Abstract
The present study aimed to investigate, the larvicidal, adult emergence inhibition and oviposition deterrent activity of aqueous leaves extract of Calotropis procera against Anopheles arabiensis and Culex quinquefasciatus as natural mosquito larvicide. The larvicidal activity was monitored against 2nd, 3rd and 4th instar larvae of each mosquito species 24 h post-treatment. Adult emergence inhibition activity was tested by exposing 3rd instar larvae of each mosquito species to different concentrations of extracts (200, 400, 600, 800 and 1000 ppm for An. arabiensis and 100, 200, 300, 400, 500 and 600 ppm for Cx. quinquefasciatus). Probit analysis was used to analyze data from bioassay experiments. The oviposition deterrent activity was tested by using three different concentrations of extracts (1000, 500 and 200 for An. arabiensis, and 1000, 500 and 100 for Cx. quinquefasciatus) that caused high, moderate and low larval mortality in the larvicidal experiment against 3rd instar larvae. It was found that, LC50–LC90 values calculated were 273.53–783.43, 366.44–1018.59 and 454.99–1224.62 ppm for 2nd, 3rd and 4th larval instars, respectively, of An. arabiensis and 187.93–433.51, 218.27–538.27 and 264.85–769.13 ppm for 2nd, 3rd and 4th larval instars, respectively, of Cx. quinquefasciatus. Fifty percent of adult emergence inhibition (EI50) was shown at 277.90 and 183.65 ppm for An. arabiensis and Cx. quinquefasciatus, respectively. The pupal stage was not affected till a concentration of 5000 ppm. The extract showed oviposition deterrence and effective repellence against both mosquito species at different concentrations, with the observation on that maximal eggs were laid in low concentration of extract. These results suggest that the leaves extract of C. procera possess remarkable larvicidal, adult emergence inhibitor, repellent and oviposition deterrent effect against both An. arabiensis and Cx. quinquefasciatus, and might be used as natural biocides for mosquito control.
Keywords: Calotropis procera, Mosquito control, Anopheles arabiensis, Culex quinquefasciatus, Larvicidal, Oviposition deterrents
1. Introduction
Mosquitoes (Diptera, Culicidae) are responsible for transmitting the most important vector-borne diseases, above all malaria, lymphatic filariasis, Japanese encephalitis, and dengue as well as yellow fever and other forms of encephalitis (WHO, 2006a). Malaria and filariasis rank amongst the world most prevalent tropical infectious diseases. An estimated 300–500 million people are infected with malaria annually, resulting in 1.5–3 million deaths (WHO, 2000). Malaria remains a major health problem in Sudan. Accordingly about 20–40% of out patient clinic visits and approximately 30% of total hospital admissions are due to malaria (WHO and UNICEF, 2005). Lymphatic filariases (LF) is probably the fastest spreading insect-borne disease of human in the tropic, about 30% (394 million) of the global population are estimated to be in the LF-endemic countries of the African region (WHO, 2006b). Lymphatic filariasis is a significant public health and economic problem in many tropical and subtropical regions of the world, including Sudan (Satti and Abdel Nur, 1974; El setouhy and Ramzy, 2003; Aiah et al., 2005). One of the effective methods to control these diseases is to target the vectors for the interrupting disease transmission. The control effort can target all stages of the mosquito life cycle, but has focused almost on adult stage by using conventional insecticides based on indoor residual house spraying (Manzava et al., 1993; Curtis, 1994) or more recently, the use of insecticide treated bed nets or curtains. The control of mosquito at the larval stage is necessary and efficient in integrated mosquitos’ management. During the immature stage, mosquitoes are relatively immobile; remaining more concentrated than they are in the adult stage (Rutledge et al., 2003). Larval control strategies against malaria vectors in sub-Saharan Africa could be highly effective, complementary to adult control interventions, and should be prioritized for further development, evaluation and implementation as an integral part of rolling back malaria (Killeen et al., 2002). Since the discovery of DDT, mosquito control approach has been almost completely based on synthetic organic insecticides. But the extensive use of synthetic organic insecticides during the last five decades have resulted in environmental pollution and also in the development of physiological resistance in major vector species in addition to the increased costs of insecticides. This has necessitated the need for search and development of environmentally safer, low cost, indigenous methods for vector control. During the last decade, various studies on natural plant products against mosquito vectors indicate them as possible alternatives to synthetic chemical insecticides (Mittal and Subbarao 2003; Rajkumar and Jebanesan, 2005a,b; Promsiri et al., 2006). In addition to application as general toxicant against mosquito larvae, botanical insecticides also have potential uses as growth and reproduction inhibitors, repellents, ovicidal and oviposition deterrents (Prajapati et al., 2005; Rajkumar and Jebanesan 2005a,b; Pushpanathan et al., 2006). Calotropis procera R. Br. (Asclepiadaceae) is a plant widely distributed in tropical and subtropical regions of Africa and Asia with a long history of use in traditional medicine. A wide range of chemical compounds including cardiac glycosides, flavonoids, phenolic compounds, terpenoides have been isolated from this species (Mueen Ahmed et al., 2005). The bioactive constituents of these plants could be either a single substance or a mixture of substances. The separation of the mixture is neither practical nor advantageous in the insect economic control strategies. The aim of the current study is to investigate the activity of aqueous leaves extract of C. procera against the larval stages of Anopheles arabienses, the main malaria vector and lymphatic filariasis as well in Sudan (WHO, 2005a) and Culex quinquefasciatus, the vector of filariasis (WHO, 2006a), and the subsequent effects of the extracts on adult emergence, and oviposition deterrent, as natural biocide for mosquito control.
