Abstract
Characterizing phenotypic differences between sexual and asexual organisms is a critical step towards understanding why sexual reproduction is so common. Because asexuals are often polyploid, understanding how ploidy influences phenotype is directly relevant to the study of sex and will provide key insights into the evolution of ploidy-level variation. The well-established association between genome size and cell cycle duration, evidence for a link between genome size and tissue regeneration rate and the growing body of research showing that ploidy influences growth rate and gene expression led us to hypothesize that healing and tissue regeneration might be affected by ploidy-level variation. We evaluated this hypothesis by measuring the rate of regeneration of antenna tissue of Potamopyrgus antipodarum, a New Zealand snail characterized by frequent coexistence between diploid sexuals and polyploid asexuals. Antennae of triploid and presumptive tetraploid asexuals regenerated more rapidly than the antennae of diploid sexuals, but regeneration rate did not differ between triploids and tetraploids. These results suggest either that ploidy elevation has nonlinear positive effects on tissue regeneration and/or that factors associated directly with reproductive mode affect regeneration rate more than ploidy level. The results of this study also indicate that the lower ploidy of sexual P. antipodarum is unlikely to confer advantages associated with more rapid regeneration.
Keywords: sexual reproduction, Potamopyrgus antipodarum, polyploidy
1. Introduction
Because only females can produce offspring, sexually reproducing females that reallocate resources from daughters to sons are expected to pay a substantial ‘cost of males’ [1]. This cost is not paid by asexually reproducing organisms that produce only daughters, leading to the expectation that asexuality should be the norm. The predominance of sexual reproduction despite the cost of males means that sex must be associated with profound advantages, which themselves remain unclear [2].
Characterizing differences between sexual and asexual organisms constitutes a critical step towards understanding why sex is so common [2]. Potamopyrgus antipodarum, a New Zealand freshwater snail, is an ideal study system in this context because obligately sexual and obligately asexual P. antipodarum coexist and are ecologically similar [3], allowing direct comparisons between sexual and asexual individuals.
Like many mixed sexual/asexual animal taxa [4], sexual P. antipodarum are diploid, whereas asexuals are triploid or more than 3× (‘tetraploid’) [5]. Because ploidy level can profoundly influence phenotype [4,6], these differences in ploidy between sexuals and asexuals could affect the maintenance of sex [7]. Rigorous evaluation of the phenotypic effects of ploidy-level variation in P. antipodarum is therefore central to understanding the maintenance and distribution of sex in P. antipodarum and in the many other mixed sexual/asexual animal taxa featuring variation in ploidy level. Importantly, because there have been multiple independent spontaneous (i.e. non-hybrid) transitions from sexual to asexual P. antipodarum and from lower to higher ploidy levels within asexual P. antipodarum, we can also use comparisons of sexuals versus asexuals and triploids versus tetraploids to decouple the effects of ploidy and sexuality [5].
Here, we address whether ploidy level affects the rate of tissue regeneration in P. antipodarum. There are multiple mechanisms by which ploidy could influence healing/regeneration; we outline three possibilities below. First, the increased cell volume typically associated with polyploidy [4,6] is itself often associated with decreased metabolic rate and slower growth and development [8]. Second, there is a well-established positive correlation between haploid genome size and cell cycle time [9] and some evidence that cell cycle time also increases with ploidy level [10]. Both of these scenarios would be expected to lead to lower rates of healing and regeneration in polyploids. No study of which we are aware has directly addressed either of these hypotheses, though indirect support comes from evidence for an inverse correlation between limb regeneration rate and nuclear genome content in salamanders [11].
Alternatively, extra genome copies may result in higher production of RNA and protein [12,13]. While relationships between gene expression levels and tissue regeneration rates are not well characterized, increased gene expression via endopolyploidy was proposed as an explanation for the rapid regrowth of Arabidopsis plants subjected to simulated herbivory [14]. With this possibility in mind, and given the positive correlation between RNA level and individual growth rate in animals [15], it is conceivable that higher RNA levels could translate into higher rates of tissue regeneration. By this logic, the higher RNA content of polyploid P. antipodarum [13] could plausibly positively influence healing and regeneration. Here, we performed the first study of which we are aware to directly address potential connections between the rate of tissue regeneration and ploidy level in animals by quantifying the rate of antenna regrowth in a diverse array of diploid, triploid and tetraploid P. antipodarum.
