Abstract
Antifeedant and larvicidal activities of rhein (1,8-dihydroxyanthraquinone-3-carboxylic acid) isolated from the ethyl acetate extract of Cassia fistula flower were studied against lepidopteron pests Spodoptera litura and Helicoverpa armigera. Significant antifeedant activity was observed against H. armigera (76.13%) at 1000 ppm concentration. Rhein exhibited larvicidal activity against H. armigera (67.5), S. litura (36.25%) and the LC50 values was 606.50 ppm for H. armigera and 1192.55 ppm for S.litura. The survived larvae produced malformed adults.
Keywords: Antifeedant, Larvicidal, Cassia fistula, Rhein, Spodoptera litura, Helicoverpa armigera
1. Introduction
The growing awareness of the hazards of excessive use of pesticides globally has led researchers to search for safer and more environment friendly alternative methods for insect pest control. Therefore, extensive studies are carried out to screen plants as insect growth control agents. Over the last two to three decades, greater attention has been focused on the bioactivity of phytochemicals for their potential as pesticides against phytophagous insects (Padmaja and Rao, 2000). Research on natural products, that could be alternatives to synthetic pesticides and fungicides, for example, plant extracts and essential oils, has greatly increased during recent years (Wilson et al., 1997; Pradhanang et al., 2003; Cohen et al., 2006).
Cassia fistula L., (Leguminosae), a semi-wild Indian Labernum (also known as the Golden Shower), is distributed in various countries including Asia, South Africa, Mexico, China, West Indies, East Africa and Brazil. It is an ornamental tree with beautiful bunches of yellow flowers. The whole plant is used to treat diarrhea; seeds, flowers and fruits are used to treat skin diseases, fever, abdominal pain and leprosy by traditional people (Perry, 1980).
Kaempferol and proanthocyanidin have been isolated from the acetone extract of C. fistula flower (Narayanan and Seshadri, 1972). A bianthraquinone glycoside, fistulin together with kaempferol and rhein has been isolated from ethanol extracts of C. fistula flowers (Kumar et al., 1966). Besides phenolics and their derivatives, a certain amount of alkaloids have also been reported in C. fistula flowers (Asseleih et al., 1990); traces of triterpenes have been observed in both flowers and fruits (Guri-Fakim et al., 1994). A diterpene, 3B-hydroxy-17-norpimar-8(9)-en-15-one was isolated from the pods of C. fistula (Misra et al., 1996).
Besides its pharmacological uses, its extract is also recommended for pest and disease control (Jaipal et al., 1983; Sharma and Basandrai, 1999; Raja et al., 2000). The search for plants with novel insecticidal constituents has been intensive. Many leads from numerous plant species have been identified, with the most promising belonging to the families of Meliaceae, Rutaceae, Annonaceae, Asteraceae, Labiatae and Piperaceae (Isman, 1995; Jacobson, 1989; Schmutterer, 1992). Higher plants are a rich source of novel natural substances that can be used to develop environmentally safe compounds for insect control (Arnason et al., 1989).Our preliminary evaluation of ethylacetate extract from C. fistula flowers showed good antifeedant activity. In the present work we report the separation and identification of rhein from C. fistula flowers and its antifeedant and larvicidal effects on insects.
2. Materials and methods
2.1. Plant material
C. fistula flowers were collected from Loyola College Campus, Chennai, India in May 2006. It was authenticated by a plant taxonomist from the Department of Botany, Loyola College, Chennai. A voucher specimen (ERIC-D-73) is deposited at the herbarium of Entomology Research Institute, Loyola College, Chennai.
2.2. Preparation of plant extract
Flowers were collected and shade dried at room temperature and ground in a manual mill. The powder (1 kg) was extracted with 3 L (1:3 w/v) of ethyl acetate for 48 h. The extract was filtered through a Buchner funnel with Whatman number 1 filter paper. The filtrate was evaporated to dryness under reduced pressure using rotary evaporator at 40 °C. The crude extracts were stored at 4 °C until further use.
2.3. Isolation of active compound
The crude ethyl acetate extract (20 g) was subjected to column chromatography over silica gel (200 g-acme’s 100–200 mesh) and eluted with hexane followed by the combination of hexane: ethyl acetate ranging from 95:5 to 100. 117 fractions were collected in 200 ml conical flasks. After checking TLC, the fractions were combined into 24 fractions. Fraction 10 showed a crystal that was subjected to crystallographic analysis identified and also reported (Duraipandiyan and Ignacimuthu, 2007). Fraction 18 showed single spot on TLC and yielded 210 mg; this fraction was eluted using hexane: ethyl acetate (55:45) solvent system. The compound was subjected to spectroscopic analysis.
