Abstract
Arms races between brood parasites and their hosts provide model systems for studying the evolutionary repercussions of species interactions. However, how naive hosts identify brood parasites as enemies remains poorly understood, despite its ecological and evolutionary significance. Here, we investigate whether young, cuckoo-naive superb fairy-wrens, Malurus cyaneus, can learn to recognize cuckoos as a threat through social transmission of information. Naive individuals were initially unresponsive to a cuckoo specimen, but after observing conspecifics mob a cuckoo, they made more whining and mobbing alarm calls, and spent more time physically mobbing the cuckoo. This is the first direct evidence that naive hosts can learn to identify brood parasites as enemies via social learning.
Keywords: brood parasitism, coevolution, cuckoo, social learning
1. Introduction
Social learning facilitates the rapid transmission of adaptive information [1], and is particularly valuable when opportunities for learning through personal experience are limited [2]. One such context is recognition of brood parasites. Parasitic cuckoos lay their eggs secretively, rapidly and at times when hosts are unlikely to be near their nest [3], providing little opportunity to learn directly about the threat they pose. Yet, recognition of cuckoos is of critical importance, because parasitism imposes severe costs on host reproductive success [3], and recognition of an adult cuckoo can result in successful deflection of parasitism [4], or more accurate decisions about rejection of cuckoo eggs and chicks [5,6].
Few studies have investigated the mechanisms by which hosts recognize adult brood parasites [4]. Langmore et al. [7] showed that recognition of cuckoos is learned in superb fairy-wrens, Malurus cyaneus; individuals at parasitized sites mobbed cuckoos intensively, whereas at nearby unparasitized sites they showed no response to cuckoos. Reed warblers, Acrocephalus scirpaceus, mob cuckoos more intensively after seeing their neighbours mob a cuckoo [8,9], and mobbing intensity is influenced more by social cues than personal experience [10]. Although reed warblers clearly modify their defences against brood parasites in response to social cues, it is unclear whether social learning alters their perception of parasitism risk or facilitates recognition of cuckoos as a threat [8]. Here, we show that naive superb fairy-wrens learn to recognize cuckoos as a threat through observation of mobbing by conspecifics.
2. Material and methods
Cooperatively breeding superb fairy-wrens are common hosts of brood parasitic Horsfield's bronze-cuckoos, Chalcites basalis, and occasional hosts of shining bronze-cuckoos, Chalcites lucidus [11]. Superb fairy-wrens are resident, whereas the cuckoos are breeding migrants to southeastern Australia [11]. Fairy-wrens produce two distinct alarm calls when mobbing: a general ‘mobbing’ alarm, and a ‘whining’ alarm which has been observed only in the context of cuckoo mobbing [7]. Unlike reed warblers [12], they do not modify mobbing of cuckoos in relation to parasitism risk [7].
Experiments were conducted in Campbell Park [13,14], Canberra, Australia, during September–November 2012. Parasitism of superb fairy-wrens by Horsfield's bronze-cuckoos at this site occurred in 8/14 years (1999–2012, 0–37% of nests in which eggs were laid each year, n = 1201 nests). In 2011, parasitism was rare (4%, n = 167 nests) and localized, which provided an opportunity to identify ‘cuckoo-naive’ juvenile superb fairy-wrens (that hatched, and were colour banded late in the 2011 breeding season, and had not shared the environment with cuckoos).
To test whether superb fairy-wrens acquire a mobbing response to cuckoos through social learning, we used freeze-dried specimens of (i) shining bronze-cuckoos and (ii) white-plumed honeyeaters Lichenostomus penicillatus (a harmless heterospecific control; [7]). Shining bronze-cuckoos are morphologically similar to Horsfield's bronze-cuckoos and elicit a highly aggressive response from superb fairy-wrens, whereas honeyeaters elicit a low-level response, even when placed near a fairy-wren nest [7]. The purpose of the honeyeater control was to investigate whether repeated presentations could affect response to a stimuli through sensitization [1]. We used two specimens of each species, which we swapped between trials to control for model effects.
