Abstract
Taxol is a microtubule inhibitor drug widely used in treatment of many types of cancer. Nephrotoxicity is the most hazardous effect complicating chemotherapy in general and kidney functions must be monitored early during any chemotherapeutic course. The main objective of the present study was to investigate the effect of acute Taxol nephrotoxicity in mice. In the present study Taxol at different doses; MD, ID and MTD (0.6, 1.15 and 1.7 mg/kg), respectively, was given by intra-peritoneal route to 54 adult male mice with an average body weight of 20–25 g. Kidney samples was taken 6, 24, 48 h following administration, fixed in 10% neutral buffered formalin, paraffin sections 5 μm thick were stained by haematoxylin and eosin and PAS and then examined for histological changes. Samples from animals treated by the maximum dose (MTD = 1.7 mg/kg) for 48 h were fixed in 3% gluteraldehyde in phosphate buffer (pH 7.4) and processed for transmission electron microscope. Taxol given for short duration was found to produce marked degenerative changes in kidney parenchyma even in minimum tolerated dose (MD = 0.6 mg/kg). Individual variations were observed regarding the degree of nephrotoxicity. There was marked loss of renal tubules epithelial lining, damage of brush border and formation of hyaline casts within the damaged tubules. The alterations were in the form of both necrotic and apoptotic changes in the kidney tubules. Focal atrophy of glomerular tufts was also observed. Vascular congestion and degenerative changes in renal blood vessels were occasionally evident in some samples. Ultrastructure study revealed damage of glomerular membrane. Proximal tubule showed loss of basal infoldings, damage of brush border, mitochondrial degeneration and nuclear changes. Distal tubules also showed demarked degenerative changes. Increased frequency of micronuclei proved that Taxol had genotoxic effects in mice bone marrow cells. In conclusion Taxol had nephrotoxic effect on mice kidney that must be considered during its use as a chemotherapeutic agent in human.
Keywords: Taxol, Nephrotoxicity, Ultra, Renal tubules, Glomerular tufts, Micronucleus assay, MTD (tolerated), ID (dose), MD
1. Introduction
Most cytotoxic drugs including Taxol interact directly with DNA or its precursors, inhibiting the synthesis of new genetic material or causing irreparable damage to DNA (Zhou et al., 2003). Although anticancer or cytotoxic drugs are advantageous in treating cancer, they also have their drawbacks. One of the greatest problems is that cytotoxic drugs may not distinguish between normal and cancer cells. Unfortunately, no currently available agent meets this criterion (Damia and Broggini, 2004).
Therefore, normal tissues that have higher rates of cellular proliferations such as bone marrow and reproductive organs are very susceptible to anticancer drugs. Liver and kidney are the most sensitive organs for chemotherapy cytotoxicity (Merouani et al., 1997).
Taxol is a new anticancer drug that is isolated from stem park of the pacific yew tree; Taxus brevifolia. Its antitumour activity against a variety of rodent tumors was discovered in 1967; when, its unique mechanism of action led to the development of a new class of chemotherapeutic agents called taxanes (). In 1991, The National Cancer Institute hailed Taxol (also known as paclitaxel) as the most important new cancer drug in the past 15 years, and it has recently been called the best new anticancer agent developed from natural agents. Although the drug was discovered forty years ago, it was not tested experimentally until 1977. It took another sixteen years to be approved by the Food and Drug Administration (FDA) for the treatment of ovarian cancer, breast cancer, and Kaposi’s sarcoma. It has been shown to be effective against solid tumors, breast, lung and colorectal cancers, which before were unaffected by most chemotherapeutic agents (Kubota et al., 1997). Taxol can either be used on its own as monotherapy or in combination with other drugs (Kolomeichuk et al., 2008). Taxol, when used on its own, produced a response in up to 60% of patients with breast and ovarian cancer (Tresukosol et al., 1995). It has been described to have some activity in head and neck gastric cancer, and hematological cancer (Crown and O’Leary, 2000). Paclitaxel (Taxol) prevents cell division by promoting the assembly of stable microtubules from α and β tubulin heterodimers and inhibiting their depolymerisation (Schiff and Horwitz, 1981). This is an opposite effect to the Vince alkaloids, which inhibit the polymerization of the microtubules (Goodsell, 2000). Taxol has been reported to induce micronuclei (Bajie et al., 2007). Moreover, investigations have shown that Taxol activates a checkpoint pathway that delays cell cycle progression and induces programmed cell death (Sorger et al., 1997; Guo et al., 2002; Ikui et al., 2005).
Nephrotoxicity of Taxol was reported with other cytotoxic drugs. However, no available literatures dealing with histological changes in kidney parenchyma evoked by Taxol administration. In the present study, acute Taxol nephrotoxicity at 3 different doses was tested. Light and electron microscopic studies were used to demonstrate cytotoxicity. Micronuclei assay was applied for testing aneuogenecity that may delay cell cycle program or induced programmed cell death (Jagetia and Adiga, 1997).
1.1. Histological studies
1.1.1. Animal preparation
Fifty-four adult male albino mice (average weight 25–30 g) were obtained from King Fahad medical research centre King Abdel Aziz University. The animals were divided into two groups; control (n = 9) and experimental (n = 45). The later was sub divided into three subgroups, G1, G2 and G3 each 15 animals for the three used doses.