2. Material and methods
We arbitrarily selected 10–15 adult or near-adult female P. antipodarum from four inbred sexual diploid lineages, one outbred sexual diploid lineage, 13 asexual triploid lineages and 13 asexual tetraploid lineages. All lineages were laboratory-raised and independently derived from a single female and were selected because they contained enough snails for the experiment. We also arbitrarily selected two to four adult female P. antipodarum of unknown ploidy collected from four New Zealand lakes (Alexandrina, Brunner, Kaniere and Selfe) containing P. antipodarum populations that comprised coexisting sexual diploids and asexual triploids. We used the largest females available for each lineage/field collection to ensure comparisons between individuals of similar life stages.
Next, we anaesthetized groups of 10–15 snails by exposing them to 2 g crushed menthol crystals [16] dissolved in 200 ml of carbon-filtered tap water. After approximately 90 min of menthol exposure, we used microscissors to sever the right antenna of one arbitrarily selected and successfully anaesthetized snail from each laboratory lineage and from each of the nine successfully anaesthetized field-collected snails. We also captured an image of each snail next to a ruler and then used ImageJ software to measure the length of the shell of each snail. We used the shell length data to address whether there were differences in body size between sexuals and asexuals or across ploidy levels and control for the possibility that snail size could affect antenna regeneration rate. Snails that were not fully anaesthetized (i.e. were still moving) after 90 min of menthol exposure were not used in the experiment.
Following antenna severing, each snail was housed individually in a plastic cup filled with 200 ml carbon-filtered tap water and fed 0.024 mg dried Spirulina every 2–3 days [17]. A pilot experiment had demonstrated that antennae regenerated to approximately 90% of their initial length within 9–10 days. Accordingly, after 10 days of regrowth, we captured an image of the antenna of each snail and then used ImageJ to measure the length of the regrown antenna.
Next, we dissected and flash-froze the head tissue of the nine field-collected snails in preparation for flow cytometric determination of ploidy level using the ‘Iowa’ protocol described in [5,18]. We also treated seven experimental snails from sexual diploid lineages in the same manner to provide examples of representative diploids.
Data uploaded to Dryad: doi:10.5061/dryad.68nj4.
(a). Statistical analyses
As previous flow cytometry-based analyses of ploidy in P. antipodarum have demonstrated [5], the 10 diploid and six triploid samples were readily distinguishable (see the electronic supplementary material, figure S1). One of the putatively diploid individuals included in the flow cytometry run was revealed to be triploid, representing either contamination from another lineage or ploidy elevation within the sexual line. We thus reclassified this individual as triploid for subsequent analyses. When multiple field-collected individuals from the same lake had the same ploidy level, we selected the largest individual from each lake/ploidy level combination for future analyses (paralleling our selection of the largest females available from the laboratory lineages), leaving us with three field-collected diploids and two field-collected triploids.
While there were no systematic differences in shell length between either sexuals and asexuals or between triploid and tetraploid asexuals (see the electronic supplementary material, figure S2), there was a positive association between shell length and antenna regrowth (linear regression, β = 0.428, F = 7.858, p = 0.008). We thus used the residuals from this regression as the response variable in a univariate ANOVA to evaluate how the fixed factor of ploidy affected antenna regrowth. All statistical analyses were conducted in IBM SPSS Statistics v. 21.