Only cuckoo-naive individuals in a breeding group with ‘cuckoo-experienced’ individuals (that have previously shared habitat with breeding cuckoos) were used in experiments, and one naive individual was tested per breeding group (11 individuals). All experiments were conducted during late nest building, when cuckoos monitor host nests and are mobbed by their hosts (W. E. Feeney & N. E. Langmore 2012, personal observations).
At least 30 min prior to each experiment, a camouflaged hide was set up 10–25 m from the nest of the focal group, and the model was placed on a perch within a protective cage of wire mesh approximately 2 m from the nest [7]. The cage was covered with a camouflage cloth, which was pulled from the cage to reveal the model to the naive individual or group. Each trial comprised three treatments: (i) presentation of the model to the naive individual at a time when it was alone (pre-training), (ii) presentation of the model to the entire group (training) and (iii) presentation of the model to the (previously) naive individual, again when it was alone (post-training; methods similar to 1), and a cuckoo and control trial was conducted on each territory. When possible, all three treatments for a given trial were accomplished in 1 day, and the following trial was conducted on the following day. The order of trials was alternated between groups. Further, Langmore et al. [7] found no effect of presentation order (cuckoo or honeyeater) on response, so any change in response is likely a result of learning rather than carry-over aggression.
Pre- and post-training trials commenced when the naive individual came within 2 m of the model and no other group members were in sight. Training trials commenced when at least one experienced group member came within 2 m of the model. In all cases, the naive individual approached within 5 m of the model within 30 s of an experienced individual, after which the training trial continued for 5 min. For each focal individual, the length (s) of the pre- and post-training trials for the cuckoo and honeyeater were standardized according to the length of the shortest trial (a trial ceased if another fairy-wren approached; average trial length = 41.18 ± 15.59 s). A minimum of 90 min was allowed between training and post-training trials for carry-over aggression to diminish.
The number of mobbing and whining calls were recorded using a Marantz PMD661 solid-state recorder and an Audio-Technica condenser microphone, and distance between the focal bird and the model (time spent < 0.5, 2, 5, and 10 m from the model; [7]) was simultaneously dictated onto the recording. Number and type of call were extracted using Raven Pro v. 1.3 [15], statistical analyses were conducted in R v. 2.13.2 [16] and mixed-effects models were conducted using the ‘lme4’ package [17]. Our data violated assumptions of mixed-effects models, so we used conservative Friedman's tests to test whether response (number of whining and mobbing alarm calls, and proportion of trial spent mobbing) by the naive individual changed following training by their group. We used linear mixed-effects models to test whether group mobbing of cuckoos was more intense than to honeyeaters with model type, trial order and model replicate as a fixed effects, and group identity as a random effect. Trial order and model replicate were non-significant and were removed from final models.
Raw data can be found in the electronic supplementary material.
3. Results
Cuckoo-naive superb fairy-wrens were unresponsive to cuckoo models in the pre-training trials (table 1). However, following training by group members, these individuals produced significantly more whining and mobbing alarm calls and spent significantly more time within 0.5 m of the model in response to the cuckoo (table 1; figures 1 and 2).
Table 1.
Responses (mean ± s.e.) of naive (n = 11) and experienced superb fairy-wrens to presentation of cuckoo and control specimens during pre-training, post-training and training trials (mean length pre- and post-training trials = 41.18 ± 15.59 s, mean length training trial = 300 ± 0 s). Italics indicates statistical significance.
