Abstract
Myrrh, Commiphora molmol has been used as anti-inflammatory and wound healing commercial product. Leukocyte count had been reliable indicator for clinicians to monitor progress of healing for their patients. We hypothesized that myrrh supplement participate in the activation of leukocyte proliferation pathway prior and post skin injury and gastric ulcer. The purpose of the present study was to examine long-term effect of myrrh on leukocytes proliferation before injury and during different stages of healing. Results showed that all types of leukocytes were significantly (p < 0.05) higher in the myrrh-treated groups before and during healing. The pretreatment with myrrh offered a time-dependent rise in leukocytes proliferation. Microscopic examination of blood smear from myrrh-treated rats with skin injury, showed an elevated count of middle-sized lymphocytes and neutrophils that were characterized with well-defined nuclear lobules and rich-granules cytoplasm. Furthermore, the microscopic examinations of the spleen and lymph nodes of myrrh-treated rats with skin injury, showed an increased thickness of lymphatic sheath around the arterioles in the white pulp that was associated with high density of the medium-sized lymphocytes in the secondary lymphoid follicles in the lymph nodes with engorged sinusoids. As myrrh enhanced leukocytes proliferation before injury, it can be concluded that myrrh posse’s antigenic-driven responses and that indicated some foreignness or toxicity of some constituents of myrrh. Because myrrh helped to maintain the relative rise of leukocytes counts throughout healing period and that implied it activated late steps of both proliferation and differentiation pathways for all types of leukocytes during effective phase of the specific immune responses.
Keywords: Commiphora molmol, Herbal product, Light microscopic of leukocytes, Leukocytes proliferations
1. Introduction
Skin injury has been used in experimental animals as model to elicit inflammatory response whereas gastric ulcer involves the degeneration of gastric mucous membranes which is one of the most important tissues that form the first line of defense of the immune system. Leukocytes proliferation, in terms of their increased count, has been used as reliable indicator to make variety of clinical decisions for both the appropriateness of treatment and for surgical intervention. Indeed leukocytes are major cellular component of the immune system and hence their response to proliferate is critical factor in evaluating the effectiveness of the immune system response. On these bases increased interest in herbal medical products and their ability to stimulate the cellular component of the immune system because of its high therapeutic importance. The use of many plants that contain substances for treatment of disease has increased dramatically over the past 15 years all-over the world (Brieskorn and Noble, 1982; Danne et al., 1993; Baumann, 1996; Habeeb et al., 2009). The roots of these interests draw back to the Arabic medicine since ancient times, but the cellular interpretations of the significance still lacking.
Commiphora molmol, known in folklore medicine as “myrrh” is one of the most common herbs in the Saudi society, which has been used in the treatment of hypertension (Abdul-Ghani and Amin, 1997; Ziyyat et al., 1997), hyperlipidemia (Verma and Bordia, 1988), respiratory infections (Tariq et al., 1985; Atta and Alkofahi, 1998), ulcer (Al-Faraj, 1995; Al-Harbi et al., 1997) and cancer (Worthen et al., 1998). Myrrh is the dried resin of several species of Commiphora, Burseraceae, of small trees of the arid and semiarid regions of East Africa, Arabia, and the Indian subcontinent. For many years, myrrh has been used for its healing qualities benefits during injuries (Duwiejua et al., 1993). Clearly, the benefits of using the oleo-gum resin, C. molmol, in the Middle East System of Medicine have been proven in scientific studies (Tariq et al., 1985; Al-Harbi et al., 1997). However, the stimulatory role of myrrh, C. molmol, on the dynamic of the cellular component of the immune system has not been examined nor the role of myrrh on the behavior of leukocytes during healing is known.
In view of the introduction presented above, the present study was designed to examine the effect of myrrh, C. molmol on leukocytes proliferation before and during the healing from gastric ulcer and skin injuries, in attempt, to elucidate its preventive and healing role.
2. Methods
2.1. Plants materials
Myrrh, C. molmol, was purchased from commercial market in Riyadh city, Saudi Arabia. It is thought that, the aerial sources of C. molmol used in this study were collected from Farasan Island of Red Sea (Saudi Arabia) and traded in the market as myrrh resin. The dry weigh of 500 mg was diluted in 1 l water and was offered as drinking water for myrrh-treated-animals. All animals were treated according to standards described in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by National Institute of Health (NIH publications 86-23 revised 1985).