2. Materials and methods
2.1. Area of study
The targeted area for this study is Shambat Village which lies in the western part of Khartoum North town, on eastern bank of the River Nile between latitude 15.40N and longitude 32.32E. The period of the study was from June 2005 to September 2007.
2.2. Collection and rearing of mosquitoes
Larvae of the mosquito were collected from breeding sites within the study area, and reared under laboratory condition at 25–28 °C. The larvae were fed by adding finely ground powdered yeast on the surface of the water. Water was changed every day to avoid scum formation; which might create toxicity. Pupae were collected daily, and transferred to small bowls containing clean water. The bowls were placed in cage 30 × 30 × 30 cm covered with mosquito net for adult emergence. From the day of emergence, Adult mosquitoes were provided with cotton soaked with a 10% sugar solution as a carbohydrate source. On the third day post emergence from pupae, the female mosquitoes were fed on pigeons for at least 10 h during the night. On the following day, Petri-dishes provided with moist cotton or filter papers were fitted at the bottom of each cage for oviposition. To rear larvae for toxicity assays single egg rafts were placed in a number of 2 l plates (30 cm diameter) containing 1 l of de-chlorinated tap water for hatching. The life cycle continued as mentioned above.
2.3. Preparation of extract
Leaves of the plant C. procera (Ait), (Family: Ascelpiadaceae), were collected from plants within the study area, during the flowering season, dried under shade and finely ground to powder. Five grams from leaves powder was soaked in separate bottle (500 ml) containing 250 ml distilled water. The solution was allowed to stand for 24 h with vigorous occasional shaking, the suspension was filtered with filter paper. The marc was washed several times with distilled water and filtered. The final volume was adjusted to 500 ml by adding distilled water to prepare stock solution of 1%. The stock solution was then serially diluted by add water to prepare the test concentrations required.
2.4. Larvicidal and pupicidal bioassay
Larvicidal and pupicidal activities of the extract were determined by following the WHO standard procedure (WHO, 2005b). Initially, mosquito larvae were exposed to a wide range of test concentrations and a control to find out the activity range of the aqueous extract of plant under test. After determining the mortality of larvae in this wide range of concentrations, a narrower range of 5–6 concentrations was used, to determine the lethal concentration of 50% (LC50) and the lethal concentration of 90% (LC90) values. Twenty-five laboratory reared 2nd, 3rd and 4th instars larvae, and twenty-five pupae of each mosquito species were transferred by means of dropper to the small test cups (250 ml), each containing 100 ml of de-chlorinated tap water to which the required concentration were added. Four replicates were setup for each test concentration. In each replicate 25 larvae were used, with four replicate of control. The experiment was performed under laboratory conditions at 25–28 °C. To determine pupicidal activity, the mouth of each cup containing pupae was covered with mosquito net to prevent the escape of any emerged adult mosquitoes. Mortality in larvae and pupae was recorded 24 h post-treatment. If more than 10% of the control larvae pupate in the course of the experiment, the test is discarded and repeated. If the control mortality is between 5% and 20%, the mortalities of treated groups should be corrected according to Abbott (1925) formula.