The drug was dissolved in normal saline and given as a single dose to G1, G2 and G3, respectively, via intra-peritoneal route at three different doses; maximum tolerated dose (MTD), intermediate dose (ID) and a medical dose (MD) (1.7, 1.15 and 0.6 mg/kg), respectively.
The control group was injected with 0.9% ml saline only. The animals were dissected after 6, 24, and 48 h following injection. The heart was perfused by normal saline followed by 10% neutral buffered formalin to insure perfect fixation. The Kidneys were removed; 2–3 mm specimens from each were refixed in the same fixative for 12, 24 h, dehydrated using the ascending grades of alcohol (70–100%) for 1–2 h and cleared in xylene. The specimens were embedded in paraffin at 58 °C, then sections of 5–7 μm thick and stained by haematoxylin and eosin and periodic acid schief (PAS) for further examination and photographing using light microscopic provided by digital camera connected to computer.
For electron microscopy small pieces (2 mm) from control and Taxol treated mice kidney were fixed in 2.5% gluteraldehyde in 0.1 m phosphate buffer (pH 7.3) for 30 min, 1% osmium tetraoxide in 0.1 M phosphate buffer (pH 7.3) for 1 h. The specimens was washed twice by 0.1 m phosphate buffer for 5 min each, the samples was immersed in propylene oxide (two changes) then in a mixture of propylene oxide and resin and embedded in (Epon). Ultra thin section, 80–100 Å thick were cut by glass knife and examined by using EM.
1.2. Micronucleus assay
Bone marrow samples from animals at the three used doses (MTD, ID and MD) were used for scoring the number of micronuclei in polychromatic erythrocyte (PCE) is performed according to the method described by Matter and Schmid (1971). With some modifications by Hedle et al. (1984). As follows; mice were sacrificed by cervical dislocation at appropriate sample time, and the femurs were removed. The bone marrow was pushed out with a pin, placed on a microscope slide, and mixed with drop of fetal calf serum. The cells were then smeared and allowed to dry. The slides were fixed in absolute methanol and stained with Giemsa stain. One thousand polychromatic erythrocytes (PCEs) were scored, and the number of micro-nucleated PCEs was recorded. Micronuclei were identified as dark staining rounded bodies in cytoplasm of PCE. Five-thousand cells per each treatment dose will be examined to score the number of micronuclei in PCEs. Precautions with regard to scoring and artifacts were taken as described by Hedle et al. (1984).
2. Results
Animals injected by Taxol become aggressive immediately after injection then looked weak and unable to move compared to control. Although animals lost their appetite to food. Animal weight was not significantly affected owing to short duration of Taxol administration.
2.1. Effect of acute Taxol administration on mice kidney parenchyma
2.1.1. Light microscopic study
2.1.1.1. Control mice
The histology of control mice renal parenchyma was normal and was similar to those described in previous literature regarding mammalian kidney including mice (Figs. 1 and 2).
Figure 1.
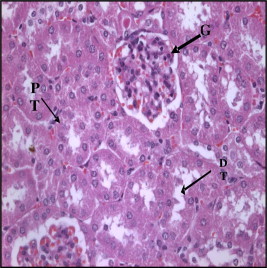
Section in control mice kidney showing normal glomeruls (G), proximal (PT) and distal (DT) tubules (H× and E× 400).
Figure 2.
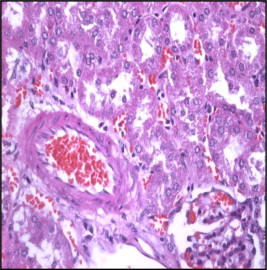
Section in control mice kidney showing arcute artery at cortico-medullary junction (H× and E× 400).
2.1.1.2. Histological change of kidney parenchyma in mice after 24–48 h of treatment by Taxol (0.6 mg/kg/BW)
Histological examination of Taxol treated kidney (24 h by 0.6 mg) revealed degenerative changes in most parenchymatous elements in comparison with control. The changes were more evident at 48 h. Atrophy of renal corpuscle and, decrease in glomerular cellularity was observed. There was wide spacing of tubules, atrophy of the lining epithelium and reticulated casts within the lumen. Proximal tubules showed histological changes in the form of widening of tubular lumen and vaculation of some of the lining cells. Marked atrophy and degeneration of distal tubules cells was also observed (Fig. 3). The arcute vessels were dilated and showed degeneration of their vascular wall. There was also perivascular edema and fibrosis (Fig. 4).
Figure 3.
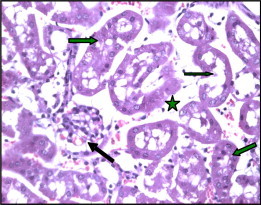
Section of kidney of Taxol treated mice (0.6 mg-24 h) showing wide spacing (star) of tubules with atrophy of their lining epithelium. Notice the reticulated casts within their lumumina (arrows). There is marked atrophy of renal corpuscle (black arrow) (H× and E× 400).
Figure 4.
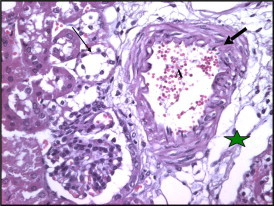
Section in mice kidney of Taxol treated mice (1.15 mg/kg) showing atrophy and degeneration of renal tubules (thin arrows), thickened arterial wall (thick arrows), and perivascular edema and fibrosis (stars).
