Abstract
Agaricus blazei Murill is one of the very popular edible medicinal mushrooms. The present study investigated the protective effect of this biologically active mushroom on the tissue peroxidative damage and abnormal antioxidant levels in carbon tetrachloride induced hepatotoxicity in male albino rats. Male albino rats of Sprague–Dawley strain weighting (120–150 g) were categorized into five groups. The first group served as the normal control, the second and the third groups were treated with Agaricus blazei Mushroom extract and carbon tetrachloride dose, respectively. Fourth group (protective group) was first treated with Agaricus blazei Mushroom extract followed by carbon tetrachloride treatment and fifth (therapeutic group) with carbon tetrachloride first followed by Agaricus blazei Mushroom treatment. The wet fruiting bodies of mushroom Agaricus blazei Murill, crushed and suspended in distilled water was administered orally to the treated groups of male albino rats. The activities of various enzymes (aspartate and alanine transaminase, lactate dehydrogenase, glutathione reductase), levels of non-enzymatic antioxidants (glutathione, vitamin C, vitamin E) and level of lipid peroxidation (malondialdehyde) were determined in the serum of all the experimental animals. Decrease in all the enzymes and non-enzymatic antioxidant, along with an increase in the lipid peroxidative index (malondialdehyde) was found in all the carbon tetrachloride treated rats as compared with normal controls. Also increase level of non-enzymatic antioxidant along with the decrease level in malondialdehyde was found in all experimental animals which were treated with Agaricus blazei Mushroom extract as compared with normal controls. The findings indicate that the extract of Agaricus blazei Murill can protect the liver against carbon tetrachloride induced oxidative damage in rats and is an efficient hepatoprotective and antioxidant agent against carbon tetrachloride induced liver injury.
Abbreviations: AbM, Agaricus blazei Murill; CCl4, carbon tetrachloride; AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase; GR, glutathione reductase; GSH, glutathione; MDA, malondialdehyde; vit. C, vitamin C; vit. E, vitamin E
Keywords: Agaricus blazei Murill, Carbon tetrachloride, Hepatoprotective, Antioxidant, Liver
1. Introduction
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions such as drug metabolism, amino acid metabolism, lipid metabolism and glycolysis. Liver is capable of detoxifying toxic substances and synthesizing useful ones. Hepatotoxic agents can cause very serious damages to the liver as they may deprive the liver from its principal functions (Subramoniam and Pushpangadan, 1999). Hepatotoxic chemicals cause the liver damages which are induced by lipid peroxidation and other oxidative damages (Muhtaseb et al., 2008; Appiah et al., 2009). Carbon tetrachloride is used extensively in experimental models to induce oxidative stress in rats (Onori et al., 2000; Nabeshima et al., 2006). It is a well known hepatotoxin that catabolizes radical induced lipid peroxidation, damage the membranes of liver cells and organelles and causes swelling and necrosis of hepatocytes. Carbon tetrachloride can induce liver damage through the formation of reactive free radicals that can bind covalently to cellular macromolecules forming nucleic acid, protein and lipid adducts; through the induction of hypomethylated ribosomal RNA, resulting in inhibition of protein synthesis. These injuries are mediated through the formation of reactive intermediates such as trichloromethyl (•CCl3) free radicals and ROS (Lin et al., 2008). Experimental and clinical results indicate that oxidative stress may be the link connecting different types of chronic liver injuries and hepatic fibrosis (Lin et al., 2008). The intracellular concentration of ROS is a consequence of both their production and their removal by various antioxidants. Thus, the antioxidant activity or the inhibition of the generation of free radicals is important in the protection against CCl4-induced hepatopathy (Weber et al., 2003; Yuan et al., 2008).