2.5. Adult emergence inhibition (EI) bioassay
Only 3rd instars larvae were used. The method of the larvicidal activity was followed. Because of the long duration of the test the larvae were fed by yeast at two days intervals until mortality counts were made. The yeast powder was prepared as stock suspension in water from which one or two drops added per cup. All the treated and control cups containing pupae were kept separately in the net cage to prevent successfully emerged adults from escaping into the environment. Mortality of the larvae and pupae was recorded at 24 h intervals. Observation was continued in treated and control cups (de-chlorinated tap water) until the complete emergence of adults. At the end of observation period, the impact is expressed as EI% based on the number of larvae that do not develop successfully into viable adults. In recording EI% for each concentration, moribund and dead larvae and pupae, as well as adult mosquitoes not completely separated from the pupal case, were considered as dead. The experiments stop when all the larvae or pupae in the controls have died or emerged as adults.
2.6. Statistical analysis
Data were subjected to probit analysis. The regression equation (Y = a + bx), lethal concentration that killed 50% and 90% of the population (LC50–LC90), fiducial limit (FL) with 95% confidence limit (CL) and regression coefficient (r2) were calculated.
2.7. Oviposition deterrent bioassay
The oviposition deterrent test was performed according to Xue et al. (2001) which has been used by Rajkumar and Jebanesan (2005b). Five cages were designed and placed side by side A, B, C, D and E for each bioassay. Fifteen gravid female of An. arabiensis and Cx. quinquefasciatus were transferred to each mosquito cage 30 × 30 × 30 cm. A 10% sucrose solution was available at all times. The concentration of leaf extract of C. procera which showed the highest, moderate and lowest mortality in the larvicidal activity (against 3rd instar) were prepared, and 100 ml from each were taken and put in the test cup in cage A, B and C. Three test cups each containing 100 ml of de-chlorinated tap water were prepared and put in cage A, B and C on the opposite place of the treated cup as control. The positions of the cups were alternated between the different replicates so as to nullify any effect of position on egg laying. In cage D, all the experimental concentrations (high, moderate and low) were placed without control. While in cage E two cups of control were placed without any treated cup. Three replicates for each concentration were run. After 48 h, the number of eggs laid in treated and control cups was recorded. In the case of An. arabiensis the test cup was replaced by Petri dishes with filter paper in the bottom.The percent effective repellency for each concentration was calculated using the following formula:
where ER is the percent effective repellency; NC is the number of eggs in control cups; and NT is the number of eggs in treated cups.
3. Results
The aqueous leaf extract of C. procera showed high level of toxicity against the larvae of mosquitoes An. arabiensis and Cx. quinquefasciatus. The results are presented in Table 1 and Fig. 1. The 50% mortality (LC50 values) was shown at 273.53, 366.44 and 454.99 ppm for 2nd, 3rd and 4th instar larvae, respectively of An. arabiensis and 187.93, 218.27 and 264.85 ppm for 2nd, 3rd and 4th instar larvae, respectively of Cx. quinquefasciatus.
Table 1.
Larvicidal activity of leaves extract of C. procera against 2nd, 3rd and 4th instar larvae of An. arabiensis and Cx. quinquefasciatus.
| Mosquito species | Larval instar | LC50 (ppm) | LC90 (ppm) | Regression equation | FL with 95%CL | r2 |
|---|---|---|---|---|---|---|
| An. arabiensis | 2nd | 273.53 | 783.43 | Y = 2.799X − 1.820 | ±2.675 | 0.997 |
| 3rd | 366.44 | 1018.59 | Y = 2.883X − 2.393 | ±2.335 | 0.993 | |
| 4th | 454.99 | 1224.62 | Y = 2.977X − 2.913 | ±2.400 | 0.970 | |
| Cx. quinquefasciatus | 2nd | 187.93 | 433.51 | Y = 3.528X − 3.024 | ±2.372 | 0.973 |
| 3rd | 218.27 | 538.27 | Y = 3.261X − 2.626 | ±2.675 | 0.984 | |
| 4th | 264.85 | 769.13 | Y = 2.77X − 1.713 | ±2.675 | 0.996 | |
FL with 95%CL = fiducial limit with 95% confidence limit.
(r2) = regression coefficient.
Figure 1.