2.2. Animal stock
Wistar albino male rats, aged 7–8 weeks and weighing 150–200 g, were obtained from the Experimental Animal Care Centre, King Saud University, Riyadh, Saudi Arabia. The animals were fed on Purina chow diet were kept under standard conditions of humidity (55 ± 5%), temperature (22 ± 2 °C) and light (12-h light/12-h dark cycle).
2.3. Experimental design
The first group (SIM) served as skin injury but treated with myrrh (500 mg/kg/day), the second served as skin injury (SI) only, the third group served as gastric ulcer and treated with myrrh (GUM), and the fourth groups was gastric ulcer (GU) only. The treatment with myrrh began 4 weeks in SIM and GUM groups prior to injury and continued to the end of the experiments. The baselines control values of leukocytes were recorded, then recorded again on the 1st day of 5th week about 1–2 h prior to injury. The post injury values were recorded on the 4th and the 7th days of the 5th week and lastly on the 4th day the 6th week.
2.4. Skin injury and gastric lesions
Animals of the skin injury groups skin of their planter aspect of their feet were cut superficially and was evaluated as severe skin injury. Whereas animals in the gastric ulcer groups were given 1 ml of necrotic agents, either 80% ethanol, 0.2 M NaOH or 25% NaCl, which are known to produce gastric lesions. In order to assure the existence of gastric ulcer, three animals were killed under anesthesia, using diethyl ether 1 h after treatment with the necrotic agents. The stomach of each of the animals was excised and opened along the greater curvature. After washing with saline the gastric lesions were quantified using a binocular magnifier.
3. Results
3.1. Descriptive findings
3.1.1. Preventive role of myrrh
Myrrh supplement in drinking water for 4 weeks (D1WK5), elevated the average (±SD) count of leukocytes, lymphocytes, neutrophils, monocytes, basophile and eosinophils, from base line control (initial) of 8.54 ± 0.95, 5.19 ± 0.61, 2.46 ± 0.28, 0.051 ± 0.10, 0.19 ± 0.07 and 0.11 ± 0.04 to 18.14 ± 2.44, 11.23 ± 1.47, 5.34 ± 0.86, 0.94 ± 0.14, 0.51 ± 0.08 and 0.19 ± 0.06 (103/mm) in group 1 (SIM, D1Wk5). The corresponding changes in group 3 (GUM, D1Wk5) were elevated from base line control values of 8.83 ± 0.70, 5.31 ± 0.62, 2.49 ± 0.16, 0.53 ± 0.10, 0.18 ± 0.06 and 0.14 ± 0.05 to 19.65 ± 3.68, 11.91 ± 2.27, 6.09 ± 1.24, 1.01 ± 0.19, 0.49 ± 0.12 and 0.21 ± 0.06. There were no observed differences between all types of leukocytes on the 1st day of the 5th week (D1WK6) among groups 1 and 3 nor there were observed differences in all types of leukocytes among groups 2 (SI) and 4 (GU). These results demonstrated that myrrh supplements in drinking water elicited leukocytes activated stimulus for all types of leukocytes proliferation prior to injury. Figs. 1a–1f show that the initial rises in all types of leukocytes in groups 1 and 3 were obvious, as compared with groups 2 and 4.
Figure 1a.
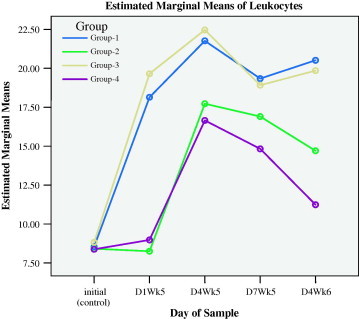
Leukocytes responses during preventive (initial to D1WK5) and healing (D4WK5 to D4WK6) periods.
Figure 1b.
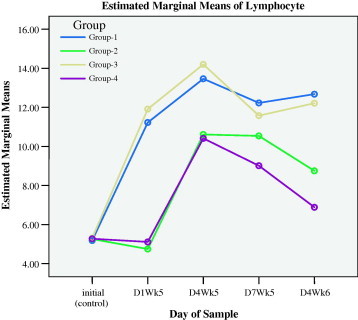
Lymphocytes responses during preventive (initial to D1WK5) and healing (D4WK5 to D4WK6) periods.