3. Results
There was a significant effect of ploidy on antenna regrowth (F2,34 = 12.382, p < 0.0001; figures 1 and 2). Pairwise post hoc Fisher's least significant difference (LSD) comparisons revealed that this effect was driven by the low rate of regrowth in diploids (mean regrowth = −1.15 ± 0.88 s.d.) relative to triploids (0.12 ± 0.68 s.d.; Bonferroni-corrected p = 0.002) and tetraploids (0.56 ± 0.81 s.d.; Bonferroni-corrected p < 0.0001). While there was somewhat higher mean regrowth of tetraploids than triploids, this difference was not significant (Bonferroni-corrected p = 0.421) and was driven in part by a tetraploid with antenna regrowth that was more than twice as rapid as any other individual (mean tetraploid regrowth without this individual: 0.4 ± 0.61 s.d.; figure 2). The power of the triploid/tetraploid comparison was only approximately 0.33 (power analysis executed in IBM SPSS Statistics v. 21), however, meaning that detection of differences in tissue regeneration between triploid and tetraploid P. antipodarum—if they exist—will require a larger experiment.
Figure 1.
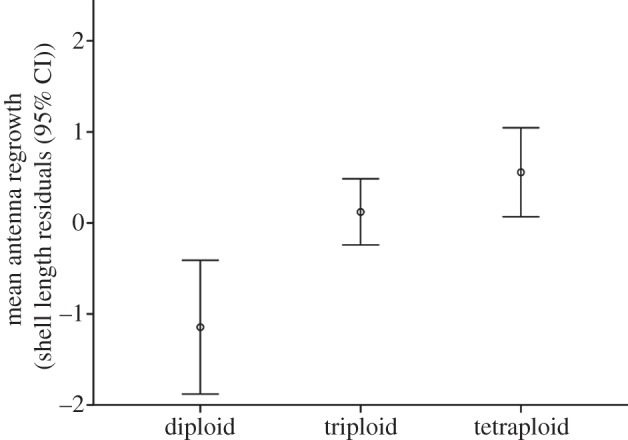
Mean antenna regrowth (shell length residuals) after 10 days.
Figure 2.
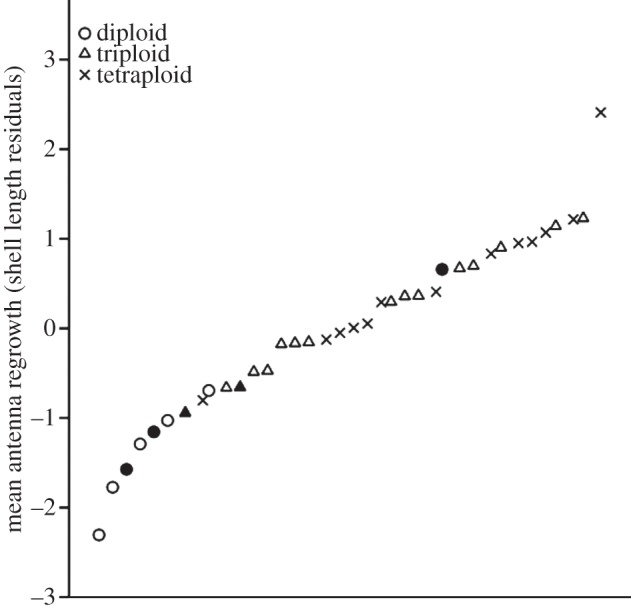
Rank-ordered antenna regrowth (shell length residuals) for each snail used in the experiment. Filled symbols represent the three field-collected diploids and the two field-collected triploids.
4. Discussion
Regeneration of antenna tissue proceeded much more rapidly in polyploid asexual P. antipodarum than in sexual diploid P. antipodarum but occurred at statistically indistinguishable rates in triploid versus tetraploid asexual P. antipodarum. While we did not have adequate power to detect the more subtle differences in rate of regeneration that may exist between triploids and tetraploids, the results of our experiment suggest that any such differences are likely to be relatively minor compared with the magnitude of difference in regeneration rate between sexuals and asexuals.
One possible explanation for the relatively high rate of tissue regeneration in asexual polyploids is that P. antipodarum experience nonlinear positive effects of ploidy elevation on the rate of tissue regeneration. While the effects of polyploidy on phenotype remain unclear, ploidy elevation often results in increased cell size [4,6] and higher levels of RNA and protein production [12,13]. Both phenomena could plausibly affect rates of healing and regeneration [14]: cell size can directly or indirectly influence many important traits [8,9], and the increased RNA/protein production associated with higher ploidy level may facilitate more rapid regeneration [14,19].
