Full text
PDF

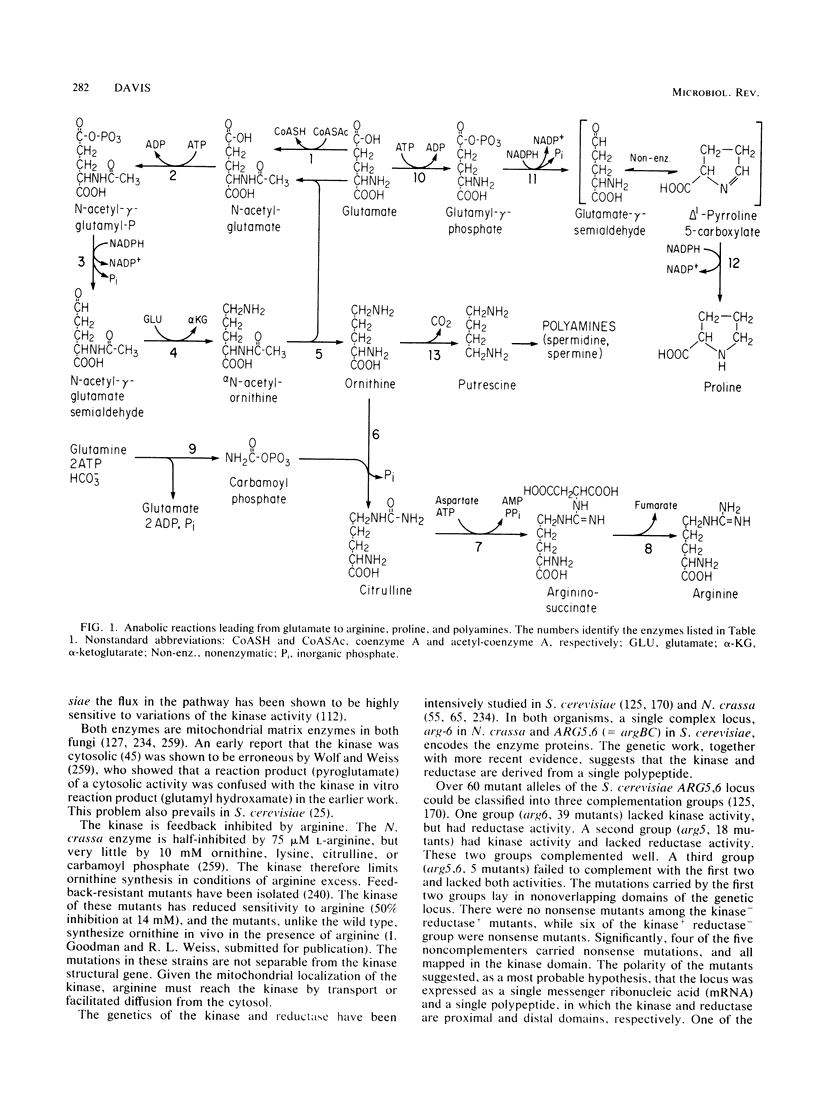
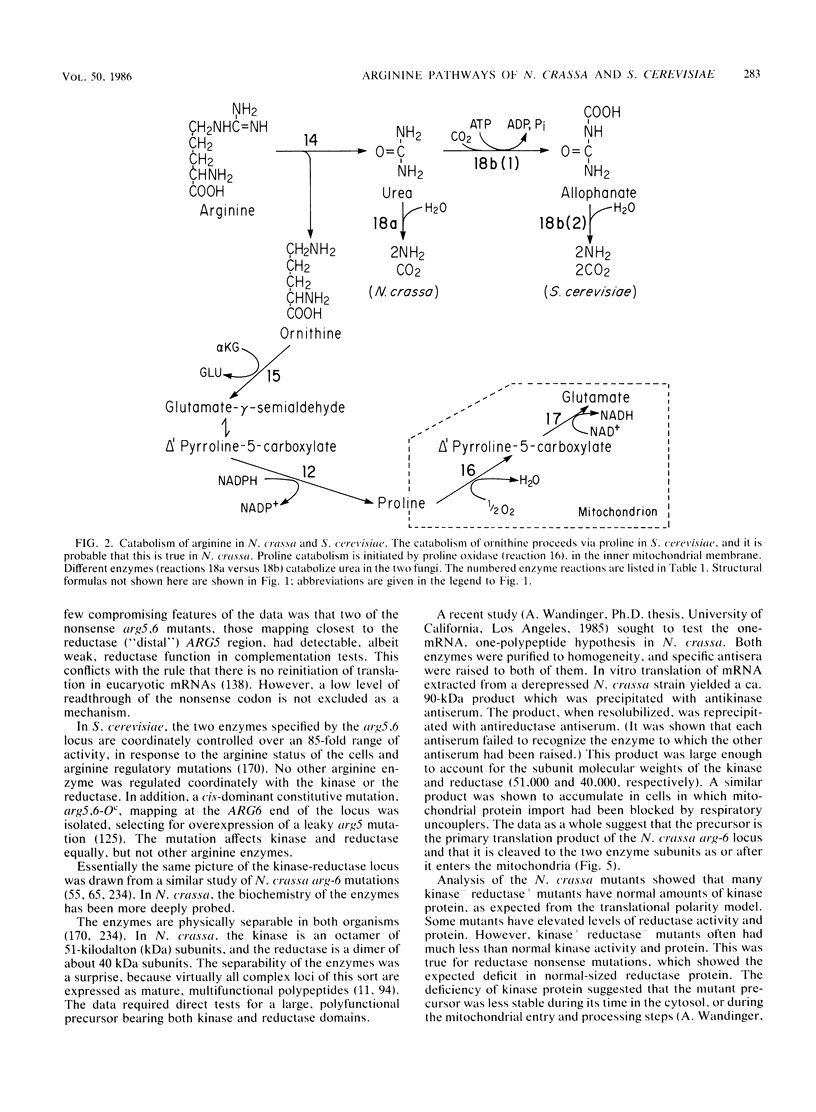
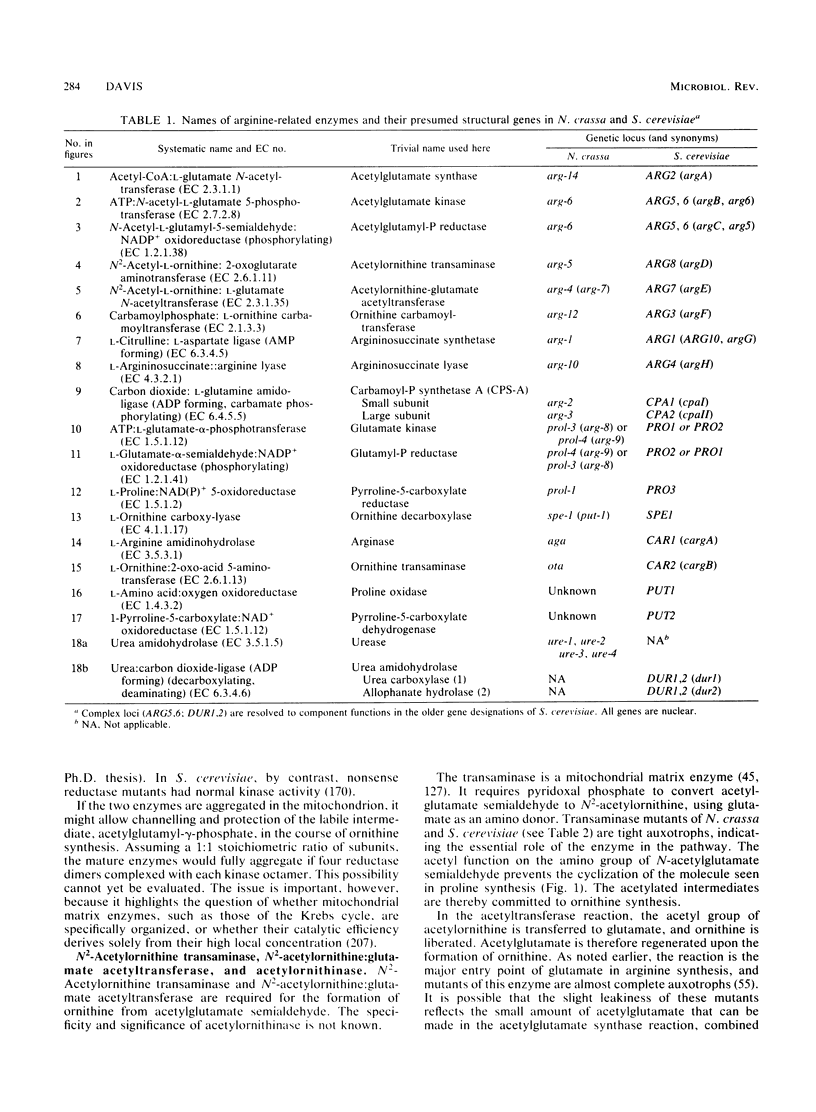
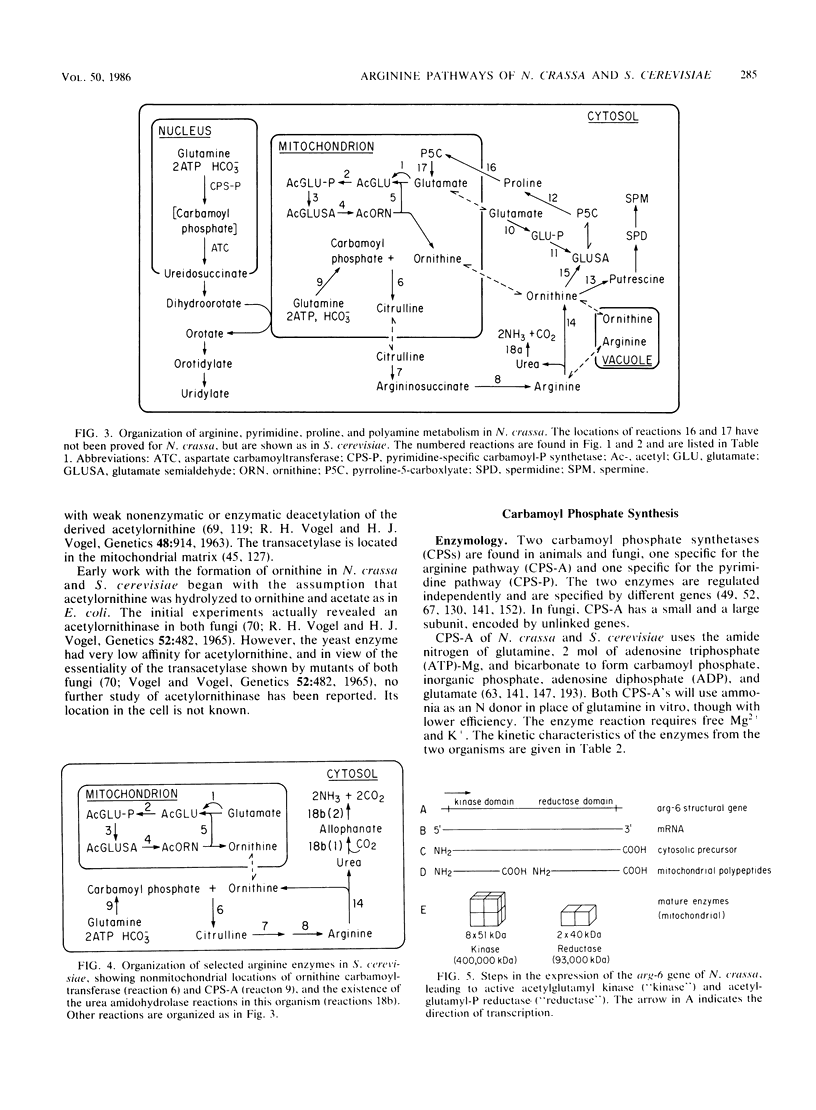






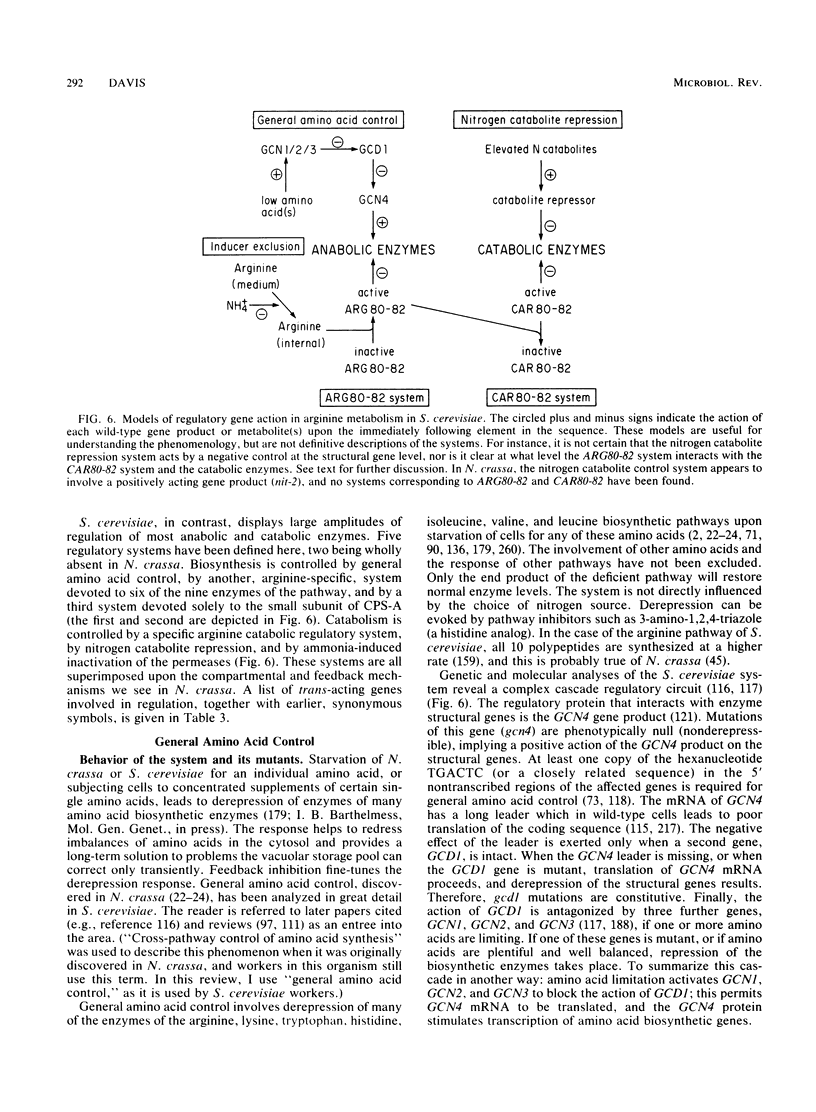









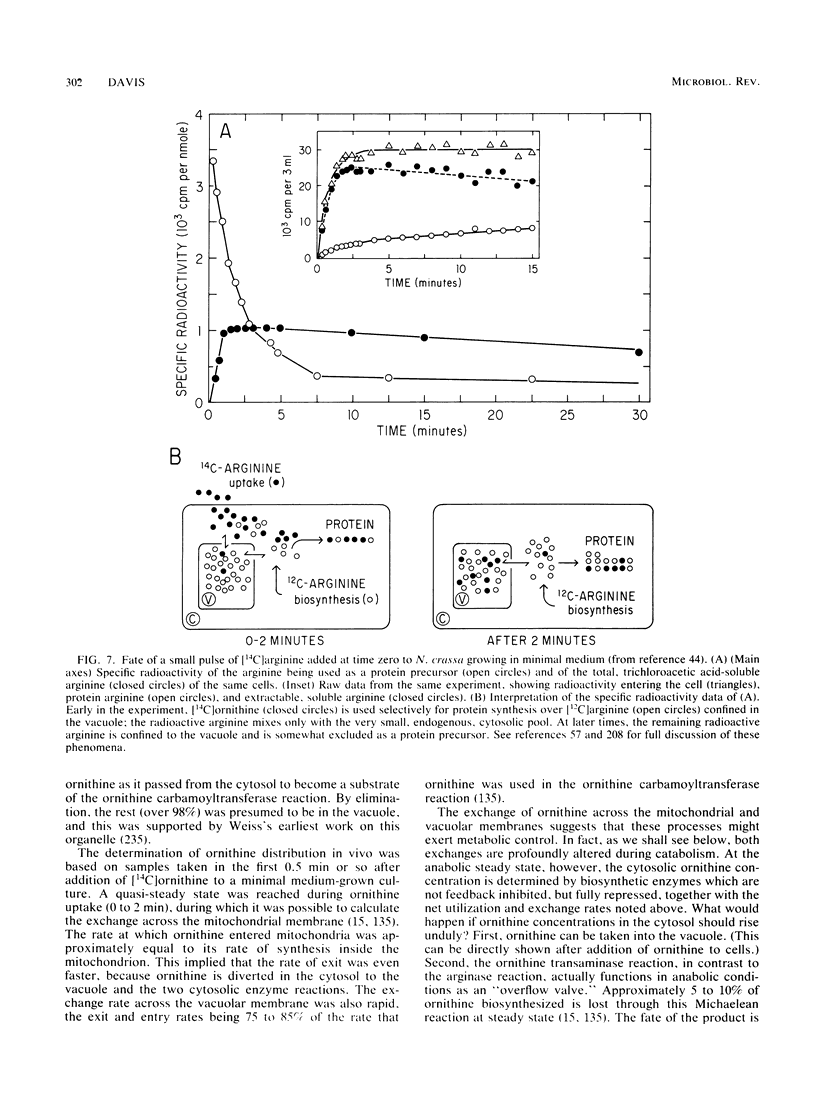

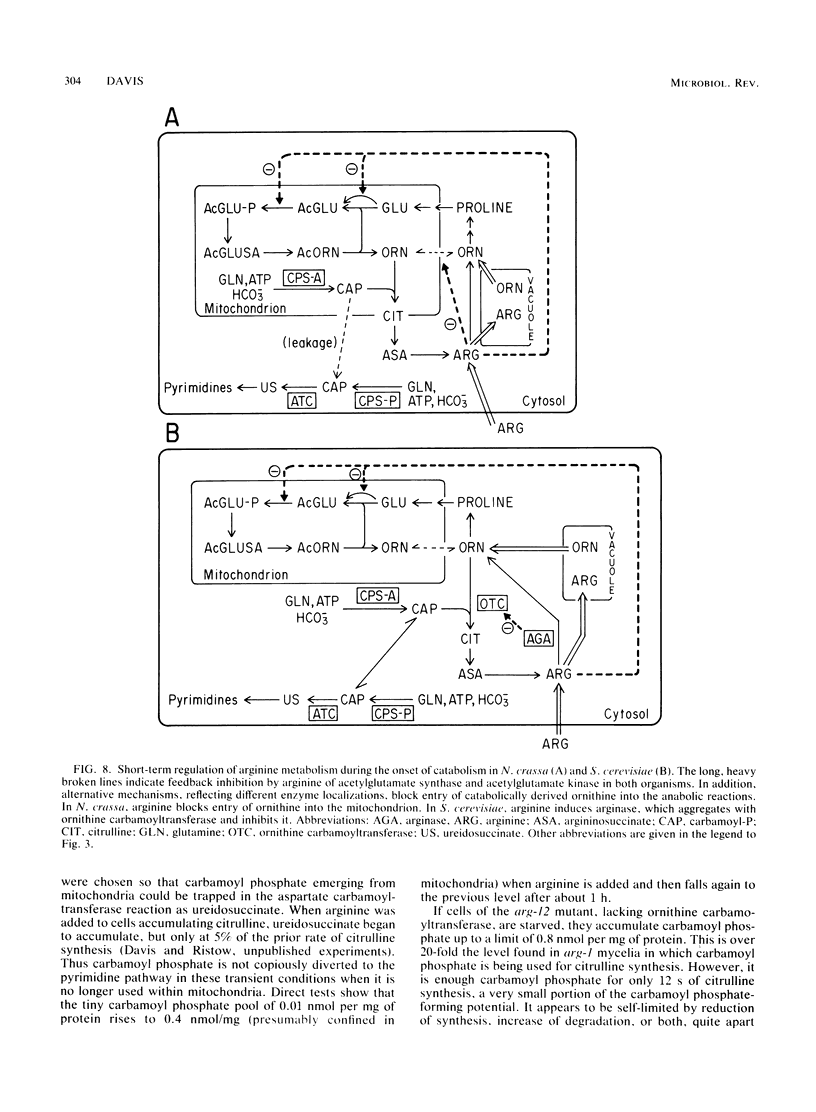









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Barthelmess I. B., Curtis C. F., Kacser H. Control of the flux to arginine in Neurospora crassa: de-repression of the last three enzymes of the arginine pathway. J Mol Biol. 1974 Aug 5;87(2):303–316. doi: 10.1016/0022-2836(74)90151-x. [DOI] [PubMed] [Google Scholar]
- Barthelmess I. B. Mutants affecting amino acid cross-pathway control in Neurospora crassa. Genet Res. 1982 Apr;39(2):169–185. doi: 10.1017/s0016672300020863. [DOI] [PubMed] [Google Scholar]
- Bates M., Weiss R. L., Clarke S. Ornithine transcarbamylase from Neurospora crassa: purification and properties. Arch Biochem Biophys. 1985 May 15;239(1):172–183. doi: 10.1016/0003-9861(85)90824-0. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Schweitzer B. W., Warrick H. M., Carbon J. The nucleotide sequence of the yeast ARG4 gene. Gene. 1984 Sep;29(3):271–279. doi: 10.1016/0378-1119(84)90056-8. [DOI] [PubMed] [Google Scholar]
- Bechet J., Greenson M., Wiame J. M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):31–39. doi: 10.1111/j.1432-1033.1970.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Benson E. W., Howe H. B., Jr Reversion and interallelic complementation at four urease loci in Neurospora crassa. Mol Gen Genet. 1978 Oct 24;165(3):277–282. doi: 10.1007/BF00332527. [DOI] [PubMed] [Google Scholar]
- Bernhardt S. A., Davis R. H. Carbamoyl phosphate compartmentation in Neurospora: histochemical localization of aspartate and ornithine transcarbamoylases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1868–1872. doi: 10.1073/pnas.69.7.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelis R., Keesey J. K., Fink G. R. The yeast his4 multifunctional protein. Immunochemistry of the wild type protein and altered forms. J Biol Chem. 1981 May 25;256(10):5144–5152. [PubMed] [Google Scholar]
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Bossinger J., Cooper T. G. Molecular events associated with induction of arginase in Saccharomyces cerevisiae. J Bacteriol. 1977 Jul;131(1):163–173. doi: 10.1128/jb.131.1.163-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger J., Lawther R. P., Cooper T. G. Nitrogen repression of the allantoin degradative enzymes in Saccharomyces cerevisiae. J Bacteriol. 1974 Jun;118(3):821–829. doi: 10.1128/jb.118.3.821-829.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Arginine catabolism in Neurospora: cycling of ornithine. J Bacteriol. 1977 Apr;130(1):285–291. doi: 10.1128/jb.130.1.285-291.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Cellular distribution of ornithine in Neurospora: anabolic and catabolic steady states. J Bacteriol. 1977 Apr;130(1):274–284. doi: 10.1128/jb.130.1.274-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J. Identification and properties of an ATPase in vacuolar membranes of Neurospora crassa. J Bacteriol. 1982 Sep;151(3):1326–1337. doi: 10.1128/jb.151.3.1326-1337.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J. Comparison of the vacuolar membrane ATPase of Neurospora crassa with the mitochondrial and plasma membrane ATPases. J Biol Chem. 1983 Dec 25;258(24):15238–15244. [PubMed] [Google Scholar]
- Brandriss M. C., Magasanik B. Proline: an essential intermediate in arginine degradation in Saccharomyces cerevisiae. J Bacteriol. 1980 Sep;143(3):1403–1410. doi: 10.1128/jb.143.3.1403-1410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss M. C., Magasanik B. Subcellular compartmentation in control of converging pathways for proline and arginine metabolism in Saccharomyces cerevisiae. J Bacteriol. 1981 Mar;145(3):1359–1364. doi: 10.1128/jb.145.3.1359-1364.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATCHESIDE D. G., OVERTON A. Complementation between alleles in heterocaryons. Cold Spring Harb Symp Quant Biol. 1958;23:137–140. doi: 10.1101/sqb.1958.023.01.017. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., McCLURE F. T. Metabolic pools and the synthesis of macromolecules. Biochim Biophys Acta. 1959 Jan;31(1):236–245. doi: 10.1016/0006-3002(59)90460-3. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., WALTON B. P. Kinetics of formation and utilization of metabolic pools in the biosynthesis of protein and nucleic acid. Biochim Biophys Acta. 1956 Aug;21(2):211–226. doi: 10.1016/0006-3002(56)90001-4. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Wesseling A. C. Cross-pathway regulation: histidine-mediated control of histidine, tryptophan, and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):893–898. doi: 10.1128/jb.119.3.893-898.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G., Cooper T. G. Isolation and characterization of mutants that produce the allantoin-degrading enzymes constitutively in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Sep;2(9):1088–1095. doi: 10.1128/mcb.2.9.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. B., Bishop J. O. Purification of argininosuccinase from Neurospora and comparison of some properties of the wild-type enzyme and an enzyme formed by inter-allelic complementation. Genet Res. 1966 Oct;8(2):243–252. doi: 10.1017/s0016672300010090. