Abstract
In the present study antioxidant activities by (1,1-diphenyl-2-picrylhydrazyl radical (DPPH), hydrogen peroxide, hydroxyl radical inhibition, hemolysis by hydrogen peroxide assay, reducing power and total antioxidant activities of polyphenolic extract of Ichnocarpus frutescens leaves were investigated. The flavonoids and total polyphenolic contents of the extract were also determined using standard methods. Phytochemical analyses revealed the presence of flavonoids, polyphenols, anthocyanins and simple phenolic acids. The results of antioxidant activities of polyphenol extract obtained by different in vitro methods were varied depending on the method used. Nevertheless, polyphenol extract showed significant inhibitory activities in all in vitro reactive oxygen species scavenging, might be attributed due to the high level of polyphenolic compound. Also, these various antioxidant activities were compared to α-tocopherol and l-ascorbic acid as reference antioxidant compounds. These findings provide evidence that the polyphenolic extract of I. frutescens is a natural source of antioxidant against oxidative damage.
Keywords: Ichnocarpus frutescens, Polyphenolic extract (PPE), Radical scavenging activity, Antioxidants, Oxidative stress
1. Introduction
Oxidative stress occurs when there is excessive free radical production and/or low antioxidant defense, which leads to chemical alterations of biomolecules causing structural and functional modifications (Hoake and Pastorino, 2002). Oxidative damage causing plays a significant pathological role in human diseases such as cancer, inflammation arthritis, diabetes and atherosclerosis (Halliwell, 1991). Currently available synthetic antioxidants showed low solubility, promote negative health and moderate antioxidant activity (Barlow, 1990). Hence, string restrictions have been placed on their application and there is trend to substitute them with naturally available antioxidants. Recently there has been an upsurge of interest in the therapeutic potential of medicinal plants as antioxidants in reducing free radical induced tissue injury. Also many other plant species have been investigated in the search of novel antioxidants (Omar et al., 2009), but there is still a demand to find more effective antioxidants from plant species. It has been mentioned that antioxidants activity of plants might be due to their phenolic compounds (Cook and Samman, 1996). However, antioxidant supplements or foods containing antioxidant may be used to help the human body from the oxidative damage (Kuhnan, 1976).
The leaves of Ichnocarpus frutescens are used extensively in the form of decoction for its antidiabetic activity by the tribal of Karnataka and Utter Pradesh states, for treating diabetes (Parinitha et al., 2004). Leaves are boiled in oil and applied in headaches and fevers; they are also applied to wounds (Anonymous, 1976). Some of the constituents of the plant, such as polyphenols and flavonoids were shown to present antidiabetic, antioxidant and related biological activities (Du Thie and Crozier, 2000). Our previous study shows that I. frutescens have anti-diabetic, anti-cancer activity and hepatoprotective (Kumarappan and Mandal, 2007). Active constituents in I. frutescens include flavonoids, simple phenolic acids, coumarins, pentacyclic triterpenoids (Singh and Singh, 1987). So far number of them, including polyphenolics, flavonoids, and various plants extracts, have been reported to be effective radical scavengers and inhibitors of lipid peroxidation (Godjevac et al., 2004). In the present study, the antioxidants activity of polyphenolic extract of I. frutescens was examined in different ROS scavenging, reducing power, hemolysis and lipid peroxidation assays in order to evaluate its natural antioxidant properties.
2. Methods and materials
2.1. Chemicals
Folin–Ciocalteu reagent, 4-dinitrophenylhydrazine, DPPH (1,1-diphenyl-2-picryl-hydrazyl, 2-deoxyribose, Thiobarbituric acid, Trichloroacetic acid, alpha tocopherol, l-ascorbic acid, pyrocatechol, naringenin, quarcetin, linoleic acid, xylenol orange, manganese chloride, HPLC grade methanol, butylated hydroxyl toluene, p-anisaldehyde and Fast blue B salt were purchased from SISCO Research Laboratory, Mumbai, India. All other chemicals and solvents were purchased from locally and used were of analytical grade.