| naive individuals |
experienced groups |
||||
|---|---|---|---|---|---|
| pre-training trial | post-training trial | Friedman's test on call rate or % time less than 0.5 m (χ2) | p | training trial | |
| cuckoo | |||||
| alarm calls | 0 (±0) | 54.18 (±30.75) | 4 | 0.006 | 324.10 (±57.80) |
| whining calls | 0 (±0) | 18.18 (±9.88) | 7.36 | 0.045 | 239.64 (±80.81) |
| per cent of time less than 0.5 m | 0.91 (±0.91) | 62.27 (±13.22) | 6.4 | 0.01 | 79.21 (±11.92) |
| honeyeater | |||||
| alarm calls | 22.64 (±6.83) | 17.18 (±10.12) | 1 | 0.32 | 185.82 (±53.80) |
| whining calls | 3.27 (±3.27) | 0 (±0) | 1 | 0.32 | 27.18 (±25.15) |
| per cent of time less than 0.5 m | 19.70 (±12.06) | 13.12 (±8.26) | 1 | 0.65 | 30.85 (±12.43) |
Figure 1.
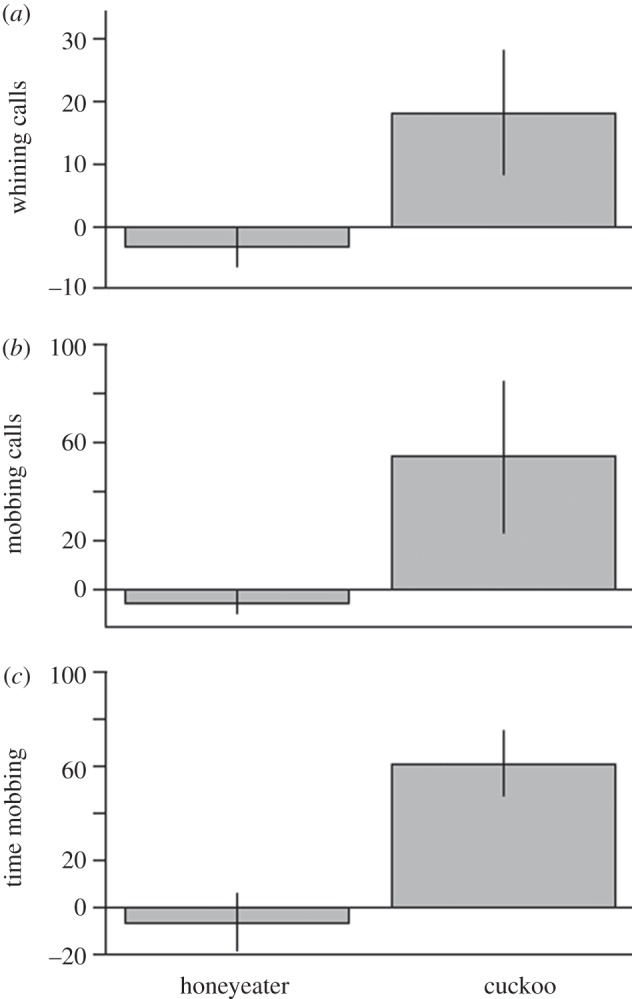
Change in response of naive superb fairy-wrens (n = 11) to honeyeater and cuckoo models following training. ‘Whining calls’, and ‘mobbing calls’ refer to the number of calls, and ‘time mobbing’ refers to the proportion of time spent within 0.5 m of the model by the naive individual. Error bars denote s.e.
Figure 2.

Mobbing of a cuckoo model by a previously naive male superb fairy-wren and a female superb fairy-wren in Campbell Park, Australia. (Online version in colour.)
During the training trial, groups were more aggressive to cuckoos than honeyeaters (LME on number of whining calls, Z =−35.16, p < 0.001; mobbing calls, Z =−20.05, p < 0.001 and proportion of time spent within 0.5 m by the closest group member, Z =−25.53, p < 0.001, table 1), and whining and mobbing alarm calls produced in the post-training trials always occurred following whining and mobbing alarm calls being produced by at least one experienced group member in the training trial.