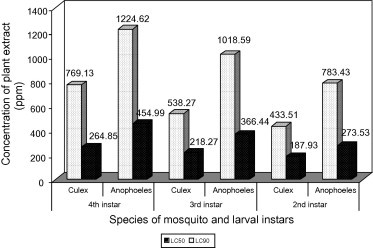
Larvicidal activity of leaves extract of C. procera against 2nd, 3rd and 4th instars larvae of An. arabiensis and Cx. quinquefasciatus expressed as LC50 and LC90.
The LC90 values (90% mortality) were shown at 783.43, 1018.59 and 1224.62 ppm for 2nd, 3rd and 4th instar larvae, respectively of An. arabiensis and 433.51, 538.27 and 769.13 ppm for 2nd, 3rd and 4th instar larvae, respectively of Cx. quinquefasciatus. From LC50 and LC90 values it was evident that 2nd instars were more susceptible than 3rd instar and the later was more susceptible than 4th instar. Also the two species of selected mosquito larvae showed different susceptibility to the leaf extract of C. procera. Cx. quinquefasciatus was found more susceptible than An. arabiensis. The leaf extract of C. procera did not show any pupal mortality till higher concentration of (5000 ppm) against the two species of mosquitoes after 24 h treatment. The statistical data of adult emergence inhibition (EI) activity of C. procera leaves extract against An. arabiensis and Cx. quinquefasciatus presented in Table 2, EI50–EI90 was shown at 277.90–677.64 ppm and 183.65–453.94 ppm, respectively. It was evident that EI50 and EI90 values for the two species of mosquitoes studied was less than LC50 and LC90 for the 3rd larval instar of the same mosquito’s species. The lower concentration of the leaf extract was required for the adult emergence inhibition than larvicidal. This reflects the activity of the extract as possible insect growth regulator against the two species of mosquito.
Table 2.
The adult emergence inhibition activity of leaves extract of C. procera against An. arabiensis and Cx. quinquefasciatus.
| Mosquito species | EI50 (ppm) | EI90 (ppm) | Regression equation | FL with 95%CL | r2 |
|---|---|---|---|---|---|
| An. arabiensis. | 277.90 | 677.60 | Y = 3.307X − 3.081 | ±2.292 | 0.981 |
| Cx. quinquefasciatus | 183.65 | 453.94 | Y = 3.258X − 2.376 | ±2.335 | 0.994 |
FL with 95%CL = fiducial limit with 95% confidence limit.
(r2) = regression coefficient.
The effect of different concentrations of the extract on the oviposition deterrence against gravid female mosquitoes of An. arabiensis is shown in Table 3. In the cage A, 280 eggs were laid in the control cup, while in the corresponding treated cup (1000 ppm) in the same cage no eggs were laid. A similar observation was shown in cage B and C, 390 and 480 eggs were laid in the control cup of cages B and C, respectively, and no eggs were laid in the corresponding treated cup in both cages. In cage D where choice of control was not found, maximum of egg laying (250 eggs) was shown in the lowest concentration (200 ppm), and no eggs were laid in the highest concentration (1000 ppm), while in the moderate larvicidal concentration (500 ppm), 115 eggs were laid. In cage E where only control was found about 550 eggs were laid.
Table 3.
Oviposition deterrent activity of leaves extract of C. procera against gravid, female An. arabiensis.
| Cage | A | B | C | D | E | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (ppm) | C | 1000 | C | 500 | C | 200 | 1000 | 500 | 200 | C | C |
| Number of eggs laid within 48 h | 280 | 0 | 390 | 0 | 480 | 0 | 0 | 115 | 250 | 290 | 260 |
| ER% | 100 | 100 | 100 |
C = control (de-chlorinated tap water).
ER = effective repellency.
The aqueous leaf extract of C. procera at different concentrations of larvicidal activity (1000, 500 and 200 ppm) showed 100% oviposition deterrence and 100% effective repellence against An. arabiensis when the extract is to be used as material of choice (treated–control). However, when all the concentrations were found without control (choice) the avoidance of egg laying was not shown except in the high concentration (1000 ppm), and maximum of eggs were laid in the low concentration (200 ppm). A similar observation was shown on Cx. quinquefasciatus, with relative difference in that Cx. quinquefasciatus showed 90.6% oviposition deterrent and effective repellency at low concentration (100 ppm) as it was shown in Tables 3 and 4.
Table 4.