2.2. Plant materials
The fresh leaves of I. frutescens (L.) R.Br. were collected from Delta region of Cauvery River, Thiruchirappalli, India, in February 2005 and was authenticated at Botanical Survey of India (BSI), Central National Herbarium (CNH), Howrah, India (REF NO: CNH/I-I/87/2005-TECH/1326). An authentic voucher specimen was deposited in the Herbarium of Division of Pharmacognosy, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India.
2.3. Preparation of polyphenolic extract (PPE)
Dried leaves of I. frutescens (500 g) were finely powdered, mixed with 70% methanol and kept at room temperature for 5 days. After 5 days it was filtered and the solvent was evaporated. The residue was dissolved in water and the aqueous layer was washed with petroleum ether several times until a clear upper layer of petroleum ether was obtained. The lower layer was then treated with ethyl acetate containing glacial acetic acid (10 mL/l). Extraction of polyphenols was carried out for 36 h at room temperature and the combined ethyl acetate layer was concentrated. The residue was lyophilized and stored at −70 °C. The total polyphenolic content and flavonoid of the extract were assayed using the standard methods. Preliminary phytochemical screening of extract of I. frutescens leaf was carried out for the detection of phytoconstituents, using standard chemical tests (Harborne, 1998).
2.4. Total phenolic and flavonoid contents
Total soluble phenolics and flavonoids in the PPE were determined with Folin–Ciocalteu reagent according to the previously reported method (Singleton et al., 1999; Chang et al., 2002). The concentration of total phenolic compounds in PPE was determined as microgram of pyrocatechol equivalent by using an equation that was obtained from standard pyrocatechol graph. Flavones and flavonols in polyphenolic extract (PPE) were expressed as quercetin equivalent. Flavanones in polyphenolic extract (PPE) were expressed as naringenin equivalent.
2.5. In vitro antioxidant assays
The in vitro antioxidant assays were carried out to determine the free radical scavenging capacity using the DPPH radical (α,α-diphenyl-β-picryl hydrazyl radicals), hydroxyl radicals and hydrogen peroxide, according to the standard methods (Blois, 1958; Chung et al., 1997; Ruch et al., 1989). Reducing power was investigated using the previously developed method (Oyaizu, 1986). The total antioxidant activity of PPE was determined using thiocynate method (Mitsuda et al., 1996).
2.6. Effect on hydrogen peroxide (H2O2) induced hemolysis
2.6.1. Isolation of erythrocytes
All experiments were performed with Human blood. Healthy human blood was collected in acid-citrate dextrose solution. The packed erythrocytes were isolated by centrifugation at 3000g for 10 min at 4 °C. The plasma and buffy coat were removed by aspiration and cells thus obtained were washed thrice with phosphate buffer saline, pH 7.4 and a suspension of packed cell were prepared in the same buffer.
2.6.2. Incubation of erythrocyte with oxidants/antioxidants
Hemolysis of erythrocytes was carried out using H2O2 by a modified method (Otomo and Fujihira, 1970). That is, 0.02 M of normal human erythrocyte was taken in 0.1 mL of 0.02 M phosphate buffer saline, pH 7.4, and mixed with 0.5 mL of the test sample (PPE and alpha tocopherol) and 0.1 mL of H2O2 in phosphate buffered saline. In the absence of a test sample, 0.5 mL of buffer was included in the assay medium. The mixture was incubated for 1 h in a shaking water bath at 37 °C and then centrifuged at 1500g for 10 min. Following this, 1.5 mL of the red cell free supernatant solution from each tube was transferred to cuvettes and the optical density was read at 540 nm in a spectrophotometer using distilled water as a bank, which gives the extent of hemolysis. An inhibitory effect (R %) of the test compounds on hemolysis was estimated by the following equation where OD is optical density:
2.7. Effect on in vitro lipid peroxidation
2.7.1. Preparation of rat homogenates
Rats were handled according international regulations, and maintained under regular conditions of humidity, food, circadian cycles, and temperature. Brain homogenates was obtained from 3-month-old male Wistar rats weighing 200–250 g. For the ferrous ion oxidation with xylenol orange (FOX) method, 40% (w/v) homogenates were prepared in HPLC-grade methanol.