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Kyan F. S., Kyan S. S., Cheung C. W., Raijman L. The apparent Km of ammonia for carbamoyl phosphate synthetase (ammonia) in situ. Biochem J. 1985 Jul 1;229(1):205–211. doi: 10.1042/bj2290205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Regulatory mutations affecting ornithine decarboxylase activity in Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):791–799. doi: 10.1128/jb.142.3.791-799.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Lam C., Turoscy V. Structural analysis of the dur loci in S. cerevisiae: two domains of a single multifunctional gene. Genetics. 1980 Mar;94(3):555–580. doi: 10.1093/genetics/94.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Lawther R. P. Induction of the allantoin degradative enzymes in Saccharomyces cerevisiae by the last intermediate of the pathway. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2340–2344. doi: 10.1073/pnas.70.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Sumrada R. A. What is the function of nitrogen catabolite repression in Saccharomyces cerevisiae? J Bacteriol. 1983 Aug;155(2):623–627. doi: 10.1128/jb.155.2.623-627.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Magasanik B. Ammonia regulation of amino acid permeases in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Apr;3(4):672–683. doi: 10.1128/mcb.3.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Huygen R., Cunin R., Glansdorff N. The promoter region of the arg3 gene in Saccharomyces cerevisiae: nucleotide sequence and regulation in an arg3-lacZ gene fusion. EMBO J. 1983;2(2):205–212. doi: 10.1002/j.1460-2075.1983.tb01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Messenguy F., Lacroute F., Glansdorff N. Cloning arg3, the gene for ornithine carbamoyltransferase from Saccharomyces cerevisiae: expression in Escherichia coli requires secondary mutations; production of plasmid beta-lactamase in yeast. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5026–5030. doi: 10.1073/pnas.78.8.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer C. L., Davis R. H. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J Biol Chem. 1984 Apr 25;259(8):5152–5157. [PubMed] [Google Scholar]
- Cramer C. L., Ristow J. L., Paulus T. J., Davis R. H. Methods for mycelial breakage and isolation of mitochondria and vacuoles of Neurospora. Anal Biochem. 1983 Feb 1;128(2):384–392. doi: 10.1016/0003-2697(83)90390-1. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Vaughn L. E., Davis R. H. Basic amino acids and inorganic polyphosphates in Neurospora crassa: independent regulation of vacuolar pools. J Bacteriol. 1980 Jun;142(3):945–952. doi: 10.1128/jb.142.3.945-952.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- DAVIS R. H. Consequences of a suppressor gene effective with pyrimidine and proline mutants of Neurospora. Genetics. 1962 Mar;47:351–360. doi: 10.1093/genetics/47.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H., THWAITES W. M. STRUCTURAL GENE FOR ORNITHINE TRANSCARBAMYLASE IN NEUROSPORA. Genetics. 1963 Nov;48:1551–1558. doi: 10.1093/genetics/48.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H., WOODWARD V. W. The relationship between gene suppression and aspartate transcarbamylase activity in pyr-3 mutants of Neurospora. Genetics. 1962 Aug;47:1075–1083. doi: 10.1093/genetics/47.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DEKEN R. H. Pathway of arginine biosynthesis in yeast. Biochem Biophys Res Commun. 1962 Aug 31;8:462–466. doi: 10.1016/0006-291x(62)90297-8. [DOI] [PubMed] [Google Scholar]
- DEDEKEN R. H. BIOSYNTH'ESE DE L'ARGININE CHEZ LA LEVURE. I. LE SORT DE LA N-ALPHA-AC'ETYLORINITHINE. Biochim Biophys Acta. 1963 Dec 13;78:606–616. doi: 10.1016/0006-3002(63)91026-6. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Bowman B. J., Weiss R. L. Intracellular compartmentation and transport of metabolites. J Supramol Struct. 1978;9(4):473–488. doi: 10.1002/jss.400090403. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. I. Preliminary characterization of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):44–53. doi: 10.1016/0304-4165(65)90387-9. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Compartmentation and regulation of fungal metabolism: genetic approaches. Annu Rev Genet. 1975;9:39–65. doi: 10.1146/annurev.ge.09.120175.000351. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Genetics of arginine biosynthesis in Neurospora crassa. Genetics. 1979 Nov;93(3):557–575. doi: 10.1093/genetics/93.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Krasner G. N., DiGangi J. J., Ristow J. L. Distinct roles of putrescine and spermidine in the regulation of ornithine decarboxylase in Neurospora crassa. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4105–4109. doi: 10.1073/pnas.82.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Ristow J. L. Control of the ornithine cycle in Neurospora crassa by the mitochondrial membrane. J Bacteriol. 1983 Jun;154(3):1046–1053. doi: 10.1128/jb.154.3.1046-1053.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Ristow J. L., Ginsburgh C. L. Independent localization and regulation of carbamyl phosphate synthetase A polypeptides of Neurospora crassa. Mol Gen Genet. 1981;181(2):215–221. doi: 10.1007/BF00268429. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Ristow J. L., Hanson B. A. Carbamyl phosphate synthetase A of Neurospora crassa. J Bacteriol. 1980 Jan;141(1):144–155. doi: 10.1128/jb.141.1.144-155.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Sources of urea in Neurospora. Biochim Biophys Acta. 1970 Aug 14;215(2):412–414. doi: 10.1016/0304-4165(70)90042-5. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Utilization of exogenous and endogenous ornithine by Neurospora crassa. J Bacteriol. 1968 Aug;96(2):389–395. doi: 10.1128/jb.96.2.389-395.