Antioxidative action plays an important role in protecting the liver against CCl4-induced liver injury (Ardanaz and Pagano, 2006). Medical treatment for acute and chronic liver diseases is often difficult to handle and has limited efficiency (Lee et al., 2007). The usage of herbal drugs for the treatment of liver diseases has increased all over the world. (Girish and Pradhan, 2008) Developing therapeutically effective agents from natural products may reduce the risk of toxicity when the drug is used clinically (Shen et al., 2009). Recent research has found that mushrooms contain a powerful antioxidant called l-Ergothioneine. Antioxidants are the heroes of cell preservation. They work by slowing or preventing the oxidative process caused by free radicals that can lead to cell damage and the onset of problems like heart disease and diabetes. Researchers at the Pennsylvania State Mushroom Research Laboratory found that mushrooms contain significant levels of Ergothioneine, which has shown antioxidant properties as a scavenger of strong oxidants. Antioxidant activity is enhanced by the presence of selenium. A 100 g serving of uncooked, sliced white mushrooms provides 13% of the Daily Value for selenium. Ergothioneine is heat-stable, meaning it is present in both raw and cooked mushrooms. Of the Agaricus variety, portabella and crimini mushrooms have the most Ergothioneine, followed closely by white mushrooms. Nowadays mushroom is becoming attractive as a useful food item as it is low in calories, high in minerals, essential amino acids, vitamins and fibers (Mattila et al., 2002). Naturally mushrooms are low in sodium but good source of fiber and contain virtually no fat or cholesterol. Some mushrooms are called as medicinal mushrooms and are important source of nutrients and non-toxic medicines (Wasser and Weis, 1999). In addition, mushrooms contain virtually no fat or cholesterol. In fact, mushrooms have been used in folk medicine throughout the world since ancient times. Many medicinal properties have been attributed to mushrooms (Borchers et al., 1999) including the inhibition of platelet aggregation, reduction of blood cholesterol concentrations (Jeong et al., 2010; Aletor and Aladetimi, 1995), prevention or alleviation of heart disease and reduction of blood glucose levels (Manzi and Pizzoferrato, 2000) and also prevention or alleviation of infections caused by bacterial, viral, fungal and parasitic pathogens (Breene, 1990).
AbM is one of the very popular edible medicinal mushrooms which belongs to the family of Basidiomycetes and has been cultivated especially for the Japanese health food market. AbM has also been reported to block induced liver lipid peroxidation (Lin et al., 1998). In addition AbM has also been used as a health food for the prevention of a range of illnesses including cancer, diabetes, arteriosclerosis and chronic hepatitis (Takaku et al., 2001; Itoh et al., 1994). Bactericidal and fungicidal effects of Agaricus sp. have also been reported (Vogel et al., 1974; Rosa et al., 2003). The present study was designed to evaluate the protective and therapeutical actions of an extract of AbM in an experimental model of CCl4-induced hepatotoxicity in male albino rats, selection of doses was based on the previous study by (Abdulmajeed, 2007).
2. Materials and methods
2.1. Animals
Male albino rats of Sprague–Dawley strain weighting (120–150 g) were used for this study. Animals were housed in cleaned metallic cages and were maintained under standard conditions (23 ± 2 °C) and 12 h light/dark cycles). They were given standard pellet diet and water ad libitum.
2.2. Preparation of mushroom extracts
The AbM (Agaricaceae) was cultivated adopting the “layer spawning” method. Freshly harvested whole mushrooms were dried in the shade and then finely powdered. Five grams of the powder were extracted with 100 ml of 95% ethanol using Soxhlet apparatus. The residue was filtered and concentrated to a dry mass by vacuum distillation; the filtrate thus obtained was used as mushroom extract.
2.3. CCl4-induced hepatotoxicity
CCl4-induced liver injury was administered intraperitoneally in different groups of rats, except the normal control groups (Gs 1 and 2), using a single necrogenic dose (1.5 ml/kg body weight of 80% CCl4 in corn oil) according to Pawa and Ali (2004), which was equivalent to 1/5 of the oral LD50 in mice (Abou Gabal et al., 2007). Liver injury was evaluated by analyzing serum obtained from rats sacrificed 48 h after receiving the Hepatotoxic CCl4.
The wet fruiting bodies of the mushroom AbM were crushed and suspended in distilled water and given orally through an intragastric tube, in a dose of 0.5 g/kg body weight daily for 30 days, 48 h either before (G4, as protective group) or after (G5, as therapeutic group) CCl4 injection.
2.4. Experimental design
Animals were divided into five groups. The experimental design and treatment schedule are as follows:
Group 1: Rats did not receive any treatment (normal healthy control).
Group 2: Rats treated with Agaricus blazei Mushroom extract only (treated group).
Group 3: Rats treated with CCl4 only (treated group).
Group 4: Rats first treated with Agaricus blazei Mushroom extract followed by CCl4 (protective group).
Group 5: Rats first treated with CCl4 followed by Agaricus blazei Mushroom (therapeutic group).
2.5. Blood collection
At the end of the experimental period, rats in the different groups were sacrificed under ether anesthesia from each rat, blood samples were collected from the inferior vena cava. The serum was separated from the blood, and stored at −80 °C until analysis.
2.6. Biochemical analysis
2.6.1. Measurement of lactate dehydrogenase (LDH)
The quantities determination of LDH5 was achieved by using pyruvate to lactate kinetic method, two-point colometric method for lactate dehydrogenase (LDH) (Wooten and freeman 1982):
2.6.2. Determination of transaminase (AST, ALT)
AST was determined on the basis of coupling reaction of malate dehydrogenase and reduced nictinamide adenine dinucleotide, and ALT was determined on the basis of coupled reaction of lactate dehydrogenase and reduced nicotinamide adenine dinucleotide.