2.7.2. Lipid peroxidation by FOX method
Lipid peroxidation was conducted for a 60 min interval at 37 °C. The mixture for lipid hydroperoxide generation contained 10 μL of Fenton’s reagent (5 μL of 5 mM manganese chloride and 5 μL of 50 mM hydrogen peroxide), 10 μL of the aqueous extract, and 80 μL of each homogenate (Mitsuda et al., 1996). Nine hundred microliters of FOX reagent (49 mg of ferrous ammonium sulfate in 50 mL of 250 mM H2SO4, 0.397 g of BHT, and 0.038 g of xylenol orange in 950 mL of HPLC grade methanol) was added to each sample and left to react for 30 min at room temperature. The absorbance was read at 560 nm (Jiang et al., 1992).
2.8. Statistical analysis
Data were analyzed using Graphat Instat Software (San Diego, CA, USA). The experimental data were expressed as mean ± SEM. The concentration of the extract (μg/mL) that was required to scavenge 50% of radicals was calculated by using linear regression method of plots of the percent of antiradical activity against the concentration of the tested compounds using Microsoft Excel Software Programme. The percent scavenging activities of five different extract concentrations. Percent scavenging activity was calculated as [1 − (Ai − Aj)/Ac] × 100. Where Ai is the absorbance measured with different PPE fractions in the particular assay with a ROS source; Aj is the absorbance measured with different PPE fractions in the particular assay but without a ROS source.
3. Results
Total phenolic contents of PPE were expressed as mg of pyrocatechol equivalent per gram of dry weight of PPE extract. 1000 μg of PPE was used to determine the amount of total polyphenolic contents. The level of total polyphenolic compounds was 184.52 mg of pyrocatechol equivalent per gram of PPE. The present study showed the flavonoid content determined by two independent colorimetric methods, one for the determination of flavones and flavonols and other for determination of flavanones, as reported by earlier. The contents of total flavonoids in the PPE of I. frutescens were expressed as the sum of two complementary methods for the determination of flavones, flavonols and flavonones and the results found to be 36.42 mg of quercetin and naringenin equivalent per gram of PPE. Major types of phenolic constituents identified in the leaves of I. frutescens are simple phenolic acids, flavonol, flavones, flavonones and flavonoid glycosides.
3.1. Effect on DPPH radicals
In the present study, the scavenging of DPPH radicals exerted by PPE as well as α-tocopherol was summarized in Fig. 1 At higher concentrations, PPE showed slightly higher scavenging activity. The linear response curves were also obtained and the IC50 were estimated 163.38 μg/mL for PPE and 142.91 μg/mL for α-tocopherol. In the presence of an electron donating antioxidant, the purple color of the DPPH free radical diminishes in intensity a change that can be followed spectrophotometrically at 517 nm. The radical scavenging activity of the extract measured as decolorizing activity following the trapping of the unpaired electron of the DPPH.
Figure 1.
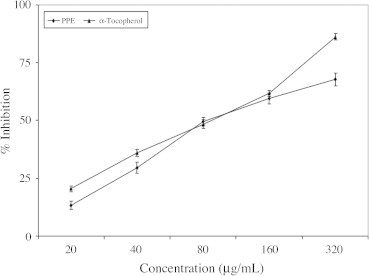
DPPH radical scavenging effect of polyphenolic extract of I. frutescens. Values are expressed as mean ± SEM. Values are mean of three experiments.