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk R. M., DeBusk A. G. Physiological and regulatory properties of the general amino acid transport system of Neurospora crassa. J Bacteriol. 1980 Jul;143(1):188–197. doi: 10.1128/jb.143.1.188-197.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delforge J., Messenguy F., Wiame J. M. The regulation of arginine biosynthesis in Saccharomyces cerevisiae. The specificity of argR- mutations and the general control of amino-acid biosynthesis. Eur J Biochem. 1975 Sep 1;57(1):231–239. doi: 10.1111/j.1432-1033.1975.tb02295.x. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Dubois E., Wiame J. M. L-Ornithine transaminase synthesis in Saccharomyces cerevisiae: regulation by inducer exclusion. Mol Gen Genet. 1979 Jul 24;174(3):225–232. doi: 10.1007/BF00267794. [DOI] [PubMed] [Google Scholar]
- Donahue T. F., Daves R. S., Lucchini G., Fink G. R. A short nucleotide sequence required for regulation of HIS4 by the general control system of yeast. Cell. 1983 Jan;32(1):89–98. doi: 10.1016/0092-8674(83)90499-3. [DOI] [PubMed] [Google Scholar]
- Drainas C., Weiss R. L. Energetics of vacuolar compartmentation of arginine in Neurospora crassa. J Bacteriol. 1982 May;150(2):770–778. doi: 10.1128/jb.150.2.770-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drainas C., Weiss R. L. Energy requirement for the mobilization of vacuolar arginine in Neurospora crassa. J Bacteriol. 1982 May;150(2):779–784. doi: 10.1128/jb.150.2.779-784.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. Release of the "ammonia effect" on three catabolic enzymes by NADP-specific glutamate dehydrogenaseless mutations in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Feb 20;50(4):967–972. doi: 10.1016/0006-291x(73)91500-3. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. The participation of the anabolic glutamate dehydrogenase in the nitrogen catabolite repression of arginase in Saccharomyces cerevisiae. Eur J Biochem. 1974 Oct 2;48(2):603–616. doi: 10.1111/j.1432-1033.1974.tb03803.x. [DOI] [PubMed] [Google Scholar]
- Dubois E., Hiernaux D., Grennon M., Wiame J. M. Specific induction of catabolism and its relation to repression of biosynthesis in arginine metabolism of Saccharomyces cerevisiae. J Mol Biol. 1978 Jul 15;122(4):383–406. doi: 10.1016/0022-2836(78)90417-5. [DOI] [PubMed] [Google Scholar]
- Dubois E., Messenguy F. Isolation and characterization of the yeast ARGRII gene involved in regulating both anabolism and catabolism of arginine. Mol Gen Genet. 1985;198(2):283–289. doi: 10.1007/BF00383008. [DOI] [PubMed] [Google Scholar]
- Dunn-Coleman N. S., Tomsett A. B., Garrett R. H. Nitrogen metabolite repression of nitrate reductase in Neurospora crassa: effect of the gln-1a locus. J Bacteriol. 1979 Aug;139(2):697–700. doi: 10.1128/jb.139.2.697-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Boller T., Wiemken A. Action of proteinases on the arginine transport system of purified vacuoles from Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1976 Nov 8;73(1):193–199. doi: 10.1016/0006-291x(76)90515-5. [DOI] [PubMed] [Google Scholar]
- Eisenstein E., Hensley P. Ligand binding-promoted conformational changes in yeast ornithine transcarbamoylase. J Biol Chem. 1986 May 15;261(14):6192–6200. [PubMed] [Google Scholar]
- Eisenstein E., Osborne J. C., Jr, Chaiken I. M., Hensley P. Purification and characterization of ornithine transcarbamoylase from Saccharomyces cerevisiae. J Biol Chem. 1984 Apr 25;259(8):5139–5145. [PubMed] [Google Scholar]
- Eversole P., DiGangi J. J., Menees T., Davis R. H. Structural gene for ornithine decarboxylase in Neurospora crassa. Mol Cell Biol. 1985 Jun;5(6):1301–1306. doi: 10.1128/mcb.5.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHAM J. R. S. Ornithine transaminase in Neurospora and its relation to the biosynthesis of proline. Biochem J. 1953 Jan;53(2):313–320. doi: 10.1042/bj0530313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHAM J. R., BOYLEN J. B. Neurospora crassa mutants lacking arginino-succinase. J Gen Microbiol. 1957 Apr;16(2):438–448. doi: 10.1099/00221287-16-2-438. [DOI] [PubMed] [Google Scholar]
- Facklam T. J., Marzluf G. A. Nitrogen regulation of amino acid catabolism in Neurospora crassa. Biochem Genet. 1978 Apr;16(3-4):343–354. doi: 10.1007/BF00484090. [DOI] [PubMed] [Google Scholar]
- Flint H. J., Dible S., Kacser H. Depression of enzyme synthesis in response to arginine limitation in Neurospora crassa. J Gen Microbiol. 1985 Nov;131(11):2891–2900. doi: 10.1099/00221287-131-11-2891. [DOI] [PubMed] [Google Scholar]
- Flint H. J., Kemp B. F. General control of arginine biosynthetic enzymes in Neurospora crassa. J Gen Microbiol. 1981 May;124(1):129–140. doi: 10.1099/00221287-124-1-129. [DOI] [PubMed] [Google Scholar]
- Flint H. J., Porteous D. J., Kacser H. Control of the flux in the arginine pathway of Neurospora crassa. The flux from citrulline to arginine. Biochem J. 1980 Jul 15;190(1):1–15. doi: 10.1042/bj1900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H. J., Tateson R. W., Barthelmess I. B., Porteous D. J., Donachie W. D., Kacser H. Control of the flux in the arginine pathway of Neurospora crassa. Modulations of enzyme activity and concentration. Biochem J. 1981 Nov 15;200(2):231–246. doi: 10.1042/bj2000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H. J., Wilkening J. Cloning of the arg-12 gene of Neurospora crassa and regulation of its transcript via cross-pathway amino acid control. Mol Gen Genet. 1986 Apr;203(1):110–116. doi: 10.1007/BF00330391. [DOI] [PubMed] [Google Scholar]