Figure 1c.
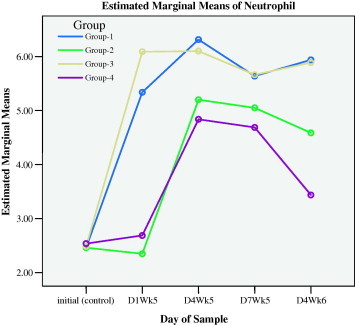
Neutrophils responses during preventive (initial to D1WK5) and healing (D4WK5 to D4WK6) periods.
Figure 1d.
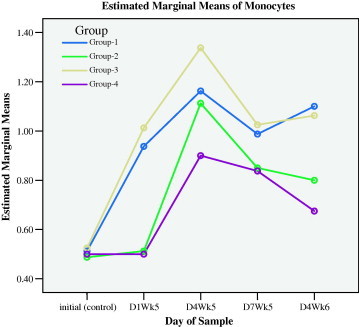
Monocytes responses during preventive (initial to D1WK5) and healing (D4WK5 to D4WK6) periods.
Figure 1e.
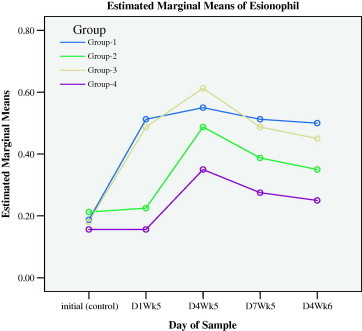
Eosinophils during preventive (initial to D1WK5) and healing (D4WK5 to D4WK6) periods.
Figure 1f.
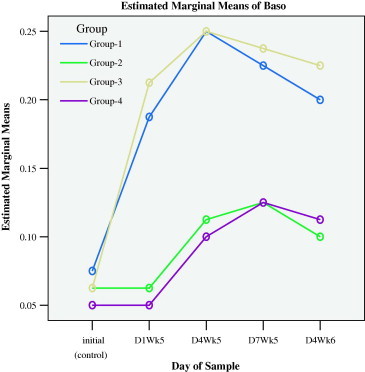
Basophils responses preventive (initial to D1WK5) and healing (D4WK5 to D4WK6) periods.
3.1.2. Healing role of myrrh
The measurements of day 4 during the fifth week (D4WK5), that is 3 days following the skin injury and ulcer injury, showed that all types of leukocytes sustained higher levels in groups 1 and 3 that were supplemented with myrrh as compared with groups 2 and 4. These results demonstrated that myrrh stimulated leukocytes proliferations and acted as anti-inflammatory stimulus during healing. Figs. 1a–1f display the behavior of all types of leukocytes at the initial point, on the 1st day of the 5th week (D1WK5) prior to injury as well as three follow up measurements, with 3 days intervals, during healing from injuries, namely 4th day of the 5th of week (D4WK5), 7th day of the 5th week (D7WK5) and 4th day of the 6th week (D4WK6). As noted from Figs. 1a–1f that all types of leukocytes sustained their rise throughout the healing period in groups 1 and 3 in whom animals were supplemented with myrrh. On the contrary, leukocytes failed to sustain their peaks in groups 2 and 4 in whom animals were not supplemented with myrrh. These findings, along with the observations of a rapid and high quality healing in groups 1 and 3, justified the healing role of myrrh.
3.1.3. Histological findings
3.1.3.1. None-myrrh-treated rats with skin injury
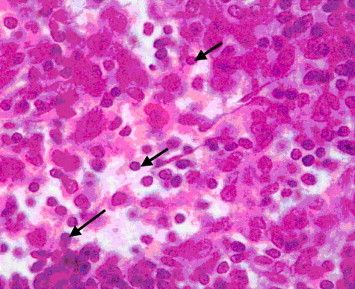
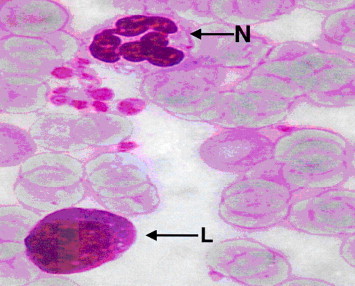
The microscopic examinations of blood smear from rat of group 2 (none-myrrh-treated rats) showed that high population density of immature neutrophils associated with the absence of lobular nucleus but rather with band shape and that signified cellular swelling or necrosis (Fig. 2), as evident by nuclear ring shaped and an overall larger than normal cell size (Fig. 3).
