carried out using the PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Kyoto, Japan) for 1 μg of total RNA with and without enzymes. Real-time PCR was carried out with StepOne Plus (Applied Biosystems, CA, USA) using THUNDERBIRD SYBR qPCR Mix (Toyobo, Tokyo, Japan) and the primers listed in the electronic supplementary material, table S1. Standard conditions (95°C for 30 s, 50 cycles of 95°C for 10 s and 60°C for 45 s) were applied to all analyses in triplicate. The quantity of mRNA for gustducin, TRPM5 and taste receptors was determined by comparative calculation with the internal marker GAPDH and an external marker for each cloned amplicon. Amplicons were also analysed by 2% agarose gel electrophoresis and confirmed as single bands.
For immunohistochemistry, autopsy specimens were embedded in paraffin. Sections were later deparaffinized and rehydrated in graded alcohols followed by water. Antigen retrieval to unmask immunogenic epitopes was performed with normal horse serum for 1 h. Sections were incubated overnight at 4°C with primary rabbit polyclonal antibody against Gαgust (I-20, sc-395) diluted in blocking solution (PBS-T, 1 : 3000 concentration). Sections were then incubated with biotinylated goat anti-rabbit secondary antibodies diluted to 1 : 250 concentrations in blocking solution for 1 h. Antibody complexes were visualized with avidin–biotin-peroxidase conjugate (ABC Elite) and 3,30-diaminobenzidine (DAB). For immunofluorescence histochemistry, rabbit polyclonal antibody against Gαgust (1 : 2000) and a mixture of goat serum against TAS2Rs (sc-163420 for TAS2R16, sc-165637 for TAS2R40 and sc-34850 for TAS2R43, 1 : 200 concentration each) were used, and visualized by alexa555-conjugated anti-rabbit IgG and alexa488-conjugated anti-goat IgG.
Data deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.s3m5s.
3. Results
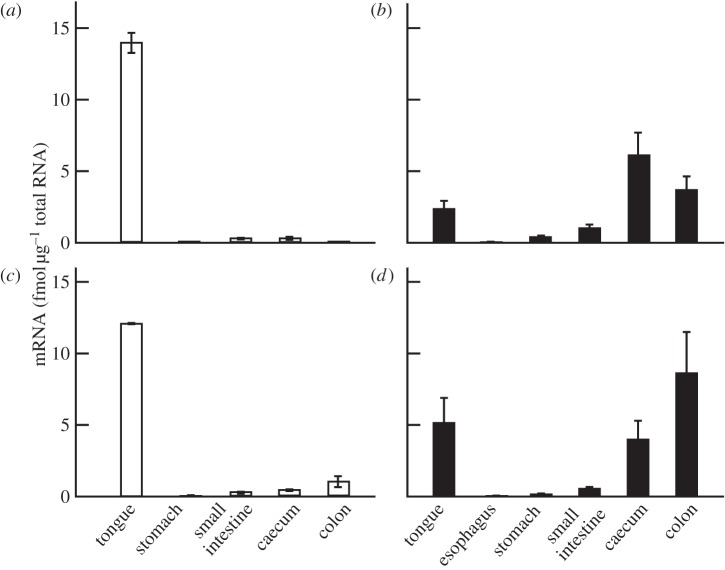
We first screened the transcripted gustducin from various organs of two types of model non-human primate: macaques and common marmosets (figure 1). We detected the expression of gustducin mRNA in both the gut and tongue of these animals. In macaques, the level of mRNA expression was highest in the tongue, with a lower level observed in the intestinal organs (figure 1a,c), a pattern similar to that found in humans [8]. Surprisingly, the level of gustducin expression was highest in the caecum and colon in newborn marmosets (figure 1b). Older marmosets showed a reduced but nevertheless significant level of transcription of these genes (figure 1d), suggesting the presence of this protein functioning in the caecum as well as the colon [9]. Similar results were obtained for downstream TRPM5 for each subject (see the electronic supplementary material, figure S1).
Figure 1.
Quantitative RT-PCR analysis of gustducin in the gastrointestinal tract of non-human primates. Plots show the expression level of gustducin mRNA in newborn (a, n = 2) and adult (c, n = 2) Japanese macaques and in newborn (b, n = 4) and adult (d, n = 3) common marmosets. Error bars indicate standard errors calculated from three independent experiments on each individual. Mean Ct values for GAPDH in each sample are 19.1 ± 1.7, 19.3 ± 1.1, 20.4 ± 1.4, 20.5 ± 1.3, 20.9 ± 1.9, 21.3 ± 1.9 for the tongue, oesophagus, stomach, small intestine, caecum and colon, respectively, of newborn marmosets. Mean Ct values for GAPDH in each sample are 16.5 ± 0.2, 17.0 ± 0.6, 19.7 ± 1.4, 18.9 ± 0.7, 19.5 ± 0.7 and 19.3 ± 0.7 for the tongue, oesophagus, stomach, small intestine, caecum and colon, respectively, of adult marmosets.