2.1.1.3. Histological change of kidney parenchyma in mice after 24–48 h of treatment by Taxol 1.15 mg/kg/BW
The kidney of mice receiving 1.5 mg/kg Taxol for 24 h showed marked congestion of renal veins in the cortical regions (Fig. 5). Both proximal and distal tubules showed disorganization and degenerated epithelial lining. The cells showed decrease in height, the cytoplasm stained dark acidophilic, and the nuclei are small, pyknotic and dark stained. Collecting distal tubules showed wide lumina. Renal vessels showed thickening with degeneration of wall components (Fig. 6).
Figure 5.
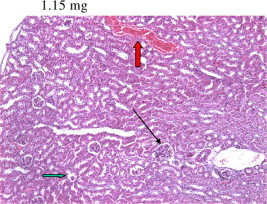
Section of Taxol treated mice kidney 24 h after 1.15 g Taxol administration showing vascular congestion (red arrow) together with atrophy of both renal corpuscle and tubular epithelium (black arrow).
Figure 6.
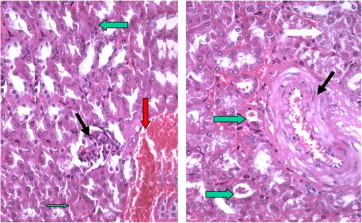
Section of Taxol treated mice kidney showing widened deformed renal tubules with intraluminal casts (thin arrow) the arcute artery (A) showing thick degenerated wall (H× and E× 400).
Occasionally sever vacuolar degeneration of tubular lining epithelium or apoptotic changes (dark stained cells with small pyknotic nuclei) were observed (Fig. 7). In other samples tubular dilatation and intraluminal casts were observed in the tubules adjacent to renal medulla (Fig. 8).
Figure 7.
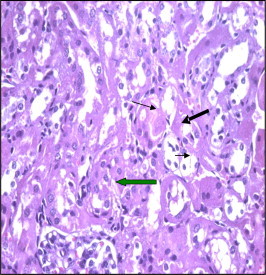
Section of mice kidney treated with 1.7 mg taxol after 48h showing disorganization and degeneration of tubules. The cytoplasm stained dark red and nuclei are small and dark (arrows).
Figure 8.
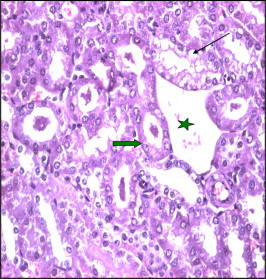
Section in Taxol treated kidney showing widening of tubular lumen due to atrophy and vaculation of the lining epithelium and appearance of casts within the lumen (arrows). Notice the marked dilated vein (star).
2.1.1.4. Histological change of kidney parenchyma in mice after 24–48 h of treatment by Taxol 1.7 mg/kg/BW
After 24 h the kidney of mice receiving 1.7 mg of Taxol showed both necrotic and apoptotic changes in the renal tubules; focal apoptotic changes involve proximal tubules were observed. The tubules showed hyaline staining; the cytoplasm was stained deeply acidophilic. The nuclei were small and deeply stained (pyknotic) (Figs. 9 and 10). Occasionally there was marked loss of normal kidney parenchymatous organizations (Fig. 11). There was also hyaline degeneration or apoptosis of most tubules. Distal tubules showed unstained cells with dark stain nuclei. The changes were more severe after 48 h where there was a large region of complete necrosis of both parenchyma and vascular elements. Cell debris and blood cells were observed in the necrotic regions (Fig. 12). The tubules had dilated lumina and contain hyaline casts. Marked atrophy of glomerular tufts was observed (Fig. 13). The renal vessels at cortico-medullary junction showed massive degeneration of vascular elements and perivascular edema (Fig. 14).
Figures 9 and 10.
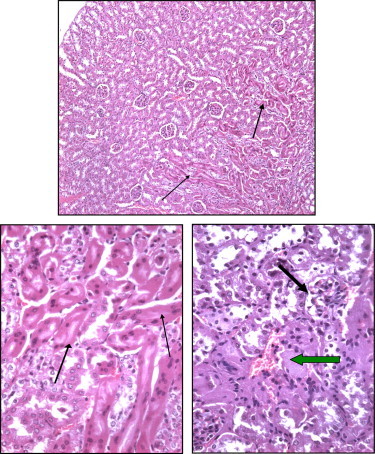
Section of Taxol treated mouse kidney showing marked apoptotic changes (thin arrows) of proximal tubules is observed 48 h following 1.7 mg/kg. The lining cells are highly acidophilic, the nuclei are small or pyknotic and deeply stained.
Figure 11.
Section of Taxol treated mouse kidney showing marked disorganization of renal tubules with necrotic (arrow) or apoptotic changes (thick arrow), glomerular atrophy is also evident (thick arrow).