Figure 2.
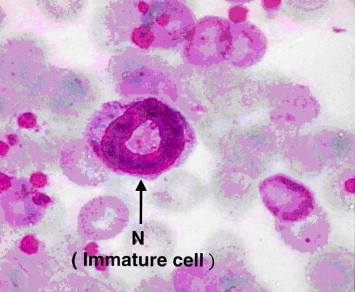
Light microscope of blood smear from none-myrrh-treated rat with skin injury. Note the presence of immature neutrophils (N) with mega ring shaped nucleus. ×100.
Figure 3.
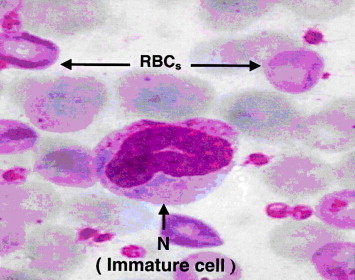
Light microscope of blood smear from none-myrrh-treated rat with skin injury. Note the presence of immature neutrophils (N) without lobular nucleus but rather with band-like shape (disk shape). ×100.
The microscopic examinations of the spleen of none-myrrh-treated rats with skin injury, showed lower number of lymphocytes population in the lymphatic sheath of the spleen and thin layer surrounding the arterioles in the white pulp as well as the absence of secondary lymphoid follicles which were obvious in myrrh-treated rats (Fig. 4). Furthermore microscopic examination of the lymph node in none-myrrh-treated rats showed lesser population density of lymphocytes in the medullar blood sinuses (Fig. 5) and small number of lymphocytes in the germinal centers (sinusoid), as compared with those myrrh-treated-animals (Fig. 6).
Figure 4.
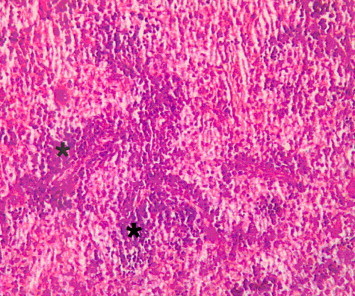
Light microscope photograph of the spleen from none-myrrh-treated rat with skin injury. Note smaller number of lymphocytes surrounding the prearteriolar sheath (*). ×200.
Figure 5.
Light microscope photograph of the lymph node from none-myrrh-treated rat with skin injury. Note smaller numbers of lymphocyte (arrow) in modularly sinuses. ×100.
Figure 6.
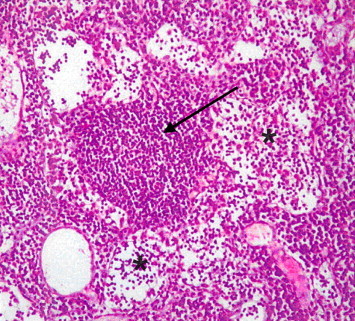
Light microscope photograph of the lymph node from none-myrrh-treated rat with skin injury. Note the formation of small granules (arrow) as an immune response but associated with lesser number of lymphocytes in the germinal centers. ×200.
3.1.3.2. Myrrh-treated rats with skin injury
The microscopic examination of blood smear from myrrh-treated rats, with skin injury, showed an increased number of middle-sized lymphocytes and the presence of relative extensive neutrophils, with well-defined nuclear lobules (Fig. 7) which reflected active chemotaxis. It was also noted in the same blood smear that there were an association between the presence of lymphocytes and neutrophils with complete lobulation of the nucleus and rich-granules cytoplasm, implying complete maturation process (Fig. 7). Besides the observed large middle-sized populations of lymphocytes (Fig. 8), there was subsequent increased population of matured lymphocytes (Fig. 9) with enhanced eurochromatin density associated with invigorated-envelop nuclear shape (Fig. 10). Furthermore, the nucleus of the neutrophils possessed complete lobulation that was associated with relative rich-granules cytoplasm (Fig. 11), as indicative of healthy maturation process.
Figure 7.
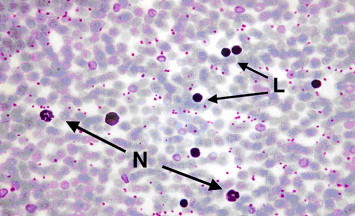
Light microscope of blood smear from myrrh-treated rat with skin injury. Note an increase number of middle-sized lymphocytes (L) and the presence of neutrophils (N) with obvious and normal lobules. ×400.