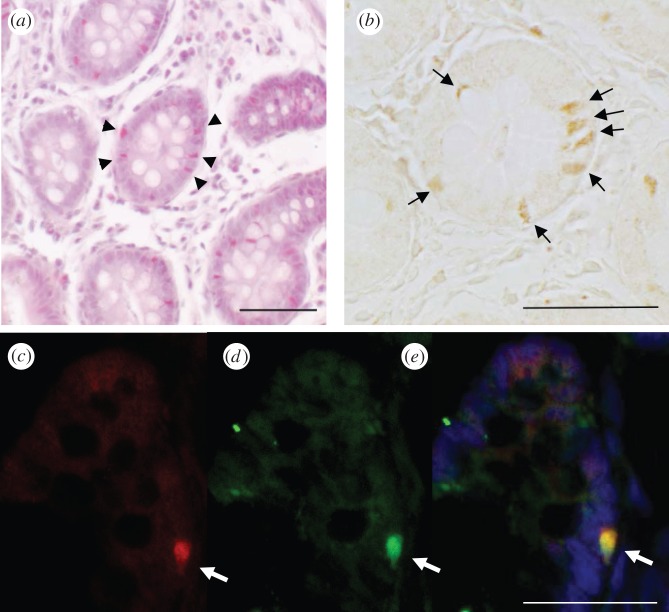
We then examined the expression of the proteins in the caecum using immunohistochemical techniques. When the tongue and caecum of the macaques were studied, gustducin expression was clearly observed in taste cells, but less so in caecal cells (data not shown). Marmoset tongue showed a similar pattern. However, we observed strong signals in marmoset caecal cells (figure 2b,c) similar to human endocrine cells [9]. To determine which receptor types exist in the gustducin-positive cells, we screened TAS1R and TAS2Rs by RT-PCR and found almost all of their transcripts in the marmoset caecum (see the electronic supplementary material, figure S2). Immunohistochemical analysis confirmed that bitter taste receptors exist in the cells, which are colocalized with gustducin (figure 2c–e). Therefore, it is probable that the cells in the marmoset caecum can respond to gustatory stimuli, unlike those in other primates.
Figure 2.
Immunohistochemistry of gustducin and bitter taste receptors in the caecum of common marmosets. (a) HE staining of the caecum of the common marmoset revealed cells containing endocrine granules (arrowheads). (b) Chromic staining using anti-gustducin antibodies showed significant signals in the cells around the proximal mucosa of the caecum (arrows). (c–e) Immunofluorescence labelling of TAS2Rs (green) and gustducin (red) revealed the colocalization of these molecules in the cells around the proximal mucosa of the caecum (arrows). Scale bar, 50 μm. In all cases, omission of the primary antibody completely negated immunostaining of these cells.
4. Discussion
This study is the first to report the presence of taste signal transduction molecules in the gastrointestinal tracts of non-human primates. The amount and transcript pattern of taste signal transduction molecules in the gastrointestinal tract appear to differ between marmosets and macaques. This might result from a phylogenetic difference or from a difference in feeding habits between the species. Unexpectedly, evidence of signal transduction components was observed in the caecum of common marmosets as well as in the colon. The specific biological significance of this system is as yet unclear. In mice and humans, gut transduction cascades are reported to detect internal bitter compounds and to secrete endocrine hormones, such as GLP-1 [10] and ghrelin [11]. In this study, we detected the colocalization of bitter taste receptors and gustducin in marmoset caecal cells, similar to human colonic cells [9]. Addition of bitter compounds to human intestinal endocrine HuTu-80 cells that express gustducin and TAS2Rs causes an increase of cellular calcium in vitro [9]. In mice, intake of bitter compounds induces plasma ghrelin production in vivo via gustducin [11]. Thus, a similar mechanism could feasibly exist in the marmoset caecum and colon. It is therefore of interest what cell types exist in the marmoset caecum and colon in order to better understand the function of the expressed taste-related proteins.
What is the origin of the difference between common marmosets and macaques? It is noteworthy that the prominent presence of gustducin and TRPM5 in the caecum and colon was not observed in other primates, including New World squirrel monkeys (see the electronic supplementary material, figure S3) and might therefore be limited to Callithrix spp. and closely related species. In the species, it is well documented that the developed caecum and colon are the organs responsible for fermentation of plant exudates (for reviews, see [12–14]). More specifically, wild common marmosets regularly consume saps or gums, which are fermented in the caecum and colon. Therefore, the detection of decomposed bioactive compounds, such as saccharides or bitter compounds, is one of the possible functions of these cells. Detection of fermenting bacteria in the airway [15] or solitary sensory [16] systems is another possible function of these cells. The recent availability of transgenic marmosets [17] may help to further elucidate the role of ‘caecal gustation’ in future research. Although the feeding behaviour of wild primates is known to be influenced by their sensory organs and upper gut through visual cues, smell, taste and digestion of foods available in their habitat [2,18], elucidating the fitness benefit of expressing taste receptors in the lower gut, caecum and colon will provide clues to understanding the complete feeding mechanisms of primates.
