Abstract
Staphylococcus aureus and Pseudomonas aeruginosa are rapidly increasing as multidrug resistant strains worldwide. In nosocomial settings because of heavy exposure of different antimicrobials, resistance in these pathogens turned into a grave issue in both developed and developing countries. The aim of this study was to investigate in vitro antibiotic synergism of combinations of β-lactam–β-lactam and β-lactam–aminoglycoside against clinical isolates of S. aureus and P. aeruginosa. Synergy was determined by checkerboard double dilution method. The combination of amoxicillin and cefadroxil was found to be synergistic against 47 S. aureus isolates, in the FICI range of 0.14–0.50 (81.03%) followed by the combination of streptomycin and cefadroxil synergistic against 44 S. aureus isolates in the FICI range of 0.03–0.50 (75.86%). The combination of streptomycin and cefadroxil was observed to be synergistic against 39 P. aeruginosa isolates in the FICI range of 0.16–0.50 (81.28%). Further actions are needed to characterize the possible interaction mechanism between these antibiotics. Moreover, the combination of streptomycin and cefadroxil may lead to the development of a new and vital antimicrobial against simultaneous infections of S. aureus and P. aeruginosa.
Abbreviations: MIC, minimum inhibitory concentration; FICI, fractional inhibitory concentration index; MRSA, methicillin resistant Staphylococcus aureus
Keywords: Antimicrobial, Synergism, S. aureus, P. aeruginosa
1. Introduction
The current clinical scenario indicates that millions of people are becoming infected with nosocomial infection leading to numerous deaths annually worldwide and to heavy economic burden in both developed and developing countries (WHO, 2002). Emerging trends in nosocomial infections signal high alerts towards multidrug resistant pathogens. Studies show that 70% of nosocomial infections are due to antibiotic resistant strains (Burke, 2003; Safdar et al., 2001). Major causative agents include antibiotic resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa. Both of these organisms are among the most common isolates from burnt patients especially under nosocomial conditions (Haghi et al., 2010).
In an increasing number of microbial infections of man, treatment with single antibiotic fails to cure. Some of these infections, however, respond favourably to combined treatment with two antibiotic drugs. Certain pairs of these drugs have proved successful in a limited number of clinical situations. The clinical significance of in vitro synergism has not yet been defined in the treatment of many infections. Therefore, this study was undertaken to investigate the effectiveness of combinations of antibiotics against clinical isolates of S. aureus and P. aeruginosa which are synergistic in vitro. It will be shown that the use of two antibiotics that are synergistic in vitro against the microorganism responsible for the infection may be associated with a significantly better outcome than that achieved with a combination which is not synergistic for the offending microorganism. Synergistic activity of antimicrobials cannot be assumed and should be tested for, prior to commencing treatment with a combination regimen (Prinsloo et al., 2008). Therefore this study was aimed to assess the in vitro synergy of three antibiotics against 58 clinical isolates of S. aureus and 48 clinical isolates of P. aeruginosa. The manuscript does not contain experiments using animals and human studies.
2. Material and methods
2.1. S. aureus and P. aeruginosa isolates
A total of 58 S. aureus and 48 P. aeruginosa isolates included in the present study were taken from the collection of the Vishakha Clinical Microbiology Laboratory, Nagpur, India. The clinical isolates were from different specimens like pus, swab, sputum, blood and wound discharge of patients attending the health centres of Nagpur district. Their species identity was confirmed by biochemical tests including catalase, coagulase, and by growth characteristics on Mannitol salt agar for S. aureus, and Glucose utilization, Indole, MR (Methyl red), VP (Voges-Proskauer), Catalase, Oxidase, and by growth characteristics on Cetrimide agar for P. aeruginosa.
2.2. Antimicrobial agents
Antimicrobial agents, namely amoxicillin and streptomycin were procured from Himedia Laboratories Pvt. Ltd., Mumbai, India, cefadroxil was procured from IFPRESS, Mumbai, India and ampicillin (Roscillin) was procured from Ranbaxy. All drugs were dissolved in their respective solvents and diluted in deionized water and filtered through 0.22 μ Millipore filter. Drug stocks were stored at −20 °C.