Figure 8.
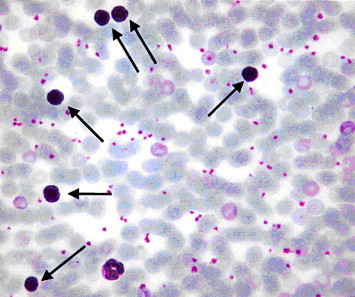
Light microscope of blood smear from myrrh-treated rat with skin injury. Note an increased numbers of middle-sized lymphocyte (arrow). ×400.
Figure 9.
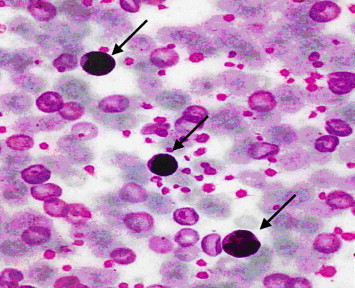
Light microscope blood smear from myrrh-treated rat with skin injury of a rat with skin injury and treated with myrrh. Note an increase in lymphocytes (arrow). ×100.
Figure 10.
Light microscopy of blood smear from myrrh-treated rat with skin injury. Note middle-sized lymphocyte has nucleus with normal density chromatin (u/h) associated with invegetation in the nuclear envelope. ×1000.
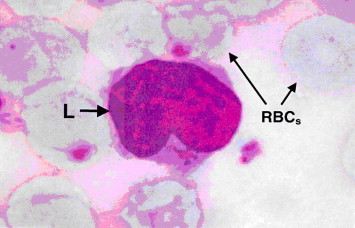
Figure 11.
Light microscope of blood smear from myrrh-treated rat with skin injury. Note, neutrophils (N) with complete nuclear lobulation and maturation. The middle-sized lymphocyte (L) with relative surplus of cytoplasmic granules. ×1000.
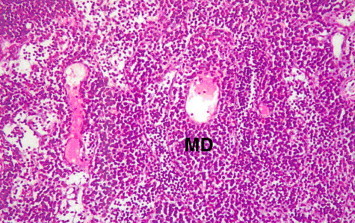
The microscopic examinations of the spleen of myrrh-treated rats with skin injury showed increase in thickness of lymphatic sheath around the arterioles (periarteriolar lymphoid sheath) mainly due to an large lymphocytes population density (Fig. 12). Note also secondary lymphoid follicles (SL) which originate from the main lymphatic sheath showed several changes in the spleen tissue that verified an increase in the efficacy of this lymphatic organ. These changes included; an increase thickness of periarteriolar lymphoid sheaths in the white pulp as a result of an increase in active lymphocytes proliferation within the sheaths that had contained both lymphoblasts (germinal lymphocyte) and the medium-sized lymphocytes as evident by the observed rise in the secondary lymphoid follicles in the main lymphatic sheets (Fig. 13) in the lymph node, with engorged sinusoids because of crowded populations of lymphocytes. Using high-power magnification the details of large-sized lymphocytes became clear to observe plasma cells, antibodies production sites (Fig. 14).
Figure 12.
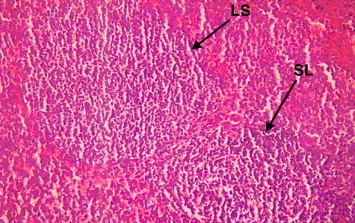
Light microscope of the spleen of myrrh-treated rat with skin injury. Note the thickness of lymphatic sheath (SL) around the arterioles (periarteriolar lymphoid sheath). Note also the thickness of the secondary lymphoid follicles (SL) which originate from the main lymphatic sheath. ×200.
Figure 13.
Light microscope of lymphoid node form myrrh-treated rat with skin injury. Note, the presence of primary germinal center in medulla (MD) of the node. Note also the appearance of secondary germinal centers in the neighboring medullas. ×200.
Figure 14.