Repeated presentations did not have an effect on the response of cuckoo-naive fairy-wrens to honeyeaters between pre- and post-training trials (table 1 and figure 1), suggesting that the increased aggression to cuckoos after training was a result of social learning rather than a change in sensitivity as a result of repeated presentations.
Two naive individuals whined briefly as they approached the honeyeater (table 1). Both individuals received the honeyeater treatment after the cuckoo, so these were likely to be brief moments of mistaken identity. Naive individuals produced more mobbing alarms in response to the honeyeater than the cuckoo during the pre-training trial (Friedman's test, χ1 = 7, p = 0.008). This is probably because honeyeaters were familiar, whereas cuckoos were novel and their level of threat was unknown.
4. Discussion
Naive superb fairy-wrens were initially unresponsive to cuckoo models. However, following a single observation of the responses of their group to a cuckoo model, they produced an aggressive response while separate from their group. By contrast, they showed no change in response to the honeyeater control between pre- and post-training trials. These results indicate that social learning is a mechanism by which this species can rapidly recognize and respond to a novel threat.
Our results are consistent with theoretical predictions about social learning. First, social learning should occur when cues from conspecifics are both reliable and specific [10]. Superb fairy-wrens produce a ‘whining’ alarm call exclusively in response to cuckoos, and are likely to use it reliably because it recruits group members and neighbours to assist in mobbing of cuckoos (W. E. Feeney & N. E. Langmore 2011, unpublished data). Thus, it provides a highly reliable source of information to naive individuals. Second, animals should rely on social learning when personal learning is more costly, such as when learning about predators [10]. The costs of successful brood parasitism are greater even than those of nest depredation; the host not only loses its entire brood, but also invests substantial time and energy in rearing the parasite. Thus, naive individuals pay a significant cost for failing to recognize and defend against brood parasites. Finally, social learning should occur rapidly if it is to provide an advantage over personal learning [18]. This was the case in our study; a single observation by a naive individual of an attack on a cuckoo by their group was sufficient for social learning to occur. This is consistent with studies showing one-event social learning of predators by birds [19], and rapid social acquisition of fear responses more generally [18].
Three other studies provide evidence of social learning in cuckoo hosts [8–10]. These studies demonstrated that mobbing of cuckoos by reed warblers is a phenotypically plastic response that is modified through social learning. However, it was unclear whether social learning led only to a change in perceived risk of parasitism or involved refinement of a template for cuckoo recognition [8]. Unlike reed warblers, fairy-wrens do not face high risks through mobbing of cuckoos, which may explain why they do not reduce their mobbing response to cuckoos with decreased risk of parasitism [7]. The absence of such phenotypic plasticity in mobbing response clarifies the role of social learning in cuckoo recognition. Our evidence that superb fairy-wrens acquire information about the threat posed by cuckoos through social learning supports the suggestion that a similar process may operate in other brood parasite hosts such as reed warblers [8].
Acknowledgements
We thank many field assistants; Leo Joseph, Robert Palmer and Gil Pfitzner for assistance with specimens; Rob Heinsohn, Becky Kilner, Rob Magrath, Trevor Murray and Justin Welbergen for helpful discussions; and Rob Magrath, Charlotte Wray and three anonymous reviewers for comments on the manuscript. W.E.F. was supported by an Australian National Geographic grant and a Canberra Birds Conservation Fund grant. N.E.L. was supported by the Australian Research Council.