3.2. Effect on hydroxyl radicals
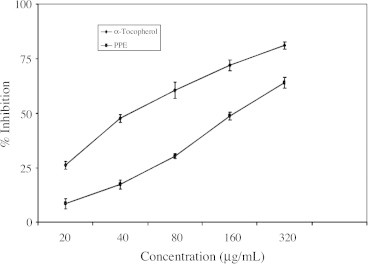
The scavenging effect against hydroxyl radicals was investigated by using 2-deoxyribose oxidation method. Fig. 2 shows the hydroxyl radical scavenging effect of PPE. The scavenging effect of PPE on hydroxyl radical was concentration dependent. The deoxyribose method is a simple assay to determine the rate constant for reactions of hydroxyl radicals. When the mixture of FeCl3–EDTA–H2O2 and ascorbate were incubated with deoxyribose in phosphate buffer (pH 7.4), the hydroxyl radicals generated attack the deoxyribose and result in a series of reaction that cause the formation of melondialdehyde. Any hydroxyl radical scavenger added to the reaction would compete with deoxyribose for the availability of hydroxyl radicals, thus reducing the amount of MDA formation. In this system we have tested the scavenging activity of PPE with positive control ascorbic acid. In Fig. 3 we found that the maximum scavenging capacity of hydroxyl radicals could be achieved with increased concentrations of PPE.
Figure 2.
Hydrogen peroxide radical scavenging effect of polyphenolic extract of I. frutescens. Values are expressed as mean ± SEM. Values are mean of three experiments.
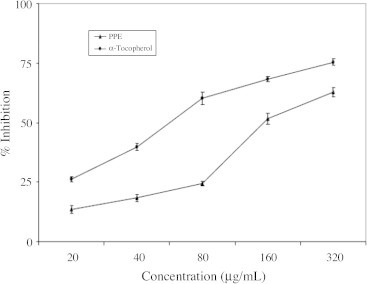
Figure 3.
Hydroxyl radical scavenging activity of polyphenolic extract of I. frutescenss. Values are expressed as mean ± SEM. All the values are mean of three experiments.
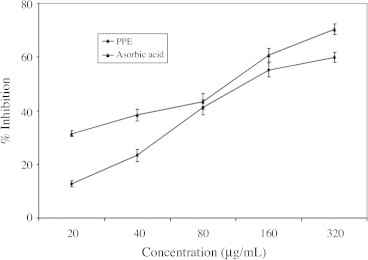
3.3. Effect on hydrogen peroxide radicals
The ability of the PPE and ascorbic acid to effectively scavenge hydrogen peroxide, determined according to the method of Ruch et al. (1989) is displayed in Fig. 3 PPE was capable of scavenging H2O2 in concentration dependent manner.
3.4. Effect on reducing power
The reducing power of the PPE and ascorbic acid are shown in Fig. 4. For the measurements of the reductive ability, we investigated the Fe3+–Fe2+ formation in the presence of PPE using the method of Oyaizu (1986). The reducing power of PPE was strong and increased steadily with increasing amount of sample. Our data on the reducing power of PPE suggested that it was likely to contribute significantly towards the antioxidant effect.
Figure 4.
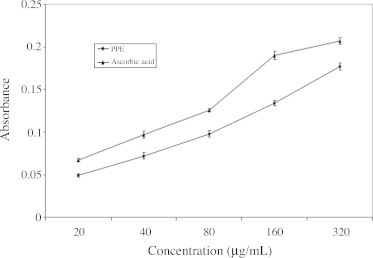
Effect of polyphenolic extract of I. frutescens on reducing power. Values are expressed as mean ± SEM. Values are mean of three experiments.
3.5. Effect on total antioxidant activity
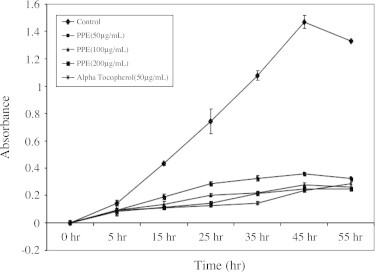
Fig. 6 shows the time course plots for the anti-oxidative activity of the different concentrations of PPE using FTC method. The amount of peroxide formed in emulsion during incubation was determined spectrophotometrically by measuring absorbance at 500 nm. High absorbance is the indication of high concentration of formed peroxide, while lowest indication of anti-oxidative activity. The anti-oxidative activities of PPE was measured in linoleic acid emulsion and compared with those of alpha tocopherol. As shown Fig. 5, the auto-oxidation of linoleic acid was effectively inhibited by the addition of PPE. Therefore, the result indicated that PPE seemed to contain some anti-oxidative phenolic compounds.