Although somatic cell size in P. antipodarum remains uncharacterized, a recent study demonstrated that the triploid males occasionally produced by asexual triploid female P. antipodarum [18] have larger sperm than diploid sexual male P. antipodarum [20]. All else being equal, larger cell sizes should equal larger body sizes, so the fact that body size does not differ across reproductive modes or ploidy levels in P. antipodarum (see the electronic supplementary material, figure S2) thus suggests either that somatic cell size does not increase with ploidy level or that cell number may decrease with ploidy level. While to the best of our knowledge, no studies have directly addressed how cell number and/or size might influence healing and regeneration in animals, Barow [19] recently suggested that endopolyploidy (i.e. genome duplication in the absence of cell division, leading to larger and fewer cells) could facilitate tissue regeneration in plants (also see [14]). Clearly, next steps towards understanding the higher rate of regeneration in asexual polyploid P. antipodarum will require quantification and comparison of cell number and sizes across ploidy levels and reproductive modes.
Triploid P. antipodarum have significantly higher per-mass RNA content than their diploid counterparts [13], providing a different but non-mutually exclusive potential explanation for the more rapid regeneration rates in polyploids. Under this hypothesis, if higher RNA production translates into more rapid tissue regrowth, the absence of a marked difference in regeneration rates between triploids and tetraploids implies that there should be little if any difference between per-mass RNA levels in triploid and tetraploid P. antipodarum.
It is also possible that the higher performance of asexual polyploid P. antipodarum, which are apomictic and feature fixed heterozygosity [21], is linked to higher heterozygosity and/or the absence of inbreeding. This possibility is especially important to consider because some of the sexual diploids in the experiment were from inbred lines. If low heterozygosity and/or inbreeding depression contributed to the relatively slow regrowth of the sexual diploids, we would predict that the field-collected sexual diploids (sampled from diverse natural populations) should experience higher regrowth than the inbred sexual diploids. We would also predict that tetraploids, which have higher heterozygosity than triploids [22], should have a higher rate of regrowth than triploids. These predictions were generally not met: antennae regeneration in two of the three field-collected sexual diploids was indistinguishable from the relatively low rate of regeneration observed in sexual diploids from inbred lines, and triploids and tetraploids experienced similar and relatively high rates of regrowth (figures 1 and 2). These results suggest that low heterozygosity and/or inbreeding depression cannot fully explain why the sexuals performed so poorly.
Acknowledgements
We thank Katelyn Larkin, Donny Warren, Claire Tucci and Haley Heniff for snail care and experimental set-up; Katelyn Larkin for assistance with flow cytometry; Daniela Vergara for snail donations; Joel Sharbrough, Laura Rice and two anonymous reviewers for comments on the manuscript; and the Secondary Student Training Program, the University of Iowa Honors Program and Lori Adams for financial and logistical support.
References
- 1.Maynard Smith J. 1978. The evolution of sex. London, UK: Cambridge University Press [Google Scholar]
- 2.Meirmans S, Meirmans PG, Kirkendall LR. 2012. The costs of sex: Facing real-world complexities. Q. Rev. Biol. 87, 19–40 (doi:10.1086/663945) [DOI] [PubMed] [Google Scholar]
- 3.Jokela J, Lively CM, Dybdahl MF, Fox JA. 1997. Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Evolution 78, 452–460 (doi:10.2307/2266021) [Google Scholar]