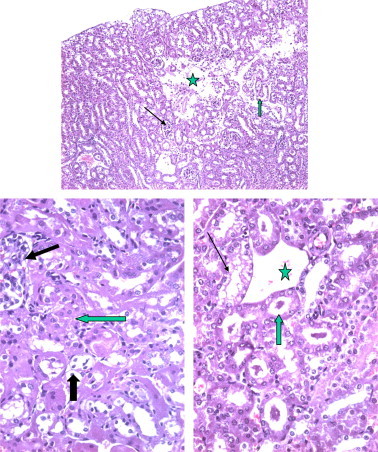
Figures 12–14.
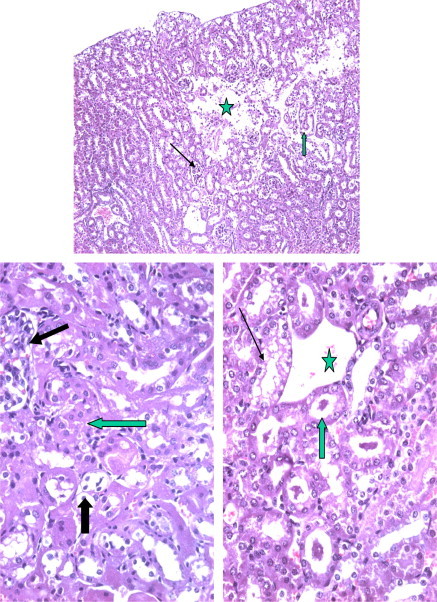
Section from mouse kidney after 48 h the kidney of mice receiving 1.7 mg/kg of Taxol showing massive regions of necrosis with complete loss (star) of renal parenchyma. The rest of tubules showed dilated (arrow) or dilated and contain hyaline.
2.1.2. Histochemical alterations
PAS +ve material was observed in the basement membrane of Bowman capsule and renal tubules. The brush border of proximal tubules (PT) lining cells showed highly PAS positive staining (Fig. 15).
Figure 15.
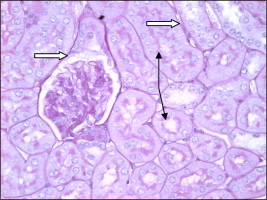
Paraffin section of control mouse kidney showing high (PAS +ve) reaction in the basement membrane of Bowman capsule and renal tubules. The brush border of proximal tubule epithelial cells is highly PAS +ve (arrow).
In Taxol treated kidney there was a decrease in PAS +ve staining of the basement membrane of altered renal tubules and Bowman capsule. Loss of brush border staining in dilated PT was also observed (Fig. 16).
Figure 16.
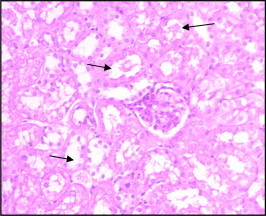
Section of mouse kidney after48 h of Taxol injection (1.7 mg/kg) showing loss of PAS +ve reaction in regions described in control due to damage of brush border of most tubules. The basement membrane is also is ill defined.
2.2. Ultrastructure alteration
Figs. 17–19 shows the semi-thin sections of parts of both control and Taxol (1.7 mg/kg for 48 h) treated mice kidney from which ultra thin sections were prepared for ultra structure studies.
Figure 17.
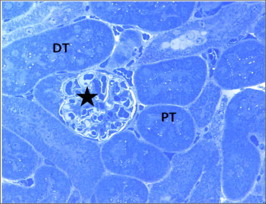
Semithin section from control mice kidney showing normal renal corpuscle and glomerulus (star), proximal (PT) and distal (DT) tubules. Toluidine blue ×400.
Figure 18.
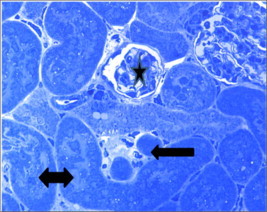
Semithin section from Taxol (1.7 mg for 48 h) treated kidney showing atrophy of a renal corpuscle and glomerulus (star), shrinked degenerated proximal tubules (black arrow) and damaged distal tubules (double head arrow). Toluidine blue ×100.
Figure 19.
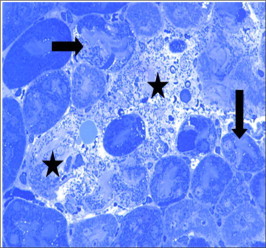
Semi thin section of Taxol (1.7 mg for 48 h) treated kidney showing marked degeneration (arrows) and necrosis of renal elements. Only ghosts of tubules and corpuscles are seen (stars).
Taxol treated kidney in a dose of 1.7 mg/kg for 48 h showed focalatrophy and shrinkage of renal corpuscles, glomeruli and tubules (Fig. 18) compared to control (Fig. 17). In some samples massive necrosis and loss or renal elements was observed (Fig. 19).
The ultrastructure study of control mice kidney showed normal appearance as described in previous literature. The renal cortex contains renal corpuscles surrounded by renal tubules (proximal and distal). Glomerular capillaries have fenestrated endothelium, thin basement membrane. Podocytes have with large vesicular nuclei. Their processes were seen ending on glomerular capillary the basement membrane (Fig. 18).
Ultrastructure showed the degenerative changes in podocyte lining the visceral layer of bowman capsule the cells had thin processes and dark or vacuolated cytoplasm (Fig. 19).