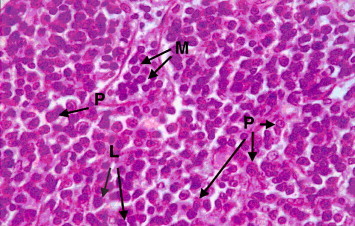
Light microscope photograph at high magnification of the lymph node from myrrh-treated rat with skin injury. Note that the lymphocytes in the secondary germinal centers are large-sized (lymphoblasts) (L) and middle-sized lymphocytes (M) that were presented in large numbers as well as the presence of plasma cells (P). ×400.
4. Discussion
In the present study, we compared myrrh-induced changes in the cellular components of the immune system in animals that were injured with skin and gastric ulcer. The major findings of the present study showed that myrrh supplementation in drinking water inducted proliferation in all types of leukocytes proliferations with high relative rate in lymphocytes, neutrophils and monocytes, respectively. Although the exact mechanisms related to myrrh-induced leukocytes proliferations processes is beyond the work of this study, however, leukocytes proliferation system originates in the bone marrow and after maturation and subsequent activation it undergoes morphological changes that contributed to increasing monocytes and neutrophils populations via meolyed pathway and also contributed to increasing lymphocytes via the lymphoid pathway (MacLennan, 1994; Liccardi et al., 1996). Therefore, myrrh stimulated early steps in maturation pathway for leukocytes. On the other hand, however, the finding of myrrh-induced leukocyte change before injury indicated some toxicity effects. However, treatment with myrrh of 250 and 500 mg/kg/day was found to be cytotoxic in tumors cells also. The anti-tumor potential of myrrh was comparable to the standard cytotoxic drug cyclophosphamide and found to be an effective anti-tumor therapy (Al-Harbi et al., 1994; Worthen et al., 1998). Toxicity of aird plants were reported previously (Keshri et al., 1995; Steinmann et al., 1997; Jacobson and Schlein, 1999). Taking into consideration that the majority of antibiotic drugs have some toxicity aspects, myrrh can be protective against some respiratory infection associated with chronic asthma, bronchitis, catarrh, coughs, gingivitis, mouth ulcers and sore throat and may also help alleviate diarrhea, dyspepsia, flatulence and hemorrhoids. It was shown that myrrh contains antibiotic compounds such as mansumbinone (1), 3,4-seco-mansumbinoic acid (2), β-elemene (3) and T-cadinol (4) have been isolated from the oleo-resin of myrrh (Brieskorn and Noble, 1982; Mukhlesur et al., 1994). The anti-inflammatory (Tariq et al., 1985; Wasfi et al., 1995; Salman et al., 1999) and anti-gastric ulcer and anti-tumor potential of myrrh (Atta and Alkofahi, 1998) were verified. Furthermore, it was shown that myrrh pretreatment at doses of 250, 500 and 1000 mg/kg provided dose-dependent protection against the ulcerogenic effects of different necrotizing agents (Al-Harbi et al., 1997; Yesilada et al., 1999), which was mainly related to the rise in mucus production, non-protein sulfhydryl and DNA. Thus the increase in leukocytes prior to injury of the present study can be attributed to an elevated rate of myrrh-antigen-driven leukocytes proliferation. Furthermore antigen stimulated T cells secrete cytokines that activate macrophages has the potential to undergoes morphological changes.
The current study focused also on the knowledge of the extent to which time (how long) the defense line of the cellular component of the immune system attempt to sustain increasing leukocytes during healing period. It should be noted that the current study, according to available information, is the first scientific assessment regarding the role of myrrh of sustaining leukocytes proliferation throughout the healing period to accomplish rapid and high healing quality. For many years, myrrh has been used for its healing qualities (Duwiejua et al., 1993; Mukhlesur et al., 1994). Clearly both skin injury and gastric ulcer injuries acted as inflammatory stimulus and contributed to macrophages stimulus. It is very important that one realizes that the principal functions of mononuclear phagocytes in innate immune response is triggered by foreign particles such as microbes, antigens, and dead tissue from both skin and ulcer injuries. Thus myrrh-induced mononuclear derived macrophages produced growth factors for fibroblast and vascular endothelium that promoted the rapid and high quality repair of both skin injury and gastric ulcer injured tissues (MacLennan, 1994. Obviously, the rise in lymphocytes throughout healing reported in the present study verified that myrrh activated cell-mediated immunity manifested by T-lymphocytes (Haq et al., 1995, 1999). Once again because the rise in leukocytes were sustained in groups 1 and 3 that were supplemented with myrrh during healing period but not in groups 2 and 4, it would be reasonable to assume that myrrh inducted activation of mononuclear phagocytes which displays foreign antigen, acting as antigen presenting cell which has an antigenic response and can further be recognized by T-lymphocytes throughout the effective phase that accelerated and improved the quality of healing.