Figure 6.
Anti-lipid peroxidation effect of polyphenolic extract of I. frutescens on Fenton’s reagent induced lipid peroxidation. Values are expresses as mean ± SEM. Values are mean of three experiments.
Figure 5.
Total antioxidant effect of polyphenolic extract of I. frutescens on linoleic acid emulsion induced lipid peroxidation. Values are expressed as mean ± SEM. Values are mean of three experiments.
3.6. Effect on H2O2 induced hemolysis
The anti-hemolytic activity of the polyphenolic extract of I. frutescens and l-ascorbic acid are shown in Table 1. The PPE and l-ascorbic acid in vitro inhibited the hemolysis of rat RBC due to H2O2 induced hemolysis in a dose dependent manner.
Table 1.
Anti-radical and in vitro antioxidant activity of polyphenolic extract (PPE) of I. frutescens, as expressed (μg/mL) by inhibitory concentration (IC50). Each value represents the mean ± SEM of three replicate experiments.
| Method | Inhibitors (μg/mL) | Inhibitory Concentration (IC50) (μg/mL) |
|---|---|---|
| DPPH radical | PPE | 163.38 |
| α-Tocopherol (20–320) | 147.91 | |
| H2O2 radical | PPE (20–320) | 217.16 |
| α-Tocopherol (20–320) | 133.89 | |
| Hydroxyl radical | PPE (20–320) | 214.43 |
| α-Tocopherol (20–320) | 76.79 | |
| H2O2 induced | PPE (6.25–100) | 15.54 |
| Hemolysis | Ascorbic acid (4.4–70.4) | 1.50 |
| FOX Method | PPE (20–320) | 163.89 |
| α-Tocopherol (20–320) | 114.53 |
3.7. Effect on in vitro lipid peroxidation
Anti-lipid peroxidation activities of the PPE and ascorbic acid were evaluated using rat brain homogenate (FOX method) as the test model (Fig. 6). We used Fenton’s reagent [H2O2 + Mn (Manganese ion because it does not react with xylenol orange in the FOX method)] as the inducer of lipid peroxidation in rat brain homogenate. The biggest percentage of induction of lipid peroxidation was observed in the rat brain homogenate. These highest percentage of lipid peroxidation allowed establishing readily the antioxidant activity of PPE. PPE significantly inhibited the Fenton’s reagent mediated lipid peroxidation of rat brain homogenate in a concentration dependent manner. The IC50 values for the anti-lipid peroxidation activity of the PPE and ascorbic acid 163.89 μg/mL and 114.53 μg/mL, respectively.
4. Discussion
DPPH radical scavenging method is standard procedure applied to the evaluation of antiradical activity. The DPPH free radicals, which are stable in ethanol shows maximum a proton donating substances such as antioxidant, the radicals would be scavenged and absorbed. PPE showed dose dependent DPPH radical scavenging activity (Fig. 1). The effect of free radical scavenging activity of PPE on DPPH radicals is thought to be due to their hydrogen donation ability of polyphenols of I. frutescens. The results showed that PPE is a free radical scavenger, as well as primary antioxidant that react with free radicals, which may limit the occurrence of free radical damage in human body.