2.4. Spectroscopic analysis
UV spectra were measured with Hitachi-2010 Spectrophotometer in ethanol. IR spectra were taken using Shimadzu by KBr pellet method. NMR studies were performed in AL-300 MHz, JEOL spectrometer.1H NMR was run at either 300 or 400 MHz and 13C NMR at 75 MHz using the solvent signal as reference. Mass spectrometric studies have been performed in Shimadzu with the temperature of EI method.
2.5. Antifeedant activity
The crude ethyl acetate extract and Rhein were tested for antifeedant activity using leaf disc no choice method (Isman et al., 1997). Different concentrations of crude extracts and compound were prepared by dissolving in acetone and tested against H. armigera and Spodoptera litura. Fresh cotton leaf discs (Gossibium hirsutum) for H. armigera and fresh castor leaf discs (Ricinus communis) for S. litura were used. Leaf discs of 4 cm diameter were punched using cork borer and dipped in 0.625%, 1.25%, 2.50% and 5.0% concentrations of crude extracts and 125, 250, 500 and 1000 ppm of isolated compound. Azadirachtin was used as positive control. Leaf discs treated with acetone and without solvent (Water) were considered as negative control. After air-drying, each leaf disc was placed in petri dish (1.5 × 9 cm) containing wet filter paper to avoid early drying of the leaf disc and single 2 h pre-starved fourth instar larva of S. litura and H. armigera was introduced into petri dishes containing the respective leaf discs. For each concentration 10 replicates were maintained. Progressive consumption of leaf area by the larva after 24 h feeding was recorded in control and treated discs using Leaf area meter (Delta-T Devices, Serial No. 15736 F 96, UK). Leaf area consumed in plant extract treatment was corrected from the control. The percent antifeedant index was calculated using the formula of Ben Jannet et al. (2000).
where, C and T represent the amount of leaf eaten by the larva on control and treated discs, respectively.
2.6. Larvicidal activity
The larvae that were fed with treated cotton (H. armigera) and castor (S. litura) leaf disc (different concentrations of compound) for 24 h were continuously maintained on untreated fresh leaves. Diet was changed every 24 h. Larval mortality was recorded after 96 h of treatment. Four replicates were maintained for each treatment with 5 larvae per replicate (total, n = 20). Per cent mortality was calculated using the formula of Abbott (1925). At the laboratory conditions were the same as in antifeedant activity study.
2.7. Statistical analysis
The data collected were represented as mean ± SD. One-way analysis of variance (ANOVA) and Significant differences between treatments were determined by Tukey’s multiple range tests (P ⩽ 0.05). LC50 value was calculated using Probit Analysis (Finney, 1971).
3. Results
The present study deals with the antifeedant and larvicidal activities of ethyl acetate extract of C. fistula flower and a compound rhein isolated from it. The active compound was identified as Rhein (1,8-dihydroxyanthraquinone-2-carboxylic acid) (Fig. 1). The structural identification of compound was carried out by IR, MS, 1H NMR and 13C NMR spectra. 1H NMR and 13C NMR spectral data corresponded to the molecular formula C15H8O6, which is of rhein (Wei et al., 2003; Liu et al., 2004).
Fig. 1.
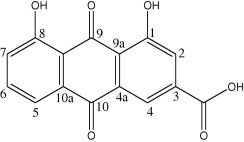
Rhein (1,8-dihydroxyanthraquinone-3-carboxylic acid) isolated from C. fistula flower.
Antifeedant activity of crude extract and isolated compound was tested against two different insect pests and the results are presented in Table 1. The compound rhein showed moderate antifeedant activity (56.79%) against S. litura at 1000 ppm, whereas it showed significant activity against H. armigera (76.13%) at 1000 ppm. Rhein exhibited larvicidal activity of 67.5 % against H. armigera with LC50 value of 606.5 ppm and 36.25% against S. litura with the LC50 value of 1192.55 ppm. The larvae after treatment with compound rhein showed malformation and mortality in larval, pupal and adult stages (Table 2).
Table 1.