Acknowledgements
All experiments conformed to the Guidelines for Care and Use of Non-human Primates, Version 3 issued by Animal Ethics Committee, Primate Research Institute, Kyoto University.
We thank members of the Department of Cellular and Molecular Biology, Primate Research Institute for valuable discussion and the use of their facilities. We thank members of the Division of Genomics Research, Life Science Research Center, Gifu University, for their supports to the experiments. We also thank Drs A. Onishi, Y. Kusakabe, Y. Ishimaru and K. Yamagishi for their valuable suggestions for immune-staining techniques. This work was supported by the Cooperation Research Program at the Primate Research Institute, Kyoto University and was financially supported by Grants-in-Aid for Scientific Research (21370109, 24370096 and 24405018) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and additional grants from the Takeda Foundation for Science and the Suzuken Memorial Foundation to H.I.
References
- 1.Yarmolinsky DA, Zuker CS, Ryba NJ. 2009. Common sense about taste: from mammals to insects. Cell 139, 234–244 (doi:10.1016/j.cell.2009.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugawara T, Imai H. 2012. Post genome biology of primates focusing on taste perception. In Post-genome biology of primates, primatology monographs (eds Hirai H, Imai H, Go Y.), pp. 79–92 Tokyo, Japan: Springer [Google Scholar]
- 3.McLaughlin SK, McKinnon PJ, Margolskee RF. 1992. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357, 563–569 (doi:10.1038/357563a0) [DOI] [PubMed] [Google Scholar]
- 4.Behrens M, Meyerhof W. 2011. Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 105, 4–13 (doi:10.1016/j.physbeh.2011.02.010) [DOI] [PubMed] [Google Scholar]
- 5.Breer H, Eberle J, Frick C, Haid D, Widmayer P. 2012. Gastrointestinal chemosensation: chemosensory cells in the alimentary tract. Histochem. Cell Biol. 138, 13–24 (doi:10.1007/s00418-012-0954-z) [DOI] [PubMed] [Google Scholar]
- 6.Janssen S, Depoortere I. 2013. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol. Metab. 24, 92–100 (doi:10.1016/j.tem.2012.11.006) [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Ishimaru Y. 2013. Oral and extra-oral taste perception. Semin. Cell Dev. Biol. 24, 240–246 (doi:10.1016/j.semcdb.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 8.Wu SV, Rozengur TN, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. 2002. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl Acad. Sci. USA 99, 2392–2397 (doi:10.1073/pnas.042617699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. 2006. Colocalization of the α-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G792–G802 (doi:10.1152/ajpgi.00074.2006) [DOI] [PubMed] [Google Scholar]
- 10.Jang HJ, et al. 2007. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl Acad. Sci. USA 104, 15 069–15 074 (doi:10.1073/pnas.0706890104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. 2011. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl Acad. Sci. USA 108, 2094–2099 (doi:10.1073/pnas.1011508108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash LT. 1986. Dietary, behavioral, and morphological aspects of gummivory in primates. Am. J. Phys. Anthropol. 29, 113–137 (doi:10.1002/ajpa.1330290505) [Google Scholar]
- 13.Ferrari SF. 1993. Ecological differentiation in the Callitrichidae. In Marmosets and Tamarins (ed. Rylands AB.), pp. 314–328 Oxford, UK: Oxford University Press [Google Scholar]
- 14.Power ML. 2010. Nutritional and digestive challenges to being a gum-feeding primate. In The evolution of exudativory in primates: developments in primatology: progress and prospects (eds Burrows AM, Nash LT.), pp. 25–44 New York: Springer [Google Scholar]
- 15.Deshpande DA, Wang WCH, McIlmoyle EL, Kathryn S, Robinett KS, Schillinger RM, An SS, Sham JSK, Liggett SB. 2010. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 16, 1299–1304 (doi:10.1038/nm.2237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tizzano M, et al. 2010. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl Acad. Sci. USA 107, 3210–3215 (doi:10.1073/pnas.0911934107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki E, et al. 2009. Generation of transgenic non-human primates with germline transmission. Nature 459, 523–527 (doi:10.1038/nature08090) [DOI] [PubMed] [Google Scholar]
- 18.Imai H, Suzuki N, Ishimaru Y, Sakurai T, Yin L, Pan W, Abe K, Misaka T, Hirai H. 2012. Functional diversity of bitter taste receptor TAS2R16 in primates. Biol. Lett. 8, 652–656 (doi:10.1098/rsbl.2011.1251) [DOI] [PMC free article] [PubMed] [Google Scholar]