Hydroxyl radical is an extremely reactive species formed in biological systems and has been implicated as highly damaging in free radical pathology, capable of damaging almost every molecule found in living cells (Hochestein and Atallah, 1988). This radical has the capacity to join nucleotides in DNA and cause strand breakage, which contribute to ageing, carcinogenesis, mutagenesis, cytotoxicity and several diseases. Among the oxygen radicals specifically, the OH radical is the most reactive and severely damages adjacent biomolecules such as, all proteins, DNA, PUFA and almost any biological molecules. In addition, this species is considered to be one of the quick initiators of the lipid peroxidation process, abstracting hydrogen atoms from unsaturated fatty acids (Kappus, 1991). Therefore, the removal of hydroxyl radical is probably one of the most effective defenses of a living body against various diseases. The ability of the PPE to quench hydroxyl radicals seems to directly relate to the prevention of propagation of the process of lipid peroxidation, and the extract seems to be a good scavenger of active radical species, thus reducing the rate of chain reaction.
Hydrogen peroxide can be formed in vivo by many oxidizing enzymes such as superoxide dismutase (SOD). Hydrogen peroxide itself is not very reactive it can sometimes be toxic to cell because it may give rise to OH radical in the cells. Addition of hydrogen peroxide to cells in culture can lead to transition metal ion dependent OH radicals mediated DNA damage. Scavenging of hydrogen peroxide by PPE may be attributed to their phenolic nature, which can donate electrons to H2O2, thus neutralizing it to water (Saurav and Kannabiran, 2012). Thus removing hydrogen peroxide is very important for life being away from damage.
The antioxidant activities of natural compounds may have a reciprocal correlation with their reducing power. The reducing power increased as the extract concentration increased, indicating some polyphenolic compounds in PPE were electron donors and could react with free radicals to convert them into more stable products and to terminate radical chain reactions. The reducing power of bioactive compounds had been reported to be associated with their antioxidant activity (Meir et al., 1995). In the FTC method, we found that the amount of peroxide in the initial stage of lipid peroxidation is greater than the amount of peroxide in the secondary stage. Furthermore, the secondary product such as melondialdehyde is not stable for a long period of time. It will turn into alcohol and acid which cannot be detected spectrophotometrically (Ottolenghi, 1959).
It has been shown that the interaction of hemoglobin and H2O2 produces reactive oxygen species and induces lipid peroxidation (D’ Souza and Prabhu, 2006). An imbalance in the oxidant/antioxidant status of a red blood cell with an excess of a free radicals leads to the formation of oxidative stress, which appear to cause several diseases. These reactions may give rise to alteration in red cell membrane structure and function, which in turn affect the survival of red cells in the circulation (Rice-evans and Hochestein, 1981). The polypenolic extract found to effectively inhibit H2O2 induced hemolysis of erythrocytes, revealing its ability to scavenge most of the free radicals generated. Polyphenolic from some fruits and vegetables have also been shown to enhance red blood cell resistance to oxidative stress, in vivo and in vitro (Alam et al., 2012). Our data on PPE extract show that increasing concentrations of PPE containing polyphenols gradually inhibits H2O2 induced hemolysis in erythrocytes. Therefore, the protective effect of PPE may be due to its association with protection of red blood cell, which prevent the membrane peroxidation and thereby RBC hemolysis.
In biological systems, MDA is a very reactive species and takes part in cross-linking of DNA with proteins and also damaging the cells (Jadhav et al., 1996). MDA, being the major product of lipid peroxidation is used to study the anti-lipid peroxidation activity in rat brain homogenates by means of reaction with TBA at high temperature and acidic conditions. Currently, a number of synthetic and natural antioxidants are used in the peroxidation and retardation of lipid peroxidation. While synthetic antioxidants have proven effective unpleasant side effects have been reported. In this study, we examined the possible in vitro antioxidant activity against Fenton’s reagent induced lipid peroxidation, PPE showed potent in vitro anti-lipid peroxidation activity and less harmful alternative to the synthetic antioxidant products.
Free radicals have been implicated in many diseases conditions, the important ones being superoxide radical, hydroxyl radical, peroxyl radical and singlet oxygen. These highly reactive species have a potential for bringing about extensive damages, including lipid peroxidation DNA lesion and protein fragmentation within the cells of biomolecules. In addition, it has been suggested that there is an inverse relationship between dietary intake of antioxidants-rich foods and the incidence of human diseases (Rice-Evans et al., 1997). In searching novel natural antioxidants, some plants have been extensively studied in the past few years for their antioxdiative and radical scavenging components, because reactive oxygen species (ROS) production and oxidative stress have been linked to ageing related illness and large number of other illness (Finkel and Holbrook, 2000).