Percent antifeedant activity of ethyl acetate extract of Cassia fistula and compound rhein against Helicoverpa armigera and Spodoptera litura (mean ± S.D.).
| Tested compounds | Concentration (%) | Test insects |
|
|---|---|---|---|
| H. armigera | S. litura | ||
| Cassia fistula (crude ethylacetate extract) | 0.625 | 33.29 ± 12.92e | 26.97 ± 13.20c |
| 1.25 | 40.25 ± 4.39cde | 29.41 ± 5.62c | |
| 2.5 | 49.81 ± 5.72cd | 32.68 ± 11.17c | |
| 5.0 | 68.27 ± 13.27a | 53.19 ± 4.98b | |
| Rhein (isolated compound) | 125 ppm | 47.82 ± 7.37fgh | 27.28 ± 12.27i |
| 250 ppm | 55.06 ± 5.79def | 33.05 ± 13.59ghi | |
| 500 ppm | 59.98 ± 5.38cde | 40.85 ± 11.20f | |
| 1000 ppm | 76.13 ± 13.43b | 56.79 ± 7.84de | |
| Azadirachtin (40%) (standard) | 125 ppm | 53.50 ± 6.34efg | 60.12 ± 7.12d |
| 250 ppm | 64.13 ± 3.11c | 70.08 ± 5.37c | |
| 500 ppm | 75.88 ± 3.99b | 78.66 ± 5.44b | |
| 1000 ppm | 86.47 ± 1.83a | 88.02 ± 3.83a | |
Values carrying different alphabets in a column are statistically significant by LSD at 5% level.
Table 2.
Percent larvicidal activity and LC50 values of tested compound rhein against Helicoverpa armigera and Spodoptera litura (mean ± SD).
| Tested compounds | Concentration (%) | Test insects |
|||
|---|---|---|---|---|---|
|
H. armigera |
S. litura |
||||
| Larvicidal activity | LC50 | Larvicidal activity | LC50 | ||
| Rhein | 125 | 22.5 ± 2.88a | 606.50 | 00.00 ± 00a | 1192.55 |
| 250 | 32.5 ± 8.66ab | 15.00 ± 10.0b | |||
| 500 | 50.0 ± 8.16bc | 21.25 ± 2.5b | |||
| 1000 | 67.5 ± 8.66c | 36.25 ± 7.5c | |||
| Azadirachtin (40%) | 125 | 38.75 ± 10.3ab | 186.16 | 52.5 ± 9.5d | 127.50 |
| 250 | 61.25 ± 10.31c | 73.75 ± 9.46e | |||
| 500 | 95.00 ± 10.00d | 100.0 ± 0.00f | |||
| 1000 | 100.0 ± 0.00d | 100.0 ± 0.00f | |||
Within the column, mean followed by the same letter do not differ significantly using Tukey’s test, P ⩽ 0.05.
4. Discussion
Rhein compound was previously reported from some other plants. Sun et al. (2000) chromatographed and purified emodin, chrysophanol and rhein from Rheum officinale extract. Wang et al. (2001) separated and determined active anthraquinone components physcion, chrysophanol, aloe-emodin, emodin, and rhein from the Chinese herb Polygonum multiflorum. Similarly Wei et al. (2003) reported the isolation and purification of rhein from Rheum officinale. Kanokmedhakul et al. (2005) have isolated seven anthraquinone and triterpenoids from Prismatomeris fragrans with antifungal activity.
Isolated rhein showed significant antifeedant activity against H. armigera. Quinones and compounds with aldehyde groups have been reported to be insect antifeedants (Blaney et al., 1987; Morimoto et al., 1999).
Georges et al. (2007) have evaluated anthraquinone (emodin, citreorosein, and emodic acid) from Cassia nigricans against Helicoverpa zea, Heliothis virescens, Bemisia tabacci (white fly) and Anopheles gambiaea (mosquito larvae) and 80% mortality was recorded on A. gambiaea. Ethyl acetate extract of C. fistula and compound rhein did not show significant activity against S. litura but showed good activity against H. armigera. Raja et al. (2005) and Pavunraj et al. (2006) found that Hyptis suaveolens and Excoecaria agallocha possessed antifeedant activity against lepidopteron pests. Antifeedant assays for anthraquinones against carpet beetles significantly inhibited feeding (Morimoto et al., 2002). Rhein showed higher antifeedant activity (76.13%) against H. armigera than S. litura (56.79%).
The larvicidal activity of rhein showed 67.5% against H. armigera and 36.25% against S. litura at 1000 ppm concentration. Similar findings were recorded in hexane fractions of A. monophylla against H. armigera (Baskar et al., 2009).