2.3. Susceptibility test
Antibiotics susceptibility test was done for all isolates of S. aureus and P. aeruginosa, using Kirby-Bauer disc diffusion method (Bauer et al., 1966). Antibiotic discs were obtained from Himedia Laboratories Pvt. Ltd., Mumbai including amoxicillin (10 μg), ampicillin (10 μg), streptomycin (10 μg) and cefadroxil (30 μg). The diameters of the zones of inhibition were interpreted in Tables 2A through 2I (Zone Diameter Interpretative Standards and equivalent Minimum Inhibitory Concentration Breakpoints) of the NCCLS M100-S12 (2002), organisms were reported as susceptible, intermediate or resistant to the agents that have been tested.
2.4. MIC and FICI determination
In vitro checkerboard studies on the activity of antibiotics alone and in combination were performed in Muller Hinton broth (Himedia Ltd.) in tube dilution. Twofold dilutions (0.125–256 μg ml−1) of each drug or drug combination were tested in two rows. One row was inoculated with 105 organisms/ml of the test organism and the second row was inoculated with the control organism. Results were read after tubes were incubated at 37 ± 2 °C for 18 h. MIC (Minimum inhibitory concentration) is determined as the lowest concentration of the drugs (alone or in combination) that inhibit growth. The fractional inhibitory concentration index (FICI) is defined as the sum of the MIC of each drug when used in combination divided by the MIC of the drug used alone. Synergistic effect was recorded when FIC indexes ⩽0.5; partial synergy when FIC >0.5 but <1.0; additive when FIC = 1.0; indifferent when FIC >1.0 but <4.0 and antagonistic when FIC ⩾4.0 (Cai et al., 2007).
3. Results
The in vitro results of interaction of cefadroxil with amoxicillin, ampicillin and streptomycin are presented in Table 1. Disc diffusion test results showed that about 90% S. aureus isolates were resistant to amoxicillin and ampicillin, however both of these antibiotics were found to be ineffective against all P. aeruginosa isolates (100% resistance). About 33% and 36% S. aureus isolates and about 29% and 23% P. aeruginosa isolates were susceptible to cefadroxil and streptomycin respectively.
Table 1.
In vitro interaction of cefadroxil with amoxicillin, ampicillin and streptomycin against S. aureus and P. aeruginosa isolates.
| Antibiotic tested | Sensitivity obtained with antibiotic alone against S. aureus (n) (%) | Antibiotic in combination with cefadroxil against S. aureus (n = 58) |
Antibiotic tested | Sensitivity obtained with antibiotic alone against P. aeruginosa (no. of isolates) (%) | Antibiotic in combination with Cefadroxil against P. aeruginosa (n = 48) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SN (%) | PS (%) | AD (%) | IN (%) | AN (%) | SN (%) | PS (%) | AD (%) | IN (%) | AN (%) | ||||
| Amoxicillin | 13.79 (8) | 81.03 | 5.17 | 5.17 | 8.62 | 0 | Amoxicillin | 0 (0) | 14.58 | 47.92 | 10.42 | 27.08 | 0 |
| Ampicillin | 8.62 (5) | 17.24 | 22.41 | 18.97 | 41.38 | 0 | Ampicillin | 0 (0) | 29.17 | 39.58 | 18.75 | 12.50 | 0 |
| Streptomycin | 36.21 (21) | 75.86 | 12.07 | 1.72 | 10.34 | 0 | Streptomycin | 22.92 (11) | 81.28 | 2.08 | 0 | 16.67 | 0 |
| Cefadroxil | 32.76 (19) | - | - | - | - | - | Cefadroxil | 29.17 (14) | - | - | - | - | - |
(a) SN: synergy, (b) PS: partial synergy, (c) AD: additive, (d) IN: indifference, (e) AN: ANTAGONISM.