The proximal tubule (PCT) in control mice is lined by cuboidal cells showing apical microvillus, and dense bodies, most probably proteins. The nucleus (N) is large, vesicular with well defined nucleolus. Basal mitochondria (M) were located between basal enfolding. The basement membrane is regular and of normal thickness (Fig. 20). In Taxol treated animals, PCT showing destruction of apical microvillus (white arrow) the nucleus (N) showed irregular shape and abnormal distribution of peripheral chromatin (star) and nucleolus (NU). Basal enfolding enclosed abnormal dark stained mitochondria (M) surrounded by light spaces. The basement membrane (BM) is irregular and thickened (Fig. 21).
Figure 20.
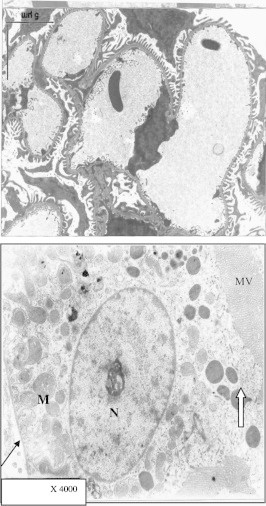
Electron micrograph of part of kidney in control mouse, showing part of a glomerulus, two podocytes (1, 2) with large vesicular nuclei (N) were observed. Notice that the foot processes ending on glomerular capillary surface. Electrograph of a magnified proximal convoluted tubule (PCT) cell of control mouse showing apical microvilli (mv) and dense bodies. The nucleus (N) is large, vesicular with well defined nucleolus. Mitochondria (M) were located between basal infolding. The basement membrane is regular and of normal thickness (thin arrow).
Figure 21.
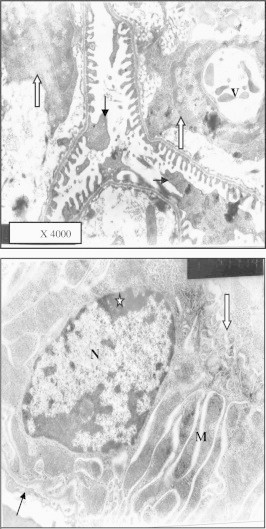
Electron micrograph of similar region after 1.7 mg Taxol administration showing degenerative changes in podocytes (arrow). Glomerular lumen is dilated and contains cells (white arrow). The cytoplasm of one cell showed large vacuole (v) compressing the nucleus, podocytes. Similar part of mice PCT showing destruction of apical microvillus (white arrow) the nucleus (N) showed irregular shape and abnormal distribution of peripheral chromatin (star) and nucleolus (NU). Basal enfolding enclosed abnormal dark stained mitochondria (M) surrounded by light spaces. The basement membrane (BM) is irregular and thickened.
Distal convoluted tubule (DCT) of control mice kidney showing normal thickness basement membrane (BM), basal infolding and mitochondria (M). The nucleus is large with normal peripheral chromatin distribution. The lumen (L) is bounded by the apical cellular membrane having few microvilli (Fig. 22). DCT lining cell from mice treated with (1.7 mg) Taxol showed thickened basement membrane (BM). Dark degenerated mitochondria (M) among abnormal basal folding. The nucleus showed chromatin clumps (Fig. 23).
Figure 22.
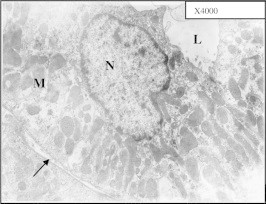
Electron micrograph of distal convoluted tubule (DCT) of control mouse kidney showing normal thickness basement membrane (black arrow), basal enfolding and mitochondria (M) The nucleus (N) is large with normal peripheral chromatin distribution. The lumen (L) is bounded by apical cellular membrane having few microvilli.
Figure 23.
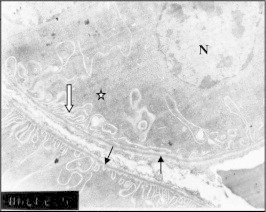
Electron micrograph of DCT lining cell from mouse treated with 1.7 mg/kg Taxol showed thickened basement membrane (BM) and dark degenerated mitochondria (M) among abnormal basal infolding. The nucleus also showed chromatin clumps.
2.3. Micronuclei formation
Micronucleus formation was known as a test for cytotoxicity. In the present study, 6 h treatment did not modify the number of micro-nucleated polychromatic erythrocytes “MN-PCEs” in samples analyzed. On the other hand, the frequencies of “MN-PCEs” were significantly higher in the treated mice at 24 and 48 h with all doses. Table 1 is compared to control.
Table 1.
Effect of Taxol “MD, ID of MTD” on the incidence of micro-nucleated polychromatic erythrocytes “MN-PCEs” in bone marrow of male mice.