Furthermore, the results of the present study showed that myrrh inducted an improvement in the efficacy of the granulacytes participation during healing in groups 1 and 3 that was not observed in groups 2 and 4. It should be pointed out that granulacytes perform major immune response during the effector phase as inflammatory cells. The mechanisms for their activation relies upon pahagotose, opsonized particles and T cell-derived cytokines. Neutrophils (polymorphocytes) are the most numerous and they respond rapidly to chemotactic stimuli, humeral activation and cytokines produced by both macrophages and endothelial cells (MacLennan, 1994; Liccardi et al., 1996; Fernandes-Carlos et al., 1997; Florido et al., 1999). Eosinophils function mainly in defense against certain types of infectious agent and posses receptor for antibody IgE.
5. Conclusions
Myrrh elevated leukocytes without injury which involved an antigenic-driven response and that indicated foreignness of some constituents of myrrh. Myrrh helped to maintain activity of leukocytes proliferation throughout healing which implied that myrrh activated late steps of both proliferation and differentiation pathways for leukocytes during effective phase of healing.
Acknowledgments
This project was supported by, the Office of Research, College of Science, King Saud University – Riyadh; Grant Number 2008/25. Special appreciation is extended to the Dean of the College of Science and the Director of Research Center in the College of Science.
References
- Abdul-Ghani A.S., Amin R. Effect of aqueous extract of Commiphora opobalsamum on blood pressure and heart rate. Journal of Ethnopharmacology. 1997;57:219–222. doi: 10.1016/s0378-8741(97)00063-9. [DOI] [PubMed] [Google Scholar]
- Al-Faraj S. Haemorrhagic colitis induced by Citrullus colocynthis. Annals of Tropical Medicine and Parasitology. 1995;89(6):695. doi: 10.1080/00034983.1995.11813006. [DOI] [PubMed] [Google Scholar]
- Al-Harbi M.M., Qureshi S. Effects of Commiphora molmol (oleo resin) on the cytological and biochemical changes induced by cyclophosphamide in mice. The American Journal of Chinese Medicine. 1994;22:77–82. doi: 10.1142/S0192415X94000103. [DOI] [PubMed] [Google Scholar]
- Al-Harbi M.M., Qureshi S., Raza M.M., Ahmed M., Afzalc M., Shah A.H. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. Journal of Ethnopharmacology. 1997;55(2):141–150. doi: 10.1016/s0378-8741(96)01488-2. [DOI] [PubMed] [Google Scholar]
- Atta A.H., Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. Journal of Ethnopharmacology. 1998;60:117–124. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- Baumann, H., 1996. The Greek Plant World in Myth, Art and Literature (W.T. Stearn, E.R. Stearn, Trans.). Timber Press, Portland.
- Brieskorn G.H., Noble P. Constituents of the essential oil of myrrh, part 2. Sesquiterpenes and furanosesquiterpenes. Planta Medica. 1982;44:87–90. doi: 10.1055/s-2007-971408. [DOI] [PubMed] [Google Scholar]
- Danne A., Peterett F., Nahrstedt A. Proanthocyanidins from Cistus incanus. Phytochemistry. 1993;34(4):1129–1133. [Google Scholar]
- Duwiejua M., Zeitlin I.J., Waterman P.G., Chapman J., Mhango G.J., Provan G.J. Anti-inflammatory activity of resins from some species of the plant family Burseraceae. Planta Medica. 1993;59(1):12–16. doi: 10.1055/s-2006-959594. [DOI] [PubMed] [Google Scholar]
- Fernandes-Carlos T., Riondel J., Glise D., Guiraud P., Favier A. Modulation of natural killer cell functional activity in athymic mice by beta-carotene, oestrone and their association. Anticancer Research. 1997;17(4a):2523–2528. [PubMed] [Google Scholar]