The results of checkerboard analysis illustrated (Table 1) that the combination of cefadroxil and amoxicillin was most effective against S. aureus isolates with 81.03% (47) synergism (FIC index 0.14–0.50) and less than 10% of other effects. FIC value distribution among 58 S. aureus isolates is presented in Fig. 1. The same combination of antibiotics found to be marginally synergistic i.e. 14.58% (7) (FIC index 0.09–0.38) but higher side towards partial synergism 47.92% (23) (FIC index 0.52–0.75) and indifference 27.08% (13) (FIC index 1.06–2.06), is presented in Fig. 2.
Figure 1.
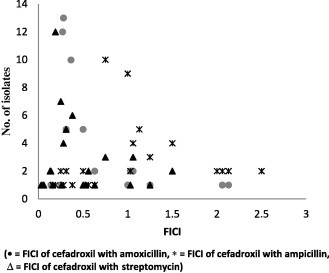
FICI of antibiotic combinations against S. aureus isolates.
Figure 2.
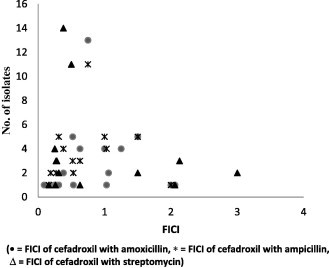
FICI of antibiotic combinations against P. aeruginosa isolates.
FIC index interpretation for activities of cefadroxil with ampicillin against S. aureus isolates predominantly showed indifference 41.38% (24) (FIC index 1.03–2.5) and same combination of antibiotics against P. aeruginosa isolates predominantly showed partial synergism 39.58% (19) (FIC index 0.52–0.75).
Cefadroxil combined with streptomycin produced predominant synergism 75.86% (44) (FIC index 0.03–0.50) against S. aureus isolates and maximum synergism i.e. 81.28% (39) (FIC index 0.16–0.50) against P. aeruginosa isolates with least other effects.
No antagonism with any combination of antibiotics was observed in the present study.
4. Discussion
Infections caused by S. aureus and P. aeruginosa are increasing both in hospitals and in general community (Hauser and Sriram, 2005; Maltezou and Giamarellou, 2006). The efficacy of many antibiotics for treatment of severe infections has become quite limited due to the development of resistance. The results from this in vitro study with 58 S. aureus clinical isolates for their susceptibility against different antibiotics indicated 8.62% and 13.79% sensitivity to ampicillin and amoxicillin respectively. Cefadroxil and streptomycin were found to be effective against 32.76% and 36.21% isolates. Our results also substantiate previous study of Adegoke and Komolafe (2009). Penicillins and Cephalosporins are the β-lactam antibiotics which inhibit cell wall synthesis. Resistance against these antibiotics revealed that the resistance is purely plasmid based since β-lactamase production is plasmid based (Rigby, 1986). Kondo et al. (1991) had reported good activities of streptomycin (MIC 1.56–6.25 μg/ml) against all tested strains of MRSA. The present study showed that 62.07% strains of S. aureus were resistant to streptomycin.
The in vitro study with 48 clinical isolates of P. aeruginosa exhibited various degrees of inhibition against different antibiotics. Total 100% resistance was observed against amoxicillin and ampicillin. Whereas, 70.83% and 77.08% resistance was observed against cefadroxil and streptomycin respectively, earlier studies also showed similar findings (Anjum and Mir, 2010). The multidrug resistance among P. aeruginosa isolates may involve reduced cell wall permeability, production of chromosomal and plasmid mediated β-lactamases (Livermore, 1989), aminoglycoside-modifying enzymes (Livermore, 1987), and an active multidrug efflux mechanism (Shahid and Malik, 2004).