References
- 1.Curio E. 1978. The adaptive significance of avian mobbing. Z. Tierpsychol. 48, 175–183 10.1111/j.1439-0310.1978.tb00254 (doi:10.1111/j.1439-0310.1978.tb00254) [DOI] [Google Scholar]
- 2.Thornton A, Clutton-Brock T. 2011. Social learning and the development of individual and group behaviour in mammal societies. Phil. Trans. R. Soc. B 366, 978–987 10.1098/rstb.2010.0312 (doi:10.1098/rstb.2010.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies NB. 2000. Cuckoos, cowbirds and other cheats. London, UK: T. & A.D. Poyser [Google Scholar]
- 4.Feeney WE, Welbergen JA, Langmore NE. 2012. The frontline of avian brood parasite–host coevolution. Anim. Behav. 84, 3–12 10.1016/j.anbehav.2012.04.011 (doi:10.1016/j.anbehav.2012.04.011) [DOI] [Google Scholar]
- 5.Langmore NE, Cockburn A, Russell AF, Kilner RM. 2009. Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav. Ecol. 20, 978–984 10.1093/beheco/arp086 (doi:10.1093/beheco/arp086) [DOI] [Google Scholar]
- 6.Davies NB, Brooke MDL. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284 10.1016/s0003-3472(88)80269-0 (doi:10.1016/s0003-3472(88)80269-0) [DOI] [Google Scholar]
- 7.Langmore NE, Feeney WE, Crowe-Riddell J, Luan H, Louwrens KM, Cockburn A. 2012. Learned recognition of brood parasitic cuckoos in the superb fairy-wren Malurus cyaneus. Behav. Ecol. 23, 798–805 10.1093/beheco/ars033 (doi:10.1093/beheco/ars033) [DOI] [Google Scholar]
- 8.Davies NB, Welbergen JA. 2009. Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320 10.1126/science.1172227 (doi:10.1126/science.1172227) [DOI] [PubMed] [Google Scholar]
- 9.Thorogood R, Davies NB. 2012. Cuckoos combat socially transmitted defenses of reed warbler hosts with a plumage polymorphism. Science 337, 578–580 10.1126/science.1220759 (doi:10.1126/science.1220759) [DOI] [PubMed] [Google Scholar]
- 10.Campobello D, Sealy SG. 2011. Use of social over personal information enhances nest defense against avian brood parasitism. Behav. Ecol. 22, 422–428 10.1093/beheco/arq225 (doi:10.1093/beheco/arq225) [DOI] [Google Scholar]
- 11.Brooker MG, Brooker LC. 1989. Cuckoo hosts in Australia. Aust. Zool. Rev. 2, 1–67 [Google Scholar]
- 12.Welbergen JA, Davies NB. 2009. Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240 10.1016/j.cub.2008.12.041 (doi:10.1016/j.cub.2008.12.041) [DOI] [PubMed] [Google Scholar]
- 13.Langmore NE, Adcock GJ, Kilner RM. 2007. The spatial organization and mating system of Horsfield's bronze-cuckoos, Chalcites basalis. Anim. Behav. 74, 403–412 10.1016/j.anbehav.2006.09.019 (doi:10.1016/j.anbehav.2006.09.019) [DOI] [Google Scholar]
- 14.Langmore NE, Kilner RM. 2007. Breeding site and host selection by Horsfield's bronze-cuckoos, Chalcites basalis. Anim. Behav. 74, 995–1004 10.1016/j.anbehav.2007.02.028 (doi:10.1016/j.anbehav.2007.02.028) [DOI] [Google Scholar]
- 15.Bioacoustics Research Program 2008. Raven Pro: interactive sound analysis software. Ithaca, NY: The Cornell Lab of Ornithology [Google Scholar]
- 16.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 17.Bates D, Maechler M. 2009. lme4: linear mixed-effects models using S4 classes. See http://lme4.r-forge.r-project.org/
- 18.Griffin AS. 2004. Social learning about predators: a review and prospectus. Anim. Learn. Behav. 32, 131–140 10.3758/bf03196014 (doi:10.3758/bf03196014) [DOI] [PubMed] [Google Scholar]
- 19.Maloney RF, Mclean IG. 1995. Historical and experimental learned predator recognition in free-living New-Zealand robins. Anim. Behav. 50, 1193–1201 10.1016/0003-3472(95)80036-0 (doi:10.1016/0003-3472(95)80036-0) [DOI] [Google Scholar]