| Treatments | Number of micronucleus (MN) |
|---|---|
| Time (h) | |
| 6 | C 6.70 c ± 1.88 |
| 24 | B 22.48 b ± 14.92 |
| 48 | A 60.03 a ± 35.30 |
| LSD at α 0.05 | 2.14 |
| LSD at α 0.01 | 2.86 |
| Conc. | |
| Control | D 3.96 d ± 0.73 |
| 0.60 | C 32.87 c ± 30.52 |
| 1.15 | A 43.20 a ± 40.11 |
| B 38.93 b ± 24.38 | |
| LSD at α 0.05 | 2.47 |
| LSD at α 0.01 | 3.30 |
| Interaction | |
| 6 h × control | GH 4.60 h ± 0.28 |
| 6 h × 0.60 | GH 6.80 gh ± 0.84 |
| 6 h × 1.15 | GH 6.40 gh ± 1.14 |
| 6 h × | G 9.00 g ± 1.58 |
| 24 h × control | H 3.13 h ± 0.03 |
| 24 h × 0.60 | F 17.80 f ± 1.48 |
| 24 h × 1.15 | E 26.80 e ± 3.42 |
| 24 h × | D 42.10 d ± 6.22 |
| 48 h × control | GH 4.13 h ± 0.56 |
| 48 h × 0.60 | B 74.00 b ± 3.16 |
| 48 h × 1.15 | A 96.40 a ± 7.23 |
| 48 h × | C 65.60 c ± 4.04 |
| LSD at α 0.05 | 4.28 |
| LSD at α 0.01 | 5.72 |
3. Discussion
Taxol, was shown to have anticancer effect in the early 1960s, where it interferes with cell division by binding to the protein tubulin, which is a key factor in mitosis, the process of cell division and growth (Jordan et al., 1993). This could affect regenerative activity of many organs including renal parenchyma. Since, Taxol has so been proven to be effective for treatment of a variety of tumor cancers (Wang et al., 2000) and is recently introduced into Kingdom of Saudi Arabia, its effect on renal functions and structure seemed to be of great clinical importance. The only study regarding nephrotoxicity was that of Merouani et al. (1997), who did a retrospective analysis of renal function in patients with gynecological cancers and found an increased nephrotoxicity in patients treated with Taxol and cisplatin as compared to cisplatin alone. However, literature in histological changes was unavailable. The present results could be considered preliminary in that subject.
In the present study, it was observed that administration of Taxol result in marked nephrotoxicity even when given in medical therapeutic doses (0.6 mg/kg). The effect was also time independent, that is to say that the drug produced its toxic effect few hours following administrations (6 h). Marked effect was observed after 24 and 48 h most probably due to cumulative effects. The changes in kidney parenchyma involved both glomerular and tubular elements. Focal necrosis of renal tubules in the form of vaculation was observed at medical dose (0.6 mg/kg), while higher doses produced wide spread massive damage in renal tubules and glomerular atrophy. Chen et al. (2008) reported that Taxol induces caspase-independent cytoplasmic vacuolization and cell death through endoplasmic reticulum (ER) swelling in ASTC-a-1 cells.
In high dose group (1.7 mg) at 48 h focal regions of proximal tubular cell apoptosis were observed. Proximal tubule cells appeared dark stained and showed pyknotic nuclei. Apoptosis was reported to be induced by different stimuli, such as death ligands, chemotherapeutic drugs or ionizing irradiation (Rodriguez-Antona et al., 2007). Park et al. (2004) reported that Taxol (paclitaxel) is known to inhibit cell growth and trigger significant apoptosis in various cancer cells. Although Taxol induces apoptosis of cancer cells, its exact mechanism of action is not yet known. Salvesen (2002) reported that apoptosis is mediated by members of a family of cysteine proteases known as caspases, whose activation follows characteristic apoptotic stimuli, and whose substrates include many proteins, the limited cleavage of which causes the characteristic morphology of apoptosis.
Chen et al. (2008) reported that Taxol induce apoptosis through autolytic activation of caspases-10 and -3 thought to be the triggering of apoptotic process in cancer cells treated with Taxol.
Apoptosis was suggested to occur via stimulation of death receptors FAS-associated death domain protein (FADD) or the activation of reactive oxygen species (ROS) (Alexandre et al., 2007). Park et al. (2004), found that in human lymphoblastic leukemia cell line; Taxol-induced apoptosis in the human leukemia HL-60 cell line by triggered the release of cytochrome c into the cytosol and induced Apaf-1-mediated caspase-3 and -9 activities known to have a role in inducing cell apoptosis (Nicholson, 1999; Audrimont et al., 2001; Assen et al., 2004).
Electron microscopic study and histochemistry done in the present study showed destruction of the brush border of tubule lining cells, degeneration of nuclei and increase nuclear peripheral chromatin. Loss and irregularity of basal infolding and degeneration of mitochondria were also observed both in proximal and distal tubules. Literature describing ultrastructure changes on the effect of Taxol on renal tissue was lacking.
Taxol was also reported to mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2 (Selimovic et al., 2008).
Park et al. (2004) showed that Taxol decreased mitochondrial membrane potential (Δψm) and result in a significant increase in ROS generation. The later were known of its damaging effect on cellular structures. In view of those reports, the damage of mitochondria observed in ultrastructure micrographs of renal tubules of mice kidney could be explained. Mitochondria were the main source of energy supply that of great importance for renal tubules to function in an optimum way.
Taxol was also known to affect microtubule assembly and stability (Chuu et al., 2007). It enhances stability of microtubules, preventing the separation of chromosomes during anaphase. This may result in further failure of regenerative processes important to keep integrity of kidney cellular activity when exposed to toxic substances. Vinblastine and paclitaxel (Taxol) are widely used chemotherapeutic drugs that inhibit the normal function of microtubules causing mitotic arrest and cell death (Gibb et al., 1997). Despite these similarities, the signaling pathways mediate and regulate cell death seemed to be different (Kolomeichuk et al., 2008).