- Gaertner F. H., Cole K. W. A cluster-gene: evidence for one gene, one polypeptide, five enzymes. Biochem Biophys Res Commun. 1977 Mar 21;75(2):259–264. doi: 10.1016/0006-291x(77)91037-3. [DOI] [PubMed] [Google Scholar]
- González A., Tenorio M., Vaca G., Mora J. Neurospora crassa mutant impaired in glutamine regulation. J Bacteriol. 1983 Jul;155(1):1–7. doi: 10.1128/jb.155.1.1-7.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman I., Weiss R. L. Control of arginine metabolism in Neurospora: flux through the biosynthetic pathway. J Bacteriol. 1980 Jan;141(1):227–234. doi: 10.1128/jb.141.1.227-234.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Crabeel M., Wiame J. M., Béchet J. Inhibition of protein synthesis and simulation of permease turnover in yeast. Biochem Biophys Res Commun. 1968 Feb 26;30(4):414–419. doi: 10.1016/0006-291x(68)90760-2. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hennaut C. Mutation affecting activity of several distinct amino acid transport systems in Saccharomyces cerevisiae. J Bacteriol. 1971 Feb;105(2):477–482. doi: 10.1128/jb.105.2.477-482.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M. Inactivation-reactivation process and repression of permease formation regulate several ammonia-sensitive permeases in the yeast Saccharomyces cerevisiae. Eur J Biochem. 1983 Jun 1;133(1):135–139. doi: 10.1111/j.1432-1033.1983.tb07438.x. [DOI] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. II. Evidence for a specific lysine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):339–346. doi: 10.1016/0304-4165(66)90388-6. [DOI] [PubMed] [Google Scholar]
- Grenson M. Study of the positive control of the general amino-acid permease and other ammonia-sensitive uptake systems by the product of the NPR1 gene in the yeast Saccharomyces cerevisiae. Eur J Biochem. 1983 Jun 1;133(1):141–144. doi: 10.1111/j.1432-1033.1983.tb07439.x. [DOI] [PubMed] [Google Scholar]
- Grove G., Marzluf G. A. Identification of the product of the major regulatory gene of the nitrogen control circuit of Neurospora crassa as a nuclear DNA-binding protein. J Biol Chem. 1981 Jan 10;256(1):463–470. [PubMed] [Google Scholar]
- HALVORSON H. O., COHEN G. N. Incorporation des amino-acides endogènes et exogènes dans les protéines de la levure. Ann Inst Pasteur (Paris) 1958 Jul;95(1):73–87. [PubMed] [Google Scholar]
- Haas D., Kurer V., Leisinger T. N-acetylglutamate synthetase of Pseudomonas aeruginosa. An assay in vitro and feedback inhibition by arginine. Eur J Biochem. 1972 Dec 4;31(2):290–295. doi: 10.1111/j.1432-1033.1972.tb02531.x. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haysman P., Howe H. B., Jr Some genetic and physiological characteristics of urease-defective strains of Neurospora crassa. Can J Genet Cytol. 1971 Jun;13(2):256–269. doi: 10.1139/g71-043. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Klig L. S., Loewy B. S. The genetic regulation and coordination of biosynthetic pathways in yeast: amino acid and phospholipid synthesis. Annu Rev Genet. 1984;18:207–231. doi: 10.1146/annurev.ge.18.120184.001231. [DOI] [PubMed] [Google Scholar]
- Hilger F., Culot M., Minet M., Pierard A., Grenson M., Wiame J. M. Studies on the kinetics of the enzyme sequence mediating arginine synthesis in Saccharomyces cerevisiae. J Gen Microbiol. 1973 Mar;75(1):33–41. doi: 10.1099/00221287-75-1-33. [DOI] [PubMed] [Google Scholar]
- Hilger F., Mortimer R. K. Genetic mapping of arg1 and arg8 in Saccharomyces cerevisiae by trisomic analysis combined with interallelic complementation. J Bacteriol. 1980 Jan;141(1):270–274. doi: 10.1128/jb.141.1.270-274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger F., Simon J. P., Stalon V. Yeast argininosuccinate synthetase. Purification; structural and kinetic properties. Eur J Biochem. 1979 Feb 15;94(1):153–163. doi: 10.1111/j.1432-1033.1979.tb12882.x. [DOI] [PubMed] [Google Scholar]
- Hinde R. W., Jacobson J. A., Weiss R. L., Davis R. H. N-acetyl-L-glutamate synthase of Neurospora crassa. Characteristics, localization, regulation, and genetic control. J Biol Chem. 1986 May 5;261(13):5848–5852. [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G., Fink G. R. Positive regulation in the general amino acid control of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5374–5378. doi: 10.1073/pnas.80.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G., Fink G. R. Repeated DNA sequences upstream from HIS1 also occur at several other co-regulated genes in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 25;258(8):5238–5247. [PubMed] [Google Scholar]
- Hoare D. S., Hoare S. L., Brame J. Deacetylation of N-acetyl-L-glutamic acid by Neurospora crassa. J Bacteriol. 1967 Sep;94(3):782–783. doi: 10.1128/jb.94.3.782-783.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet P. P., Wiame J. M. On the nature of argR mutations is Saccharomyces cerevisiae. Eur J Biochem. 1974 Mar 15;43(1):87–92. doi: 10.1111/j.1432-1033.1974.tb03388.x. [DOI] [PubMed] [Google Scholar]
- Indge K. J. Polyphosphates of the yeast cell vacuole. J Gen Microbiol. 1968 May;51(3):447–455. doi: 10.1099/00221287-51-3-447. [DOI] [PubMed] [Google Scholar]
- JONES M. E. Carbamyl phosphate. Science. 1963 Jun 28;140(3574):1373–1379. [PubMed] [Google Scholar]
- Jacobs E., Dubois E., Wiame J. M. Regulation of ureaamidolyase synthesis in Saccharomyces cerevisiae, RNA analysis, and cloning of the positive regulatory gene DURM. Curr Genet. 1985;9(5):333–339. doi: 10.1007/BF00421602. [DOI] [PubMed] [Google Scholar]