The rational for combination therapy is essential to reduce the chances of selection of resistant mutants during therapy, as well as to exploit the potential synergistic activity of some agents. We observed that the combination of cefadroxil and amoxicillin (both are β-lactam drugs) was most synergistic against S. aureus inhibiting more than 80% isolates. Previous studies also illustrated synergism between two β-lactam antibiotics against different organisms (Gutmann et al., 1986; Pasticci et al., 2008; Jones and Johnson, 1998; Matsumoto, 1998; Tascini et al., 2004; Desbiolles et al., 2001).
Against P. aeruginosa, the combination of cefadroxil and streptomycin (β-lactam and aminoglycoside) was found to be most effective, showed more than 80% inhibition. It was also observed that this combination was also synergistic against more than 75% isolates of S. aureus.
Gram-negative bacteria such as P. aeruginosa are often simultaneously isolated from patients with MRSA infections (Konno, 1995). Therefore, monotherapy with agents with activities against gram-positive bacteria, such as vancomycin, is not effective against polymicrobial infections caused by both MRSA and P. aeruginosa (Shimizu et al., 1996). A better approach to the development of a new antibiotic combination would be to aim for broad and potent activities against a wide range of Gram-positive and Gram-negative bacteria including S. aureus and P. aeruginosa. Our results demonstrated that the combination of cefadroxil and streptomycin was synergistic against both S. aureus and P. aeruginosa.
5. Conclusion
Our data clearly state that the use of synergistic combinations for therapy of polymicrobial infections and infection of multidrug resistant strains is coupled with a better clinical response than the use of nonsynergistic combinations. Each situation in which it seems prudent or necessary to use antibiotics in combination must be carefully evaluated. The dangers of toxicity, sensitization, superinfection, or increase in resistant organisms that may follow the use of an additional antibiotic, should be minimized. However, the mortality in severe nosocomial infections is extremely high, especially in debilitated patients, and the use of synergistic combinations of antibiotics might prove to be a valuable means for reducing this high mortality rate. Therefore, when one is dealing with a serious infection presumably caused by S. aureus and P. aeruginosa, it might be useful to test routinely combinations of antibiotics for a potential synergistic action. Routine use of combinations of antibiotics should, however, be practiced with caution after prior in vitro trials.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adegoke A.A., Komolafe A.O. Multi-drug resistant Staphylococcus aureus in clinical cases in Ile-Ife, Southwest Nigeria. Int. J. Med. Sci. 2009;1:68–72. [Google Scholar]
- Anjum F., Mir A. Susceptibility pattern of Pseudomonas aeruginosa against various antibiotics Afr. J. Microbiol. Res. 2010;4:1005–1012. [Google Scholar]
- Bauer R.W., Kirby M.D.K., Sherris J.C., Turck M. Antibiotic susceptibility testing by standard single disc diffusion method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Burke J.P. Infection Control—A Problem for Patient Safety. New Engl. J. Med. 2003;348:651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- Cai Y., Wang R., Pei F., Liang B. Antibacterial activity of Allicin alone and in combination with β-lactams against Staphylococcus spp. and Pseudomonas aeruginosa. J. Antibiot. 2007;60:335–338. doi: 10.1038/ja.2007.45. [DOI] [PubMed] [Google Scholar]
- Desbiolles N., Piroth L., Lequeu C., Neuwirth C., Portier H., Chavanet P. Fractional maximal effect method for in vitro synergy between amoxicillin and ceftriaxone and between vancomycin and ceftriaxone against Enterococcus faecalis and penicillin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2001;45:3328–3333. doi: 10.1128/AAC.45.12.3328-3333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Kitiz M.D., Acar J.F. Synergism and antagonism in double beta-lactam antibiotic combinations. Am. J. Med. 1986;30:21–29. [PubMed] [Google Scholar]