- 4.Otto SP, Whitton J. 2000. Polyploid incidence and evolution. Ann Rev. Genet. 34, 401–437 (doi:10.1146/annurev.genet.34.1.401) [DOI] [PubMed] [Google Scholar]
- 5.Neiman M, Paczesniak D, Soper DM, Baldwin AT, Hehman G. 2011. Wide variation in ploidy level and genome size in a New Zealand freshwater snail with coexisting sexual and asexual lineages. Evolution 65, 3203–3216 (doi:10.1111/j.1558-5646.2011.01360.x) [DOI] [PubMed] [Google Scholar]
- 6.Comai L. 2005. The advantages and disadvantages of being polyploid. Nature 6, 836–846 (doi:10.1038/nrg1711) [DOI] [PubMed] [Google Scholar]
- 7.Bierzychudek P. 1985. Patterns in plant parthenogenesis. Experientia 41, 1255–1264 (doi:10.1007/BF01952068) [DOI] [PubMed] [Google Scholar]
- 8.Hessen DO, Daufresne M, Leinaas HP. 2013. Temperature-size relations from the cellular-genomic perspective. Biol. Rev. 88, 476–488 (doi:10.1111/brv.12006) [DOI] [PubMed] [Google Scholar]
- 9.Greilhuber J, Leitch IJ. 2013. Genome size and the phenotype. In Plant genome diversity, vol. 2 (eds Greilhuber J, Dolezal J, Wendel JF.), pp. 323–344 Vienna, Austria: Springer [Google Scholar]
- 10.Davies POL, Rees H. 1975. Mitotic cycles in Triticum species. Heredity 35, 337–345 (doi:10.1038/hdy.1975.104) [Google Scholar]
- 11.Sessions SK, Larson A. 1987. Developmental correlates of genome size in plethodontid salamanders and their implications for genome evolution. Evolution 41, 1239–1251 (doi:10.2307/2409090) [DOI] [PubMed] [Google Scholar]
- 12.De Godoy LMF, Olsen JV, Cox J, Nielsen ML, Hubner NC, Frohlich F, Walther TC, Mann M. 2008. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature 455, 1251–1254 (doi:10.1038/nature07341) [DOI] [PubMed] [Google Scholar]
- 13.Neiman M, Theisen KM, Mayry ME, Kay AD. 2009. Higher nucleic acid content in asexual vs. sexual snails: a cost of asexuality? J. Evol. Biol. 22, 1359–1363 (doi:10.1111/j.1420-9101.2009.01748.x) [DOI] [PubMed] [Google Scholar]
- 14.Scholes DR, Paige KN. 2011. Chromosomal plasticity: mitigating the impacts of herbivory. Ecology 92, 1691–1698 (doi:10.1890/10-2269.1) [DOI] [PubMed] [Google Scholar]
- 15.Hessen DO, Jeyasingh PD, Neiman M, Weider LJ. 2010. Genome streamlining and the elemental costs of growth. Trends Ecol. Evol. 25, 75–80 (doi:10.1016/j.tree.2009.08.004) [DOI] [PubMed] [Google Scholar]
- 16.McCraw BM. 1958. Relaxation of snails before fixation. Nature 181, 575 (doi:10.1038/181575a0)13517228 [Google Scholar]
- 17.Neiman M, Warren D, Rasmussen B, Zhang S. In press. Complex consequences of increased density for reproductive output in an invasive freshwater snail. Evol. Ecol. (doi:10.1007/s10682-013-9632-4) [Google Scholar]
- 18.Neiman M, Larkin K, Thompson AR, Wilton P. 2012. Male offspring production by asexual Potamopyrgus antipodarum, a New Zealand snail. Heredity 109, 57–62 (doi:10.1038/hdy.2012.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barow M. 2006. Endopolyploidy in seed plants. Bioessays 28, 271–281 (doi:10.1002/bies.20371) [DOI] [PubMed] [Google Scholar]
- 20.Soper DM, Neiman M, Savytskyy OP, Zolan ME, Lively CM. 2013. Spermatozoa production by triploid males in the New Zealand freshwater snail Potamopyrgus antipodarum. Biol. J. Linn. Soc. (doi:10.1111/bij.12085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips NR, Lambert DM. 1989. Genetics of Potamopyrgus antipodarum (Gastropoda: Prosobranchia): evidence for reproductive modes. N Z J. Zool. 16, 435–445 (doi:10.1080/03014223.1989.10422911) [Google Scholar]
- 22.Paczesniak D, Jokela J, Larkin K, Neiman M. In press. Discordance between nuclear and mitochondrial genomes in sexual and asexual lineages of the freshwater snail Potamopyrgus antipodarum. Mol. Ecol . [DOI] [PubMed] [Google Scholar]