- Jacobs P., Jauniaux J. C., Grenson M. A cis-dominant regulatory mutation linked to the argB-argC gene cluster in Saccharomyces cerevisiae. J Mol Biol. 1980 Jun 5;139(4):691–704. doi: 10.1016/0022-2836(80)90055-8. [DOI] [PubMed] [Google Scholar]
- Jauniaux J. C., Dubois E., Vissers S., Crabeel M., Wiame J. M. Molecular cloning, DNA structure, and RNA analysis of the arginase gene in Saccharomyces cerevisiae. A study of cis-dominant regulatory mutations. EMBO J. 1982;1(9):1125–1131. doi: 10.1002/j.1460-2075.1982.tb01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux J. C., Urrestarazu L. A., Wiame J. M. Arginine metabolism in Saccharomyces cerevisiae: subcellular localization of the enzymes. J Bacteriol. 1978 Mar;133(3):1096–1107. doi: 10.1128/jb.133.3.1096-1107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. E. Regulation of pyrimidine and arginine biosynthesis in mammals. Adv Enzyme Regul. 1970;9:19–49. doi: 10.1016/s0065-2571(71)80036-5. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kanamori K., Legerton T. L., Weiss R. L., Roberts J. D. Nitrogen-15 spin-lattice relaxation times of amino acids in Neurospora crassa as a probe of intracellular environment. Biochemistry. 1982 Sep 28;21(20):4916–4920. doi: 10.1021/bi00263a013. [DOI] [PubMed] [Google Scholar]
- Karlin J. N., Bowman B. J., Davis R. H. Compartmental behavior of ornithine in Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):3948–3955. [PubMed] [Google Scholar]
- Kolmark H. G. Urease defective mutants in Neurospora crassa. Mol Gen Genet. 1969 Jul 3;104(3):219–234. doi: 10.1007/BF02539286. [DOI] [PubMed] [Google Scholar]
- Kulaev I. S., Vagabov V. M. Polyphosphate metabolism in micro-organisms. Adv Microb Physiol. 1983;24:83–171. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- LEVENBERG B. Role of L-glutamine as donor of carbamyl nitrogen for the enzymatic synthesis of citruline in Agaricus bisporus. J Biol Chem. 1962 Aug;237:2590–2598. [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- Legerton T. L., Kanamori K., Weiss R. L., Roberts J. D. 15N NMR studies of nitrogen metabolism in intact mycelia of Neurospora crassa. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1495–1498. doi: 10.1073/pnas.78.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Kanamori K., Weiss R. L., Roberts J. D. Measurements of cytoplasmic and vacuolar pH in Neurospora using nitrogen-15 nuclear magnetic resonance spectroscopy. Biochemistry. 1983 Feb 15;22(4):899–903. doi: 10.1021/bi00273a029. [DOI] [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of sequestered metabolities into degradative reactions by nutritional stress in Neurospora. J Bacteriol. 1979 Jun;138(3):909–914. doi: 10.1128/jb.138.3.909-914.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of vacuolar arginine in Neurospora crassa. Mechanism and role of glutamine. J Biol Chem. 1984 Jul 25;259(14):8875–8879. [PubMed] [Google Scholar]
- Lemoine Y., Dubois E., Wiame J. M. The regulation of urea amidolyase of Saccharomyces cerevisiae: mating type influence on a constitutivity mutation acting in cis. Mol Gen Genet. 1978 Nov 9;166(3):251–258. [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Aggregation states of a regulatory enzyme complex catalyzing the early steps of pyrimidine biosynthesis in bakers' yeast. Can J Biochem. 1971 Apr;49(4):403–411. doi: 10.1139/o71-059. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Metabolic compartmentation at the molecular level: the function of a multienzyme aggregate in the pyrimidine pathway of yeast. Biochim Biophys Acta. 1970 Dec 16;220(3):365–372. doi: 10.1016/0005-2744(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Widgren E. E., Broglie K. E., Nyunoya H. Yeast carbamyl phosphate synthetase. Structure of the yeast gene and homology to Escherichia coli carbamyl phosphate synthetase. J Biol Chem. 1983 Dec 10;258(23):14466–14477. [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. PHYSIOLOGICAL CHANNELING OF TRYPTOPHAN IN NEUROSPORA CRASSA. Biochim Biophys Acta. 1964 Apr 4;86:91–99. doi: 10.1016/0304-4165(64)90162-x. [DOI] [PubMed] [Google Scholar]
- Mackay E. M., Pateman J. A. Nickel requirement of a urease-deficient mutant in Aspergillus nidulans. J Gen Microbiol. 1980 Jan;116(1):249–251. doi: 10.1099/00221287-116-1-249. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Radford A. Genetics and biochemistry of carbamoyl phosphate biosynthesis and its utilization in the pyrimidine biosynthetic pathway. Microbiol Rev. 1978 Jun;42(2):307–328. doi: 10.1128/mr.42.2.307-328.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf G. A. Regulation of nitrogen metabolism and gene expression in fungi. Microbiol Rev. 1981 Sep;45(3):437–461. doi: 10.1128/mr.45.3.437-461.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Messenguy F. Concerted repression of the synthesis of the arginine biosynthetic enzymes by aminoacids: a comparison between the regulatory mechanisms controlling aminoacid biosyntheses in bacteria and in yeast. Mol Gen Genet. 1979 Jan 16;169(1):85–95. doi: 10.1007/BF00267549. [DOI] [PubMed] [Google Scholar]
- Messenguy F., Delforge J. Role of transfer ribonucleic acids in the regulation of several biosyntheses in Saccharomyces cerevisiae. Eur J Biochem. 1976 Aug 16;67(2):335–339. doi: 10.1111/j.1432-1033.1976.tb10696.x. [DOI] [PubMed] [Google Scholar]
- Messenguy F., Dubois E. Participation of transcriptional and post-transcriptional regulatory mechanisms in the control of arginine metabolism in yeast. Mol Gen Genet. 1983;189(1):148–156. doi: 10.1007/BF00326068. [DOI] [PubMed] [Google Scholar]