- Haghi M., Maadi H., Delshad R., Nezhady M.A.M. Antibiotic resistance pattern of Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa isolated from burnt patients Urmia. Iran. Inter. J. Aca. Res. 2010;2:377–380. [Google Scholar]
- Hauser A.R., Sriram P. Severe Pseudomonas aeruginosa infections. Tackling the conundrum of drug resistance. Postgrad. Med. 2005;117:41–48. doi: 10.3810/pgm.2005.01.1571. [DOI] [PubMed] [Google Scholar]
- Jones R.N., Johnson D.M. Combinations of orally administered beta-lactams to maximize spectrum and activity against drug-resistant respiratory tract pathogens: I. Synergy studies of amoxicillin and cefixime with Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 1998;31:373–376. doi: 10.1016/s0732-8893(98)00011-x. [DOI] [PubMed] [Google Scholar]
- Kondo S., Ikeda Y., Hattori S., Hamada M. Susceptibility of methicillin-resistant Staphylococcus aureus to various antibiotics. Classification by aminoglycoside-modifying enzymes and antibiotics active against MRSA. Jpn. J. Antibiot. 1991;44:1211–1215. [PubMed] [Google Scholar]
- Konno M. Nosocomial infections caused by methicillin-resistant S. aureus in Japan. J. Infect. Chemother. 1995;1:30–39. [Google Scholar]
- Livermore D.M. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur. J. Clin. Microbiol. 1987;6:439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- Livermore D.M. Role of Beta-lactamase and impermeability in the resistance of P. aeruginosa. Antibiot. Chemother. 1989;42:257–263. doi: 10.1159/000417628. [DOI] [PubMed] [Google Scholar]
- Maltezou H.C., Giamarellou H. Community-acquired methicillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents. 2006;27:87–96. doi: 10.1016/j.ijantimicag.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y. Combination cefixime/amoxicillin against penicillin-resistant Streptococcus pneumonia infection. Chemotherapy. 1998;44:6–9. doi: 10.1159/000048456. [DOI] [PubMed] [Google Scholar]
- National committee for Clinical Laboratory Standards, 2002. Performance standard for antimicrobial susceptibility testing; Twelfth Information Supplement. NCCLS Document M100–S12.
- Pasticci M.B., Mencacci A., Moretti A., Palladino N. In vitro Antimicrobial Activity of Ampicillin-Ceftriaxone and Ampicillin-Ertapenem Combinations Against Clinical Isolates of Enterococcus faecalis with High Levels of Aminoglycoside Resistance. Open Microbiol. J. 2008;2:79–84. doi: 10.2174/1874285800802010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsloo A., Straten V.A.M.S., Weldhagen G.F. Antibiotic synergy profiles of multidrug-resistant Pseudomonas aeruginosa in a nosocomial environment. South Afr. J. Epidemiol. Infect. 2008;2:07–09. [Google Scholar]
- Rigby A. Thermonuclease testing: the rapid identification of Staphylococcus aureus in blood culture Med. Lab. Science. 1986;43:196–198. [PubMed] [Google Scholar]
- Safdar N., Crnich C.J., Maki D.G. Nosocomial infections in the intensive care unit associated with invasive medical devices. Curr. Infect. Dis. Rep. 2001;1:487–495. doi: 10.1007/s11908-001-0085-5. [DOI] [PubMed] [Google Scholar]
- Shahid M., Malik A. Plasmid mediated amikacin resistance in clinical isolates of Pseudomonas aeruginosa. Ind. J. Med. Microbiol. 2004;22:182–184. [PubMed] [Google Scholar]
- Shimizu K., Orizu M., Kanno H., Kitamura S. Clinical studies on vancomycin in the treatment of MRSA infection. Jpn. J. Antibiot. 1996;49:782–799. [PubMed] [Google Scholar]
- Tascini C., Doria R., Leonildi A., Martinelli C., Menichetti F. Efficacy of the combination ampicillin plus ceftriaxone in the treatment of a case of enterococcal endocarditis due to Enterococcus faecalis highly resistant to gentamicin: efficacy of the “ex vivo” synergism method. J. Chemother. 2004;16:400–403. doi: 10.1179/joc.2004.16.4.400. [DOI] [PubMed] [Google Scholar]
- Prevention of hospital-acquired infections A practical guide 2nd edition, WHO/CDS/CSR/EPH/2002.12.