- Florido J.F., Delgado P.G., de San Pedro B.S., Quiralte J., de Saavedra J.M., Peralta V., Valenzuela L.R. High levels of Olea europaea pollen and relation with clinical findings. International Archives of Allergy and Immunology. 1999;119(2):133–137. doi: 10.1159/000024188. [DOI] [PubMed] [Google Scholar]
- Habeeb S., El-namaky A.H., Salama M.A. Efficiency of Alllium cepa and Commiphora molmol as larvicidal agent against fourth stage larva of Cullex pipiens (Diptera: Culicidae) American–Eurasian Journal of Agricultural and Environment Science. 2009;5(2):196–203. [Google Scholar]
- Haq A., Abdullatif M., Lobo P.I., Khabar K.S.A., Sheth K.V., Al-Sedairy S.T. Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity. Immunopharmacology. 1995;30(2):147–155. doi: 10.1016/0162-3109(95)00016-m. [DOI] [PubMed] [Google Scholar]
- Haq A., Lobo P.I., Al-Tufail M., Rama N.R., Al-Sedairy S.T. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. International Journal of Immunopharmacology. 1999;21(4):283–295. doi: 10.1016/s0192-0561(99)00010-7. [DOI] [PubMed] [Google Scholar]
- Jacobson R.L., Schlein Y. Lectins and toxins in the plant diet of Phlebotomus papatasi (Diptera: Psychodidae) can kill Leishmania major promastigotes in the sandfly and in culture. Annals of Tropical Medical Parasitology. 1999;93(4):351–356. doi: 10.1080/00034989958348. [DOI] [PubMed] [Google Scholar]
- Keshri G., Singh M.M., Lakshmi V., Kamboj V.P. Post-coital contraceptive efficacy of the seeds of Nigella sativa in rats. Indian Journal of Physiological Pharmacology. 1995;39(1):59–62. [PubMed] [Google Scholar]
- Liccardi G., D’Amato M., D’Amato G. Oleaceae pollinosis: a review. International Archives of Allergy and Immunology. 1996;111(3):210–217. doi: 10.1159/000237370. [DOI] [PubMed] [Google Scholar]
- MacLennan I.C.M. Germinal centers. Annual Review of Immunology Today. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Mukhlesur M.R., Garvey M., Piddock L.J.V., Gibbons S. Antibacterial terpenes from the oleo-resin of Commiphora molmol, Engl. Chemotherapy. 1994;40(5):337–347. doi: 10.1002/ptr.2501. [DOI] [PubMed] [Google Scholar]
- Salman H., Bergman M., Bessler H., Punksy I., Djaldetti M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. International Journal of Immunopharmacology. 1999;21(9):589–597. doi: 10.1016/s0192-0561(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Steinmann A., Schatzle M., Agathos M., Breit R. Allergic contact dermatitis from black cumin (Nigella sativa) oil after topical use. Contact Dermatitis. 1997;36(5):268–269. doi: 10.1111/j.1600-0536.1997.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Tariq M., Ageel A.M., A-Yahia M.A. Anti-inflammatory activity of Commiphora molmol. Agents and Actions. 1985;17:381–382. doi: 10.1007/BF01982655. [DOI] [PubMed] [Google Scholar]
- Verma S.K., Bordia A. Effect of Commiphora mukul (gum guggulu) in patients of hyperlipidemia with special reference to HDL-cholesterol. Indian Journal of Medical Research. 1988;87:356–360. [PubMed] [Google Scholar]
- Wasfi I.A., Bashir A.K., Abdala A.A., Bannna N.R., Tanir M.O.M. Anti-inflammatory activity of some medicinal plants of the United Arab Emirates. International Journal of Pharmacognosy. 1995;33(2):124–128. [Google Scholar]
- Worthen D.R., Ghosheh O.A., Crooks P.A. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Research. 1998;18(3A):1527–1532. [PubMed] [Google Scholar]
- Yesilada E., Gurbuz I., Shibata H. Screening of Turkish anti-ulcerogenic folk remedies for anti-Helicobacter pylori activity. Journal of Ethnopharmacology. 1999;66(3):289–293. doi: 10.1016/s0378-8741(98)00219-0. [DOI] [PubMed] [Google Scholar]
- Ziyyat A., Legssyer A., Mekhfi H., Dassouli A., Serhrouchni M., Benjelloun W. Phytotherapy of hypertension and diabetes in oriental Morocco. Journal of Ethnopharmacology. 1997;58(1):45–54. doi: 10.1016/s0378-8741(97)00077-9. [DOI] [PubMed] [Google Scholar]