- Messenguy F., Feller A., Crabeel M., Piérard A. Control-mechanisms acting at the transcriptional and post-transcriptional levels are involved in the synthesis of the arginine pathway carbamoylphosphate synthase of yeast. EMBO J. 1983;2(8):1249–1254. doi: 10.1002/j.1460-2075.1983.tb01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Penninckx M., Wiame J. M. Interaction between arginase and ornithine carbamoyltransferase in Saccharomyces cerevisiae. The regulatory site for ornithine. Eur J Biochem. 1971 Sep 24;22(2):277–286. doi: 10.1111/j.1432-1033.1971.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Messenguy F. Regulation of arginine biosynthesis in Saccharomyces cerevisiae: isolation of a cis-dominant, constitutive mutant for ornithine carbamoyltransferase synthesis. J Bacteriol. 1976 Oct;128(1):49–55. doi: 10.1128/jb.128.1.49-55.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Wiame J. -M. The control of ornithinetranscarbamylase activity by arginase in Saccharomyces cerevisiae. FEBS Lett. 1969 Apr;3(1):47–49. doi: 10.1016/0014-5793(69)80093-1. [DOI] [PubMed] [Google Scholar]
- Mestichelli L. J., Gupta R. N., Spenser I. D. The biosynthetic route from ornithine to proline. J Biol Chem. 1979 Feb 10;254(3):640–647. [PubMed] [Google Scholar]
- Middelhoven W. J. The derepression of arginase and of ornithine transaminase in nitrogen-starved baker's yeast. Biochim Biophys Acta. 1968 Mar 11;156(2):440–443. doi: 10.1016/0304-4165(68)90284-5. [DOI] [PubMed] [Google Scholar]
- Middelhoven W. J. The ferrous ion as the cofactor of arginase in vivo. I. Properties of yeast arginase metallo-complexes of known composition and of native arginase. Biochim Biophys Acta. 1969 Sep 30;191(1):110–121. doi: 10.1016/0005-2744(69)90320-9. [DOI] [PubMed] [Google Scholar]
- Middelhoven W. J., de Waard M. A., Mulder E. G. The ferrous ion as the cofactor of arginase in vivo. II. Experiments on the replacement of ferrous ions in native yeast arginase by other cations in vivo. Biochim Biophys Acta. 1969 Sep 30;191(1):122–129. doi: 10.1016/0005-2744(69)90321-0. [DOI] [PubMed] [Google Scholar]
- Minet M., Jauniaux J. C., Thuriaux P., Grenson M., Wiame J. M. Organization and expression of a two-gene cluster in the arginine biosynthesis of Saccharomyces cerevisiae. Mol Gen Genet. 1979 Jan 11;168(3):299–308. doi: 10.1007/BF00271500. [DOI] [PubMed] [Google Scholar]
- Morgan D. H. Selection and characterisation of mutants lacking arginase in Neurospora crassa. Mol Gen Genet. 1970;108(4):291–302. doi: 10.1007/BF00267766. [DOI] [PubMed] [Google Scholar]
- NEWMEYER D. Genes influencing the conversion of citrulline to argininosuccinate in Neurospora crassa. J Gen Microbiol. 1962 Jun;28:215–230. doi: 10.1099/00221287-28-2-215. [DOI] [PubMed] [Google Scholar]
- Nagy M., Laporte J., Penverne B., Hervé G. Nuclear localization of aspartate transcabamoylase in Saccharomyces cerevisiae. J Cell Biol. 1982 Mar;92(3):790–794. doi: 10.1083/jcb.92.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario M. The accumulation of argininosuccinate in Neurospora crassa. II. Inhibition of arginyl-tRNA synthesis by argininosuccinate. Biochim Biophys Acta. 1967 Aug 22;145(1):146–152. doi: 10.1016/0005-2787(67)90663-6. [DOI] [PubMed] [Google Scholar]
- Ness S. A., Weiss R. L. Carbamoyl-phosphate synthetases from Neurospora crassa. Immunological relatedness of the enzymes from Neurospora, bacteria, yeast, and mammals. J Biol Chem. 1985 Nov 15;260(26):14355–14362. [PubMed] [Google Scholar]
- Nicolay K., Scheffers W. A., Bruinenberg P. M., Kaptein R. In vivo 31P NMR studies on the role of the vacuole in phosphate metabolism in yeasts. Arch Microbiol. 1983 Jul;134(4):270–275. doi: 10.1007/BF00407801. [DOI] [PubMed] [Google Scholar]
- Niederberger P., Miozzari G., Hütter R. Biological role of the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jul;1(7):584–593. doi: 10.1128/mcb.1.7.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie-Villa S., DeBusk R. M., DeBusk A. G. Characterization of 2-aminoisobutyric acid transport in Neurospora crassa: a general amino acid permease-specific substrate. J Bacteriol. 1981 Sep;147(3):944–948. doi: 10.1128/jb.147.3.944-948.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Okorokov L. A., Lichko L. P., Kulaev I. S. Vacuoles: main compartments of potassium, magnesium, and phosphate ions in Saccharomyces carlsbergenis cells. J Bacteriol. 1980 Nov;144(2):661–665. doi: 10.1128/jb.144.2.661-665.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. I. Properties of two amino acid transport systems. Biochim Biophys Acta. 1969 Jan 28;173(1):113–127. doi: 10.1016/0005-2736(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. II. Properties of a basic amino acid transport system. Biochim Biophys Acta. 1970 Mar 17;203(1):139–149. doi: 10.1016/0005-2736(70)90044-1. [DOI] [PubMed] [Google Scholar]
- Pall M. L., Kelly K. A. Specificity of transinhibition of amino acid transport in neurospora. Biochem Biophys Res Commun. 1971 Mar 5;42(5):940–947. doi: 10.1016/0006-291x(71)90521-3. [DOI] [PubMed] [Google Scholar]