Needleman et al. (2005) reported that the cancer chemotherapeutic drug Taxol, suppresses microtubule dynamics and inhibits depolymerization that ultimately result in loss of ability of cells to regenerate if exposed to mild toxic substances.
In an attempt to explain the mechanism by which Taxol induce cytotoxicity micronucleus test was done in the present study using bone marrow cells. It was observed that the frequencies of micro-nucleated cells in bone marrow were significantly higher in Taxol treated groups in all doses. Micronucleus formation was taken as a sign of cytotoxicity of many drugs including Taxol (Jagetia and Adiga, 1997).
In the present study induction of micronuclei in bone marrow cells in vivo are in agreement with the observation of Kopjar et al., (2002). who reported the induction of micronuclei in mouse bone marrow cells following Taxol administration. Also, the present results are in agreement with investigations on A 549 cells (Chen and Horwitz, 2002), human T. lymphocytes (Digue et al., 2002), and peripheral blood lymphocytes (Bajie et al., 2007). As MNs are formed out of whole chromosome and Taxol found to increase significantly the micro-nucleated lymphocyte rates and over 85% of those micronuclei contained one or more whole chromosomes, Taxol is said to be aneugenic (Goodsell, 2000).
Future in vitro study using cultured renal tubular cells or other kidney parenchymatous tissues are needed for more understanding the mechanism by which Taxol induce cell necrosis or apoptosis in kidney parenchyma.
In conclusion, the present light, histochemical and ultrastructural studies together with micronucleus assay indicated an eugenic and apoptotic potential of Taxol in mice, which should be considered in the risk–benefit analysis of its increasing clinical use in human.
References
- Alexandre J., Hu Y., Lu W., Pelicano H., Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67(8):3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- Assen W., Abid-Essafi S., Achour A., Guezzah N., Zakhama A., Ellouz F., Creppy E.E., Bacha H. Karyomegaly of tubular kidney cells in human chronic interstitial nephropathy in Tunisia: respective role of ochratoxin. A and possible genetic predisposition. Hum. Exp. Toxicol. 2004;23(7):339–346. doi: 10.1191/0960327104ht458oa. [DOI] [PubMed] [Google Scholar]
- Audrimont I., Sostaric B., Yenot C., Betbeder A.M., Dano-Djedje S., Sanni A., Steyn P.S., Creppy E.E. Aspartame prevents the karyomegaly induced by ochratoxin A in rat kidney. Arch. Toxicol. 2001;75(3):176–183. doi: 10.1007/s002040100229. [DOI] [PubMed] [Google Scholar]
- Bajie V., Spremo-Potparevie B., Milieevie Z., Zivkovie L. Deregulated sequential motion of centromeres induced by antitumor agents may lead to genome instability in human peripheral blood lymphocytes. J. BOUN. 2007;12:77–83. [PubMed] [Google Scholar]
- Chen J.G., Horwitz S.B. Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res. 2002;62:1935–1938. [PubMed] [Google Scholar]
- Chen T.-S., Wang X.-P., Sun L., Wang L.-X., Xing D., Mok M. Taxol induces caspase-independent cytoplasmic vacuolization and cell death through endoplasmic reticulum (ER) swelling in ASTC-a-1 cells. Cancer Lett. 2008;270(1):164–172. doi: 10.1016/j.canlet.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Chuu J.J., Liu J.M., Tsou M.H., Huang C.L., Chen C.P., Wang H.S., Chen C.T. Effects of paclitaxel and doxorubicin in histocultures of hepatocelular carcinomas. J. Biomed. Sci. 2007;14(2):233–244. doi: 10.1007/s11373-006-9141-3. (Epub January 6) [DOI] [PubMed] [Google Scholar]
- Crown J., O’Leary M. The taxanes: an update. Lancet. 2000;355:1176–1178. doi: 10.1016/S0140-6736(00)02074-2. [DOI] [PubMed] [Google Scholar]
- Damia G., Broggini M. Cell cycle checkpoint proteins and cellular response to treatment by anticancer agents. Cell Cycle. 2004;3(1):46–50. [PubMed] [Google Scholar]
- Digue L., Orsiere T., Baciuchka-Palmaro M. Interest of studying the in vitro genotoxicity of an antineoplastic drug on healthy human: paclitaxel example. Bull. Cancer. 2002;89:887–892. [PubMed] [Google Scholar]
- Gibb R.K., Taylor D.D., Dennis M.T.W., David L.O., Gerçel-Taylor D.C. Apoptosis as a measure of chemosensitivity to cisplatin and Taxol therapy in ovarian cancer cell lines. Gynecol. Oncol. 1997;65(1):13–22. doi: 10.1006/gyno.1997.4637. [DOI] [PubMed] [Google Scholar]
- Goodsell D.S. The molecular perspective: microtubules and the taxanes. Stem Cells. 2000;18:382–383. doi: 10.1634/stemcells.18-5-382. [DOI] [PubMed] [Google Scholar]
- Guo W., Zeng C., Dong F., Lei W. Paclitaxel-induced apoptosis in osteosarcoma cell line U-2 OS. Chin. Med. J. 2002;115:1796–1801. [PubMed] [Google Scholar]
- Hedle J.A., Stuart E., Salamone M.F. The bone marrow micronucleus test. Elsevier; New York: 1984. (In Handbook of Mutagenicity Test Procedures). pp. 441–457. [Google Scholar]