Herbal drugs containing radical scavengers are gaining importance in treating oxidative stress related diseases. Many plants exhibit efficient antioxidant properties owing to their phenolic constituents (Larson, 1988). In the present experiments preliminary phytochemical screening of I. frutescens leaves showed the presence of flavonoids, phenolic compounds and anthocyanins. TLC Finger print analysis also suggested the presence of phenolic and flavonoid compounds. We investigated the antioxidant activity of the polyphenolic extract and the possible mechanism involved, basing on the response obtained in the six different in vitro and ex vivo models covering major radicals viz, DPPH, hydroxyl and hydrogen peroxide including hemolysis and total antioxidant activity by ferric thiocynate (FTC) method.
In conclusion from the above investigation, using several in vitro and in vivo models, polyphenolics extract was found to scavenge DPPH radicals, hydroxyl radicals hydrogen peroxide and inhibits lipid peroxidation. The total antioxidant and free radical scavenging of the polyphenolic extract of I frutescens can be attributed to the presence of enriched polyphenolic compounds. Polyphenols, anthocyanins and flavonoids are very valuable plant constituents in the scavenging of free radicals, due to their several phenolic hydroxyl groups (Hatano et al., 1988). Combining this fact with obtained results we could suggest that the amount of polyphenolic compound increases, antioxidant activity increase as well. This study clearly suggested that the polyphenolic extract of I. frutescens leaf had significant antioxidant activity, which might be helpful in preventing or slowing the process of various oxidative stress related diseases of major organs. However, further work is needed to develop a method for fractionation of polyphenols and identification, and to determine the most active antioxidant compounds in this polyphenolic extract.
Acknowledgements
The authors grateful to All India Council of Technical Education (AICTE), Government of India, New Delhi, India, for providing financial support (File No: FD/NDF/S/2005-2006) to carrying out this work.
References
- Alam N., Ki N.Y., Lee J.S., Cho H.J., Lee T.S. Consequence of the antioxidant activities and tyrosinase inhibitory effects of various extractsfrom the fruiting bodies of Pleurotus ferulae, Saudi. J. Biol. Sci. 2012;19:111–118. doi: 10.1016/j.sjbs.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous, 1976. The Wealth of India, vol. 5. Council of Scientific and Industrial Research, New Delhi, pp. 162–163.
- Barlow S.M. Toxicological aspects of antioxidants uses as food additives. In: Hudson B.J.F., editor. Food Antioxidants. Elsevier; London: 1990. pp. 253–307. [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chung S.K., Osawa T., Kawakishi S. Hydroxyl radical scavenging effects of spices and scavengers from Brown Mustard (Brassica nigra) Biosci. Biotech. Biochem. 1997;61:118–123. [Google Scholar]
- Cook N.C., Samman S. Flavonoids – chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996;7:66–76. [Google Scholar]
- D’ Souza H.P., Prabhu R.H. In vitro inhibition of lipid peroxidation in fish by turmeric (curcuma longa) Ind. J. Clin. Biochem. 2006;29:305–312. doi: 10.1007/BF02912929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Thie G., Crozier A. Plant derived polyphenolic antioxidants. Curr. Opin. Nutr. Metab. 2000;3:447–451. doi: 10.1097/00075197-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Godjevac D., Vajs V., Menkovic N., Tesevic V., Janakovic P., Milosavljevic S. Flavonoids from flowers of Cephalaria pastricensis and their anti-radical activity. J. Serb. Chem. Soc. 2004;69:883–886. [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: sources, biochemistry and role in human diseases. Am. J. Med. 1991;91:14–22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Harborne J.B. Phytochemical Methods. Kluwer Academic/Plenum Publishers; New York: 1998. A Guide to Modern Techniques of Plant Analysis; pp. 60–66. [Google Scholar]