- Paulus H. The evolutionary history of the ornithine cycle as a determinant of its structure and regulation. Curr Top Cell Regul. 1983;22:177–200. doi: 10.1016/b978-0-12-152822-5.50010-5. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Cramer C. L., Davis R. H. Compartmentation of spermidine in Neurospora crassa. J Biol Chem. 1983 Jul 25;258(14):8608–8612. [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Regulation of polyamine synthesis in relation to putrescine and spermidine pools in Neurospora crassa. J Bacteriol. 1981 Jan;145(1):14–20. doi: 10.1128/jb.145.1.14-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn M. D., Galgoci B., Greer H. Identification of AAS genes and their regulatory role in general control of amino acid biosynthesis in yeast. Proc Natl Acad Sci U S A. 1983 May;80(9):2704–2708. doi: 10.1073/pnas.80.9.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn M. D., Thireos G., Greer H. Temporal analysis of general control of amino acid biosynthesis in Saccharomyces cerevisiae: role of positive regulatory genes in initiation and maintenance of mRNA derepression. Mol Cell Biol. 1984 Mar;4(3):520–528. doi: 10.1128/mcb.4.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx M., Simon J. P., Wiame J. M. Interaction between arginase and L-ornithine carbamoyltransferase in Saccharomyces cerevisiae. Purification of S. cerevisiae enzymes and evidence that these enzymes as well as rat-liver arginase are trimers. Eur J Biochem. 1974 Nov 15;49(2):429–442. doi: 10.1111/j.1432-1033.1974.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Piérard A., Messenguy F., Feller A., Hilger F. Dual regulation of the synthesis of the arginine pathway carbamoylphosphate synthase of Saccharomyces cerevisiae by specific and general controls of amino acid biosynthesis. Mol Gen Genet. 1979 Jul 13;174(2):163–171. doi: 10.1007/BF00268353. [DOI] [PubMed] [Google Scholar]
- Piérard A., Schröter B. Structure-function relationships in the arginine pathway carbamoylphosphate synthase of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):167–176. doi: 10.1128/jb.134.1.167-176.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. W., Holwell J. H., Abdelal A. T. Purification and properties of the arginine-specific carbamoyl-phosphate synthase from Saccharomyces cerevisiae. J Gen Microbiol. 1978 May;106(1):145–151. doi: 10.1099/00221287-106-1-145. [DOI] [PubMed] [Google Scholar]
- Rao E. Y., Rao T. K., Debusk A. G. Isolation and characterization of a mutant of Neurospora crassa deficient in general amino acid permease activity. Biochim Biophys Acta. 1975 Nov 17;413(1):45–51. doi: 10.1016/0005-2736(75)90057-7. [DOI] [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- Roon R. J., Hampshire J., Levenberg B. Urea amidolyase. The involvement of biotin in urea cleavage. J Biol Chem. 1972 Dec 10;247(23):7539–7545. [PubMed] [Google Scholar]
- Roon R. J., Levenberg B. An adenosine triphosphate-dependent, avidin-sensitive enzymatic cleavage of urea in yeast and green algae. J Biol Chem. 1968 Oct 10;243(19):5213–5215. [PubMed] [Google Scholar]
- Roon R. J., Levenberg B. CO2 fixation and the involvement of allophanate in the biotin-enzyme-catalyzed cleavage of urea. J Biol Chem. 1970 Sep 10;245(17):4593–4595. [PubMed] [Google Scholar]
- Roon R. J., Levenberg B. Urea amidolyase. I. Properties of the enzyme from Candida utilis. J Biol Chem. 1972 Jul 10;247(13):4107–4113. [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. An arginine/histidine exchange transport system in vacuolar-membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1984 Sep 25;259(18):11509–11511. [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Schlenk F., Dainko J. L., Svihla G. The accumulation and intracellular distribution of biological sulfoninum compounds in yeast. Arch Biochem Biophys. 1970 Sep;140(1):228–236. doi: 10.1016/0003-9861(70)90027-5. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R. A., Cooper T. G. Isolation of the CAR1 gene from Saccharomyces cerevisiae and analysis of its expression. Mol Cell Biol. 1982 Dec;2(12):1514–1523. doi: 10.1128/mcb.2.12.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R. A., Cooper T. G. Nucleotide sequence of the Saccharomyces cerevisiae arginase gene (CAR1) and its transcription under various physiological conditions. J Bacteriol. 1984 Dec;160(3):1078–1087. doi: 10.1128/jb.160.3.1078-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R. A., Cooper T. G. Point mutation generates constitutive expression of an inducible eukaryotic gene. Proc Natl Acad Sci U S A. 1985 Feb;82(3):643–647. doi: 10.1073/pnas.82.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R. A., Cooper T. G. Urea carboxylase and allophanate hydrolase are components of a multifunctional protein in yeast. J Biol Chem. 1982 Aug 10;257(15):9119–9127. [PubMed] [Google Scholar]
- Sumrada R., Cooper T. G. Control of vacuole permeability and protein degradation by the cell cycle arrest signal in Saccharomyces cerevisiae. J Bacteriol. 1978 Oct;136(1):234–246. doi: 10.1128/jb.136.1.234-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surdin Y., Sly W., Sire J., Bordes A. M., Robichon-Szulmajster H. Propriétés et contrôle génétique du système d'accumulation des acides aminés chez Saccharomyces cerevisiae. Biochim Biophys Acta. 1965 Oct 18;107(3):546–566. [PubMed] [Google Scholar]
- Thireos G., Penn M. D., Greer H. 5' untranslated sequences are required for the translational control of a yeast regulatory gene. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5096–5100. doi: 10.1073/pnas.81.16.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuriaux P., Ramos F., Piérard A., Grenson M., Wiame J. M. Regulation of the carbamoylphosphate synthetase belonging to the arginine biosynthetic pathway of Saccharomyces cerevisiae. J Mol Biol. 1972 Jun 20;67(2):277–287. doi: 10.1016/0022-2836(72)90241-0. [DOI] [PubMed] [Google Scholar]
- Thwaites W. M. A mutation reducing feedback regulation by arginine in suppressed pyr-3 mutants in Neurospora. Genetics. 1967 Apr;55(4):769–781. doi: 10.1093/genetics/55.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites W. M., Knauert F. K., Carney S. S. Complementation Analysis of Metabolite-Resistant Mutations with Forced Heterokaryons of NEUROSPORA CRASSA. Genetics. 1973 Aug;74(4):581–593. doi: 10.1093/genetics/74.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites W. M., Pendyala L. Regulation of amino acid assimilation in a strain of Neurospora crassa lacking basic amino acid transport activity. Biochim Biophys Acta. 1969 Dec 30;192(3):455–461. doi: 10.1016/0304-4165(69)90394-8. [DOI] [PubMed] [Google Scholar]