Oviposition deterrent activity of leaves extract of C. procera against gravid, female Cx. quinquefasciatus.
| Cage | A | B | C | D | E | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (ppm) | C | 1000 | C | 500 | C | 200 | 1000 | 500 | 200 | C | C |
| Number of eggs laid within 48 h | 405 | 0 | 490 | 0 | 520 | 20 | 56 | 173 | 304 | 370 | 410 |
| ER% | 100 | 100 | 90.6 |
C = control (de-chlorinated tap water).
ER = effective repellency.
4. Discussions
In this study it was observed that, leaves extract of C. procera has showed larvicidal, adult emergence inhibition and oviposition deterrent activity against the mosquitoes An. arabiensis and Cx. quinquefasciatus. The biological activity of this plant extract may be due to various compounds, including phenolics, terpenoides, flavonoids and alkaloids existing in plant, these compounds may jointly or independently contribute to produce larvicidal, adult emergence inhibition, and oviposition deterrent activity against both species of mosquitoes. The obtained results agree with some previous studies. One plant species may possess substances with a wide range of activities, e.g. Neem (Azadirachta indica) products showed antifeedant, oviposition deterrence, repellency, growth disruption, sterility and larvicidal action against insects (Schmutterer, 1990; Mulla and Su, 1999). The leaf extract of five species of Cucurbitaceous plants, Momordica charntia, Trichosanthes anguina, Luffa acutangula, Benincasa cerifera and Citrullus vulgaris showed larvicidal activity at LC50 of 465.85, 567.81, 839.81, 1189.30 and 1636.04 ppm, respectively (after 24 h treatment) against the 3rd instar larvae of Cx. quinquefasciatus (Prabakar and Jebanesan, 2004). The leaf extracts of Pavonia zeylanica and Acacia ferrugginea showed larval mortality at LC50 of 2214.7 and 5362.6 ppm, respectively against the third larval instar of Cx. quinquefasciatus after 24 h treatment (Vahitha et al., 2002). Also the result agree with the finding of Pushpanathan et al. (2006) who had reported that 2nd instar larvae of Cx. quinquefasciatus was more susceptible than 3rd instar, and the later was more susceptible than 4th instar larvae to the essential oils extracted from Cymbopogan citratus plant, with LC50–LC90 of 144.54–284.27 ppm, 165.70–318.48 ppm and 184.18–359.01 ppm for 2nd, 3rd and 4th larval instar, respectively. Also it was found that Cx. quinquefasciatus was more susceptible than An. arabiensis to the leaf extract of C. procera. The varying susceptibility of the two species of mosquitoes is probably due to differences in the physiological characteristics of the two species of mosquito. This agree with (Thekkevilayil et al., 2004) who had reported that the four mosquitoes Cx. tritaeniorhynchus, An. stphensi, Aedes aegypti and Cx. quinquefasciatus larvae showed different susceptibility to the oils extract of Ipomoea cairica Linn., higher concentration was required for Cx. quinquefasciatus followed by Ae. aegypti, Anopheles stphensi and lower concentration for Culex tritaeniorhynchus, with the LC50–LC90 of 58.9–161.6 ppm for Cx. quinquefasciatus, 22.3–92.7 ppm for Ae. aegypti, 14.9–109.9 ppm for Anopheles stphensi, and 14.8–78.3 ppm for Culex tritaeniorhynchus.
The leaf extract of C. procera did not show any pupal mortality till higher concentration of (5000 ppm) against the two species of mosquitoes, suggesting that the effects of the extract on the pupal stage appear after more than 24 h exposure.
The whole latex of C. procera was shown to cause 100% mortality of 3rd instar larvae of Ae. aegypti within five minutes, and most of individual growing under experimental conditions died before reaching 2nd instars or stayed in 1st instars (Marcio et al., 2006). The effect of alkaloid extracts of C. procera leaves at the vegetative stage on the survival of fifth instar larvae and on ovarian growth of Shistocerca gregaria have revealed that a mortality rate of 100% was reached in the hoppers on the 15th day after the beginning of the treatment. In the adult the arrest of ovarian growth in females and the absence of sexual maturity in males have been observed (Abbassi et al., 2004). In laboratory the leaf extract of Solanum trilobatum greatly reduced the number of eggs laid by gravid Anopheles stephensi at several concentrations. At the highest concentrations (1–0.075%) the extract reduced eggs laying by 90–99%. Lower concentrations (0.01%) also had deterrent activity of 18.4% (Rajkumar and Jebanesan, 2005b). These findings prove that, mosquitoes are known to perceive visual, thermal and olfactory stimuli which enable them to detect light source, odour and several other volatile chemicals emanating from the skin, breath and waste products of their hosts (Takken, 1991; Davis and Bowen, 1994).