The present findings show that the isolated compound rhein exhibited a significant antifeedant activity against H. armigera. It also has the potential to act as feeding deterrent against H. armigera.
Acknowledgments
The authors are grateful to the Entomology Research Institute, Loyola College, Chennai for financial assistance.
References
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–266. [Google Scholar]
- Arnason, J.T., Philogene, B.J.R., Morand, P. 1989. Insecticides of plants origin. American Chemical Society Symposium Series, vol. 387. Washington.
- Asseleih L. M.C., Hernandez O.H., Sanchez J.R. Seasonal variation in the content of Sennosides in leaves and pods of two Cassia fistula populations. Phytochemistry. 1990;29:3095–3099. [Google Scholar]
- Baskar K., Kingsley S., Ezhil Vendan S., Gabrial Paulraj M., Duraipandiyan V., Ignacimuthu S. Antifeedant, larvicidal and pupicidal activities of Atalantia monophylla (L) Correa against Helicoverpa armigera Hubner (Lepidoptera: Noctuidae) Chemosphere. 2009;75:355–359. doi: 10.1016/j.chemosphere.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Ben Jannet H., Skhiri H.F., Mighri Z., Simmonds M.S.J., Blaney W.M. Responses of Spodoptera littoralis larvae to Tunisian plant extracts and to neo-clerodane diterpenoids isolated from Ajuga pseudoiva leaves. Fitoterapia. 2000;71:105–112. doi: 10.1016/s0367-326x(99)00146-x. [DOI] [PubMed] [Google Scholar]
- Blaney W.M., Simmonds M.S.J., Ley S.V., Katz R.B. An electrophysiological and behavioural study of insect antifeedant properties of natural and synthetic drimane-related compounds. Physiol. Entomol. 1987;12:281–291. [Google Scholar]
- Cohen Y., Wang W., Ben-Daniel B., Ben-Daniel Y. Extracts of Inula viscosa control downy mildew of Grapes caused by Plasmopara viticola. Phytopathology. 2006;96:417–424. doi: 10.1094/PHYTO-96-0417. [DOI] [PubMed] [Google Scholar]
- Finney D.J. 3rd ed. Cambridge University Press; London, UK: 1971. Probit Analysis. pp. 383. [Google Scholar]
- Georges K., Jayaprakasam B., Dalavoy S.S., Muraleedharan G.N. Pest-managing activities of plant extracts and anthraquinones from Cassia nigricans from Burkina Faso. Biores. Technol. 2007;99:2037–2045. doi: 10.1016/j.biortech.2007.02.049. [DOI] [PubMed] [Google Scholar]
- Guri-Fakim, A., Gueho, J., Sewraj, M.D., Dulloo, E. 1994. Plantes Medicinales de lile Maurice, Editions de L’Ocean Indien, Mauritius, p. 580.
- Isman B., Koul O., Lucyzynski A., Kaminski J. Insecticidal and antifeedant bioactivities of neem oils and their relationship to Azadirachtin contrent. J. Agric. Food Chem. 1997;38:1407–1411. [Google Scholar]
- Isman M.B. Leads and prospects for the development of new botanical insecticides. In: Roe R.M., Kuhr R.J., editors. vol. 3. Toxicology Communications Inc.; Raleigh, NC: 1995. pp. 1–20. (Reviews in Pesticide Toxicology). [Google Scholar]
- Jacobson M. Botanical insecticides. Past, present and future. In: Arnason J.T., Philogene B.J.R., Morand P., editors. Insecticides of Plant Origin. American Chemical Society Symposium Series No. 387; Washington, D.C: 1989. pp. 1–10. [Google Scholar]
- Jaipal S., Sing Z., Chauhan R. Juvenile hormone like activity in extracts of some common Indian plants. Ind. J. Agric. Sci. 1983;53:730–733. [Google Scholar]
- Kanokmedhakul K., Kanokmedhakul S., Phatchana R. Biological activity of Anthraquinones and Triterpenoids from Prismatomeris fragrans. J. Ethnopharmacol. 2005;100:284–288. doi: 10.1016/j.jep.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Kumar A., Pande C.S., Kaul R.K. Chemical examination of Cassia fistula flowers. Ind. J. Chem. 1966;4:460. [Google Scholar]