- Ikui A.E., Yang C.P., Matsumoto T., Horwitz S.B. Low concentrations of Taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BurbR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle. 2005;4:1385–1388. doi: 10.4161/cc.4.10.2061. [DOI] [PubMed] [Google Scholar]
- Jagetia G.C., Adiga S.K. Correlation between micronuclei induction and cell survival in V79 cells exposed to paclitaxel (Taxol) in conjunction with radiation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1997;377(1):105–113. doi: 10.1016/s0027-5107(97)00067-5. [DOI] [PubMed] [Google Scholar]
- Jordan M.A., Toso R.J., Thrower D., Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by Taxol at low concentrations. Proc. Natl. Acad. Sci. USA. 1993;90(20):9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeichuk Sergey N., Terrano David T., Lyle Christopher S., Sabapathy Kanaga, Chambers Timothy C. Distinct signaling pathways of microtubule inhibitors – vinblastine and Taxol induce JNK-dependent cell death but through AP-1-dependent and AP-1-independent mechanisms, respectively. FEBS. 2008;275(8):1889–1899. doi: 10.1111/j.1742-4658.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- Kopjar N., Garaj-Vrhovac V., Milas I. Acute cytogenetic effects of antineoplastic drugs on peripheral blood lymphocytes in cancer patients chromosome aberrations and micronuclei. Tumori. 2002;88(4):300–312. doi: 10.1177/030089160208800412. [DOI] [PubMed] [Google Scholar]
- Kubota I., Matsuzaki S.W., Hoshiya Y. Antitumour activity of pactilaxel against human breast carcinoma xenografts serially transplanted into nude mice. J. Surg. Oncol. 1997;64:115–121. doi: 10.1002/(sici)1096-9098(199702)64:2<115::aid-jso5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Matter B., Schmid W. Trenimon-induced chromosomal damage in bone-marrow cells of six mammalian species, evaluated by the micronucleus test. Mutat Res. 1971;12(4):417–425. doi: 10.1016/0027-5107(71)90092-3. [DOI] [PubMed] [Google Scholar]
- Merouani A., Davidson S.A., Schrier R.W. Increased nephrotoxicity of combination Taxol and cisplatin chemotherapy in gynecologic cancers as compared to cisplatin alone. Am. J. Nephrol. 1997;17:53. doi: 10.1159/000169072. –Perry (2001) [DOI] [PubMed] [Google Scholar]
- Needleman D.J., Ojeda-Lopez M.A., Raviv U., Ewert K., Miller H.P., Wilson L., Safinya C.R. Radial compression of microtubules and the mechanism of action of Taxol and associated proteins. Biophys. J. 2005;89(5):3410–3423. doi: 10.1529/biophysj.104.057679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D.W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6(11):1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Park S.-J., Wu c.-H., Gordon j.D., Zhong X., Emami A., Safa A.R. Taxol induces caspase-10-dependent apoptosis. J. Biol. Chem. 2004;279:51057–51067. doi: 10.1074/jbc.M406543200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Antona C., Niemi M., Backman J.T., Kajosaari L.I., Neuvonen P.J., Robledo M., Ingelman-Sundberg M. Characterization of novel CYP2C8 haplotypes and their contribution to paclitaxel and repaglinide metabolism. Pharmacogenomics J. 2007 doi: 10.1038/sj.tpj.6500482. [DOI] [PubMed] [Google Scholar]
- Salvesen G.S. Caspases and apoptosis. Essays Biochem. 2002;38:9–19. doi: 10.1042/bse0380009. [DOI] [PubMed] [Google Scholar]
- Schiff P.B., Horwitz S.B. Taxol assembles tubulin in the absence of exogenous guanosine 5’-triphosphate or microtubule-associated proteins. Biochemistry. 1981;20(11):3247–3252. doi: 10.1021/bi00514a041. [DOI] [PubMed] [Google Scholar]
- Selimovic D., Hassan M., Haikel Y., Hengge U.R. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell. Signal. 2008;20(2):311–322. doi: 10.1016/j.cellsig.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Sorger P.K., Dobles M., Tournebize R., Hyman A.A. Coupling cell division and cell death to microtubule dynamics. Curr. Opin. Cell Biol. 1997;9:807–814. doi: 10.1016/s0955-0674(97)80081-6. [DOI] [PubMed] [Google Scholar]
- Tresukosol A.P., Kudelka C.L., Edwards C., Charnsangavej N., Narboni J. Recurrent ovarian granulosa cell tumor: a case report of a dramatic response to Taxol international. J. Gynecol. Cancer. 1995;5(2):156–159. doi: 10.1046/j.1525-1438.1995.05020156.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Li Guiling, Lu Huaying, Zheng Zhonghui, Huang Yaojian, Su Wenjin. Taxol from Tubercularia sp, strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol. Lett. 2000;193(2):249–253. doi: 10.1111/j.1574-6968.2000.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Zhou B.B., Anderson H.J., Roberge M. Targeting DNA checkpoint kinases in cancer therapy. Cancer Biol. Ther. 2003;2(4 Suppl. 1):S16–S22. [PubMed] [Google Scholar]