- Hatano T., Kagawa H., Yasuhara T., Okuda Two new flavonoids and other constituents in licorice root their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988;36:2090–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- Hoake J.B., Pastorino J.G. Ethanol, oxidative stress and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Hochestein P., Atallah A.S. The nature of oxidant and antioxidant systems in the inhibition of mutation and cancer. Mutat. Res. 1988;202:363–375. doi: 10.1016/0027-5107(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Jadhav S.J., Nimbalkar S.S., Kulkarni A.D., Madhavi D.L. Lipid oxidation in biological and food systems. In: Madhavi D.L., Deshpande S.S., Salunkhe D.K., editors. Food Antioxidants: Technological, Toxicological, and Health Perspectives. Marcel Dekker Inc.; New York: 1996. pp. 5–63. [Google Scholar]
- Jiang Z., Hunt J., Wolf S. Ferrous ion oxidation in the presence of xyleneol orange for detection of lipid peroxidation in low density lipoprotein. Anal. Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Kappus H. Free Radicals and Food Additives. Taylor and Francis; London: 1991. Lipid-peroxidation mechanism and biological relevance; pp. 59–75. [Google Scholar]
- Kuhnan J. The flavonoids: a class of semi-essential food components; their role in human nutrition. World Rev. Nutr. Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- Kumarappan C.T., Mandal S.C. Anti-tumor activity of polyphenolic extract of Ichnocarpus frutescens. Exp. Oncol. 2007;29:94–101. [PubMed] [Google Scholar]
- Larson R.A. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. [Google Scholar]
- Meir S., Kanner J., Akiri B., Hadas S.P.J. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. Agric. Food Chem. 1995;43:1813–1819. [Google Scholar]
- Mitsuda H., Yuasumoto K., Iwami K. Antioxidant action of indole compounds during the autoxidation of linoleic acid. Eiyoto Shokuryo. 1996;19:210–214. [Google Scholar]
- Omar A.A., Hawazin H.M., Asmita V.P., Gerald B. Chemical composition and antioxidant activities of Jeddah corniche algae, Saudi Arabia, Saudi. J. Biol. Sci. 2009;16:23–29. doi: 10.1016/j.sjbs.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo S., Fujihira E. Stabilizing effects of anti-inflammatory drugs on erythrocyte membrane: on the haemolysis and lipid peroxidation induced H2O2 in canine erythrocytes. Yakugaku Zasshi. 1970;90:1347–1354. doi: 10.1248/yakushi1947.90.11_1347. [DOI] [PubMed] [Google Scholar]
- Ottolenghi A. Interaction of ascorbic acid and mitochondria lipids. Arch. Biochem. Biophys. 1959;79:355–363. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- Parinitha M., Harish G.U., Vivek N.C., Mahesh T., Shivanna M.B. Ethno-botanical wealth of Bhadra wild life sanctuary in Karnataka. Ind. J. Trad. Knowledge. 2004;31:37–50. [Google Scholar]
- Rice-Evans C., Hochestein P. Alterations in erythrocyte membrane fluidity by phenylhydrazine induced lipid peroxidation of lipids. Biochem. Biophys. Res. Commun. 1981;100:1537–1542. doi: 10.1016/0006-291x(81)90693-8. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Sampson J., Bramley P., Hollowa D.E. Why do we accept carotenoids to be antioxidants in vivo. Free Radical Res. 1997;26:381–398. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- Ruch R.J., Cheng S.J., Klainig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechin isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Saurav K., Kannabiran K. Cytotoxicity and antioxidant activity of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp., Saudi. J. Biol. Sci. 2012;19:81–86. doi: 10.1016/j.sjbs.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.P., Singh R.P. Flavonoids of the flowers of Ichnocarpus frutescens. J. Ind. Chem. Soc. 1987;14:715–756. [Google Scholar]
- Singleton V.L., Orthofer R.M., Ramuela-Raventos R.M. Analysis of total phenols and antioxidants and other substrates by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]