- Liu R., Li A., Sun A. Preparative isolation and purification of hydroxyl anthraquinones and cinnamic acid from the Chinese medicinal herb Rheum officinale Baill. by high-speed counter-current chromatography. J. Chromatogr., A. 2004:1052: 217–221. doi: 10.1016/j.chroma.2004.08.101. [DOI] [PubMed] [Google Scholar]
- Misra T.N., Singh R.S., Pandev H.S., Pandev R.P. Chemical constituents of hexane fraction of Cassia fistula pods. Fitoterapia. 1996;57:173–174. [Google Scholar]
- Morimoto M., Tanimoto K., Sakatani A., Komai K. Antifeedant activity of an anthraquinone aldehyde in Galium aparine L. against Spodoptera litura F. Phytochemistry. 2002;60:163–166. doi: 10.1016/s0031-9422(02)00095-x. [DOI] [PubMed] [Google Scholar]
- Morimoto M., Fujii Y., Komai K. Antifeedants in Cyperaceae: Coumaran and quinones from Cyperus spp. Phytochemistry. 1999;51:605–608. [Google Scholar]
- Narayanan V., Seshadri T.R. Proanthocyanidins of Cassia fistula. Ind. J. Chem. 1972;10:379–381. [Google Scholar]
- Padmaja P.G., Rao P.J. Efficacy of certain plant oils on the American bollworm Helicoverpa armigera. Pest. Res. J. 2000;12:107–111. [Google Scholar]
- Pavunraj M., Subramaniyan K., Muthu C., Prabu Seenivasan S., Duraipandiyan V., Maria Packiam S., Ignacimuthu S. Bioefficacy of Excoecaria agallocha (L) leaf extract against armyworm Spodoptera litura (Fab.) (Lepidoptera:Noctuidae) Entomon. 2006;31:37–40. [Google Scholar]
- Perry L.M. MIT Press; Cambridge: 1980. Medicinal Plants of East and South East Asia. [Google Scholar]
- Pradhanang P.M., Momol M.T., Olson S.M., Jones J.B. Effects of plant essential oils on Ralstonia solanacearum population density and bacterial wilt incidence in tomato. Plant Dis. 2003;87:423–427. doi: 10.1094/PDIS.2003.87.4.423. [DOI] [PubMed] [Google Scholar]
- Raja N., Albert S., Ignacimuthu S. Effect of solvent residues of Vitex negundo Linn. and Cassia fistula Linn. on pulse beetle, Callosobruchus maculates Fab. and its larval parasitoid, Dinarmusvagabundus (Timberlake) Ind. J. Exp. Biol. 2000;38:290–292. [PubMed] [Google Scholar]
- Raja N., Jeyasankar A., Venkatesan Jeyakumar S., Ignacimuthu S. Efficacy of Hyptis suaveolens against lepidopteran pests. Curr. Sci. 2005;88:220–222. [Google Scholar]
- Schmutterer H. Higher plants as sources of novel pesticides. In: Otto D., Weber B., editors. Insecticides: Mechanisms of Action and Resistance. Intercept Ltd.; Andover: 1992. pp. 3–15. [Google Scholar]
- Sharma B.K., Basandrai A.K. Efficacy of some plant extracts for the management of Karnal bunt [ Neovossia (Tilletia) indica] of wheat Triticum aestivum. Ind. J. Agr. Sci. 1999;69:837–839. [Google Scholar]
- Sun M., Sakakibara H., Ashida H., Danno G., Kanazawa K. Cytochrome P4501A1-inhibitory action of antimutagenic anthraquinones-activity relationship. Biosci. Biotech. Biochem. 2000;64:1373–1378. doi: 10.1271/bbb.64.1373. [DOI] [PubMed] [Google Scholar]
- Wang D., Yang G., Engelhardt H., Liu H., Zhao J. Separation by capillary zone electrophoresis of the active anthraquinone components of the Chinese herb Polygonum multiflorum Thunb. Chromatographia. 2001;53:185–189. [Google Scholar]
- Wei Y., Zhang T., Ito Y. Preparative separation of rhein from Chinese traditional herb by repeated high-speed counter-current chromatography. J. Chromatogr., A. 2003;1017:125–130. doi: 10.1016/j.chroma.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Wilson C.L., Solar J.M., El-Ghaouth A., Wisniewski M.E. Rapid evaluation of plant extracts and essential oils for antifungal activity. Plant Dis. 1997;81:204–210. doi: 10.1094/PDIS.1997.81.2.204. [DOI] [PubMed] [Google Scholar]