- Turoscy V., Cooper T. G. Pleiotropic control of five eucaryotic genes by multiple regulatory elements. J Bacteriol. 1982 Sep;151(3):1237–1246. doi: 10.1128/jb.151.3.1237-1246.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urech K., Dürr M., Boller T., Wiemken A., Schwencke J. Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch Microbiol. 1978 Mar;116(3):275–278. doi: 10.1007/BF00417851. [DOI] [PubMed] [Google Scholar]
- Urrestarazu L. A., Vissers S., Wiame J. M. Change in location of ornithine carbamoyltransferase and carbamoylphosphate synthetase among yeasts in relation to the arginase/ornithine carbamoyltransferase regulatory complex and the energy status of the cells. Eur J Biochem. 1977 Oct 3;79(2):473–481. doi: 10.1111/j.1432-1033.1977.tb11830.x. [DOI] [PubMed] [Google Scholar]
- Vaca G., Mora J. Nitrogen regulation of arginase in Neurospora crassa. J Bacteriol. 1977 Sep;131(3):719–725. doi: 10.1128/jb.131.3.719-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn L. E., Davis R. H. Purification of vacuoles from Neurospora crassa. Mol Cell Biol. 1981 Sep;1(9):797–806. doi: 10.1128/mcb.1.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampler D. E., Fairley J. L. Argininosuccinate synthetase of Neurospora crassa. Arch Biochem Biophys. 1967 Sep;121(3):580–586. doi: 10.1016/0003-9861(67)90041-0. [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A. U., Ness S. A., Weiss R. L. Simultaneous purification of three mitochondrial enzymes. Acetylglutamate kinase, acetylglutamyl-phosphate reductase and carbamoyl-phosphate synthetase from Neurospora crassa. J Biol Chem. 1986 Apr 15;261(11):4820–4827. [PubMed] [Google Scholar]
- Wandinger-Ness A. U., Wolf E. C., Weiss R. L., Davis R. H. Acetylglutamate kinase-acetylglutamyl-phosphate reductase complex of Neurospora crassa. Evidence for two polypeptides. J Biol Chem. 1985 May 25;260(10):5974–5978. [PubMed] [Google Scholar]
- Weiss R. L., Anterasian G. P. Control of arginine metabolism in Neurospora. Induction of ornithine aminotransferase. J Biol Chem. 1977 Oct 25;252(20):6974–6980. [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Control of arginine utilization in Neurospora. J Bacteriol. 1977 Feb;129(2):866–873. doi: 10.1128/jb.129.2.866-873.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Weiss R. L., Lee C. A. Isolation and characterization of Neurospora crassa mutants impaired in feedback control of ornithine synthesis. J Bacteriol. 1980 Mar;141(3):1305–1311. doi: 10.1128/jb.141.3.1305-1311.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M., Feller A., Piérard A. Nucleotide sequence of yeast gene CP A1 encoding the small subunit of arginine-pathway carbamoyl-phosphate synthetase. Homology of the deduced amino acid sequence to other glutamine amidotransferases. Eur J Biochem. 1985 Jan 15;146(2):371–381. doi: 10.1111/j.1432-1033.1985.tb08663.x. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G., Magasanik B. The induction of urea carboxylase and allophanate hydrolase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6203–6209. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Requirement for HCO3- by ATP: urea amido-lyase in yeast. Biochem Biophys Res Commun. 1970 Aug 24;40(4):814–819. doi: 10.1016/0006-291x(70)90975-7. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. Urea carboxylase from Saccharomyces cerevisiae. Evidence for a minimal two-step reaction sequence. J Biol Chem. 1973 Jan 10;248(1):325–330. [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiame J. M., Grenson M., Arst H. N., Jr Nitrogen catabolite repression in yeasts and filamentous fungi. Adv Microb Physiol. 1985;26:1–88. doi: 10.1016/s0065-2911(08)60394-x. [DOI] [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S. A., Davis R. H. Evidence for two discrete carbamyl phosphate pools in Neurospora. J Biol Chem. 1971 Feb 25;246(4):973–978. [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S., Davis R. H. Copurification of pyrimidine-specific carbamyl phosphate synthetase and aspartate transcarbamylase of Neurospora crassa. Biochemistry. 1970 Oct 27;9(22):4329–4335. doi: 10.1021/bi00824a013. [DOI] [PubMed] [Google Scholar]
- Wimmer M. J., Rose I. A., Powers S. G., Meister A. Evidence that carboxyphosphate is a kinetically competent intermediate in the carbamyl phosphate synthetase reaction. J Biol Chem. 1979 Mar 25;254(6):1854–1859. [PubMed] [Google Scholar]
- Wipe B., Leisinger T. Regulation of activity and synthesis of N-acetylglutamate synthase from Saccharomyces cerevisiae. J Bacteriol. 1979 Dec;140(3):874–880. doi: 10.1128/jb.140.3.874-880.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E. C., Weiss R. L. Acetylglutamate kinase. A mitochondrial feedback-sensitive enzyme of arginine biosynthesis in Neurospora crassa. J Biol Chem. 1980 Oct 10;255(19):9189–9195. [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]
- ZALOKAR M. Kinetics of amino acid uptake and protein synthesis in Neurospora. Biochim Biophys Acta. 1961 Jan 29;46:423–432. doi: 10.1016/0006-3002(61)90573-x. [DOI] [PubMed] [Google Scholar]
- Zacharski C. A., Cooper T. G. Metabolite compartmentation in Saccharomyces cerevisiae. J Bacteriol. 1978 Aug;135(2):490–497. doi: 10.1128/jb.135.2.490-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