In conclusion, leave extract of C. procera can be suggested as a natural larvicidal for controlling mosquitoes in Sudan. Since it is considered environmentally safe, less expensive and economical, as well as practical in application with minimum care by individuals and communities.
Acknowledgements
The authors are grateful to the staff members of Preventive Medicine Department, Faculty of Veterinary Medicine, University of Khartoum for laboratory facilities provided and their helpful aids. Thanks are also due to Dr. Abd Elgabar Nasir, University of Khartoum for identification and classification of the plant used in this study.
Footnotes
The work was carried out at the Department of Preventive Medicine, Faculty of Veterinary Medicine, Khartoum University, P.O. Box 32, Khartoum North, Sudan.
References
- Abbassi K., Kadiri Z.A., Ghaout S. Biological activity of Calotropisprocera (Ait. R. Br.) leaves on the desert locust (Shistocercagregaria, Forsk. 1775) Zoologica Baetica. 2004;15:153–166. [Google Scholar]
- Abbott W.S. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Aiah A.G., Maxwell A.A., Samuel D., Collins K., Samuel O.S., Wilmot A.B., Johnny G., Scott A.L. Lymphatic filariasis in Ghana: establishing the potential for an urban cycle of transmission. Tropical Medicine and International Health. 2005;10(4):387–392. doi: 10.1111/j.1365-3156.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- Curtis C.F. Should DDT continue to be recommended for malaria vector control? Medical and Veterinary Entomology. 1994;8:107–112. doi: 10.1111/j.1365-2915.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Davis E.E., Bowen M.F. Sensory physiological basis for attraction in mosquitoes. Journal of American Mosquito Control Association. 1994;10:316–325. [PubMed] [Google Scholar]
- El setouhy M., Ramzy R.M.R. Lymphatic filariasis in the Eastern Mediterranean Region: current status and prospects for elimination. Eastern Mediterranean Health Journal. 2003;9(4):534–541. [PubMed] [Google Scholar]
- Killeen, G.F., Fillinger, U., Knols, B.G., 2002. Advantages of larval control for African malaria vectors: low mobility and behavioral responsiveness of immature mosquito stages allow high effective coverage. Malaria Journal 1 (1). doi:10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed]
- Manzava A.E., Rwegoshora R.T., Tanner M., Msuya F.H., Curtis C.F., Irare S.G. The house spraying with DDT or Lambda-cyhalothrine against Anopheles arabiensis on measures of malaria morbidity in children in Tanzania. Acta Tropica. 1993;54:141–151. doi: 10.1016/0001-706x(93)90060-o. [DOI] [PubMed] [Google Scholar]
- Marcio V.R., Glais de Paiva B., Cleverson D.T., Nadia A.P.N., Nylane M.N.A., Petronio A.S., Ana F.U.C. Latex constituents from Calotropisprocera (R. Br.) display toxicity upon egg hatching and larvae of Aedesaegypti (Linn.) Memorias Instituto. 2006;101(5):503–510. doi: 10.1590/s0074-02762006000500004. [DOI] [PubMed] [Google Scholar]
- Mittal P.K., Subbarao S.K. Prospects of using herbal products in the control of mosquito vectors. Indian Council of Medical Research ICMR Bulletin. 2003;33(1):1–10. [Google Scholar]
- Mueen Ahmed K.K., Rana A.C., Dixit V.K. Calotropis species (Ascelpediaceae) a comprehensive review. Pharmacognosy Magazine. 2005;1(2):48–52. [Google Scholar]
- Mulla M.S., Su T. Activity and biological effects of neem products against arthropods of medical and veterinary importance. Journal of American Mosquito Control Association. 1999;15:133–152. [PubMed] [Google Scholar]
- Prabakar K., Jebanesan A. Larvicidal efficacy of some Cucurbitaceous plant leaf extracts against Culexquinquefasciatus (Say) Bioresource Technology. 2004;95(1):113–114. doi: 10.1016/j.biortech.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Prajapati V., Tripathi A.K., Aggarwal K.K., Khanuja S.P. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anophelesstephensi, Aedesaegypti and Culexquinquefasciatus. Bioresource Technology. 2005;96(16):1749–1757. doi: 10.1016/j.biortech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Promsiri S., Naksathit A., Kruatrachue M., Thavara U. Evaluations of larvicidal activity of medicinal plant extracts to Aedesaegypti (Diptera: Culcidae) and other effects on a non target fish. Insect Science. 2006;13(3):179–188. [Google Scholar]
- Pushpanathan T., Jebanesan A., Govindarajan M. Larvicidal, ovicidal and repellent activities of Cymbopogancitratus Stapf. (Graminae) essential oil against the filarial mosquito Culexquinquefasciatus (Say) (Diptera: Culicidae) Tropical Biomedicine. 2006;23(2):208–212. [PubMed] [Google Scholar]
- Rajkumar S., Jebanesan A. Larvicidal and adult emergence inhibition effect of Centellaasiatica Brahmi (Umbelliferae) against mosquito Culexquinquefasciatus Say (Diptera: Culcidae) African Journal of Biomedical Research. 2005;8:31–33. [Google Scholar]
- Rajkumar S., Jebanesan A. Oviposition deterrent and skin repellent activities of Solanumtrilobatum. Leaf extract against the malarial vector Anophelesstephensi. Journal of Insect Science. 2005;5(15):p3. doi: 10.1093/jis/5.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge C.R., Clarke F., Curtis A., Sackett S. Larval mosquito control. Technical Bulletin of the Florida Mosquito Control Association. 2003;4:16–19. [Google Scholar]
- Satti M.H., Abdel Nur O. Bancroftian filariasis in the Sudan. Bulletin of the World Health Organization. 1974;51:314–315. [PMC free article] [PubMed] [Google Scholar]
- Schmutterer H. Properties and potential of natural pesticides from the neem tree, Azadirachtaindica. Annual Review of Entomology. 1990;35:271–297. doi: 10.1146/annurev.en.35.010190.001415. [DOI] [PubMed] [Google Scholar]
- Takken W. The role of olfaction in host-seeking of mosquitoes: a review. Insect Science and its Application. 1991;12:287–2995. [Google Scholar]
- Thekkevilayil G.T., Sunder R., Lal Shiv. Mosquito larvicidal properties of essential oil of an indigenous plant, Ipomoeacairica Linn. Japanese Journal of Infectious Diseases. 2004;57(4):176–177. [PubMed] [Google Scholar]
- Vahitha R., Venkatachalam M.R., Murugan K., Jebanesan A. Larvicidal efficacy of Pavoniazeylanica L. and Acaciaferruginea D.C. against Culexquinquefsciatus Say. Bioresource Technology. 2002;82(2):203–204. doi: 10.1016/s0960-8524(01)00175-4. [DOI] [PubMed] [Google Scholar]
- WHO, 2000. World Health Organization, Expert Committee on Malaria. Technical Report Series 892, Geneva. [PubMed]
- WHO, 2005a. Communicable disease toolkit Sudan. World Health Organization, Communicable Disease Working Group on Emergencies, WHO Regional Office for the Eastern Mediterranean, WHO Country Office, Khartoum, WHO/CDS/20050.26.
- WHO, 2005b. Guide lines for laboratory and field testing of mosquito larvicides. World Health Organization Communicable Disease Control, Prevention and Eradication, WHO Pesticides Evaluation Scheme, WHO/CDS/WHOPES/GCDPP/2005.13.
- WHO and UNICEF, 2005. World Malaria Report 2005, WHO/HTM/MAL/2005.1102. Roll Back Malaria, World Health Organization and UNICEF. <http://rbm.who.int/wmr2005>.
- WHO, 2006a. Pesticides and their Application for the Control of Vectors and Pests of Public Health Importance. World Health Organization, WHO Pesticides Evaluation Scheme, WHO/CDS/WHOPES/GCDPP/2006.1.
- WHO, 2006b. Global programme to eliminate lymphatic filariasis. World Health Organization, Geneva. Weekly Epidemiological Record 81 (22), 221–232. <http://www.who.int/wer>. [PubMed]
- Xue R.D., Barnard D.R., Ali A. Laboratory and field evaluation of insect repellents as oviposition deterrents against the mosquito Aedesalbopictus. Medical and Veterinary Entomology. 2001;15:126–131. doi: 10.1046/j.0269-283x.2001.00301.x. [DOI] [PubMed] [Google Scholar]