Both AST and ALT activities were determined by measuring the rate of oxidation of NADH at 340 nm, the rate of decrease in absorbance at 340 nm was proportional to ALT and AST activities in the sample (Henry, 1974).
2.6.3. Determination of glutathione reductase (GR)
The GR activity was calculated using the method of Mohandas et al. (1984). GR was assayed by following the oxidation of NADPH at 340 nm.
2.6.4. Determination of lipid peroxides (MDA)
Endogenous lipid peroxidation was assessed by measuring MDA at 532 nm. Malondialdehyde concentration was calculated using extinction coefficient value (€) of 156∗105 M−1cm−1 (Buege and Aust, 1978).
2.6.5. Determination of glutathione (GSH)
The reduced glutathione (GSH) was measured by the method of Bentler et al. (1963) based on its reaction with 5,5′-dithiobis (2-nitrobenzoic acid) to yield the yellow chromophore, 5-thio-2-nitrobenzoic acid at 412 nm. GSH level is expressed as μmol g–1 tissue.
2.6.6. Determination of vitamin C
Vitamin C was estimated using Folin phenol reagent and the developed blue color was read at 760 nm (Jagota and Dani, 1982).
2.6.7. Determination of vitamin E
Vitamin E was determined according to Desai and Machlin (1985) using Bathophenanthroline reagent and the red color produced was read at 536 nm against blank.
2.7. Statistical analysis
Statistical analysis was done using one-way analysis of variance (ANOVA). The values are expressed as mean ± S.D. P value < 0.05 was considered as significant.
3. Results and discussion
The effects of AbM on CCl4-induced hepatotoxicity in rats were evaluated by recording changes in serum LDH5, ALT, AST, GR, GSH, MDA, vitamin C and vitamin E levels. The activities of LDH5, ALT, AST and GR in normal and all treated groups are shown in Table 1 and the activity of GSH, MDA, vitamin C and vitamin E is shown in Table 2. The data in Tables 1 and 2 demonstrate a trend of decreased levels of serum LDH5, ALT, AST, GR, GSH vitamin C and vitamin E in the CCl4-treated animals compared to the control. This effect was reversed in the animal groups that were given AbM only or and during treatment with CCl4 (therapeutic group, protective group). Malondialdehyde serum levels were significantly elevated in CCl4-treated groups as compared with control groups. Also in therapeutic group and protective group where CCl4 was given side by side with mushroom, levels of malondialdehyde were found higher than control group. On the other hand malondialdehyde, of animals treated with AbM alone remained within the levels of the control group. Percentage change of all the above mentioned enzymes in all the five groups are shown in Figs. 1 and 2.
Table 1.
One-way ANOVA test between the control, CCl4-treated, CCl4-treated + then Mushroom dose, Protected by Mushroom + then CCl4-treated and Protected by Mushroom groups for LDH5, ALT, AST and GR.
| Parameters | Groups | Min. | Max. | Mean ± S.D. | P value |
|---|---|---|---|---|---|
| LDH5 (nmol/min/mg prot.) | Control | 54.76 | 65.89 | 59.56 ± 4.32 | 0.000 |
| CCl4-treated | 26.82 | 33.65 | 29.33 ± 2.70a | ||
| CCl4-treated + then Mushroom dose | 47.49 | 54.10 | 50.68 ± 2.49a | ||
| Protected by Mushroom + then CCl4-treated | 55.75 | 60.30 | 58.40 ± 1.67 | ||
| Protected by Mushroom | 63.98 | 68.80 | 65.64 ± 1.86a | ||
| ALT (nmol/min/mg prot.) | Control | 31.90 | 37.02 | 34.16 ± 1.88 | 0.000 |
| CCl4-treated | 14.27 | 18.62 | 16.25 ± 1.57a | ||
| CCl4-treated + then Mushroom dose | 27.75 | 30.05 | 28.67 ± 0.87a | ||
| Protected by Mushroom + then CCl4-treated | 33.27 | 39.06 | 35.71 ± 2.19 | ||
| Protected by Mushroom | 37.32 | 43.45 | 40.25 ± 2.83a | ||
| AST (nmol/min/mg prot.) | Control | 11.25 | 13.63 | 12.29 ± 0.88 | 0.000 |
| CCl4-treated | 4.60 | 7.17 | 05.70 ± 0.93a | ||
| CCl4-treated + then Mushroom dose | 9.70 | 11.70 | 10.49 ± 0.79a | ||
| Protected by Mushroom + then CCl4-treated | 10.40 | 12.14 | 11.06 ± 0.70 | ||
| Protected by Mushroom | 9.62 | 10.68 | 10.11 ± 0.40a | ||
| GR (nmol/min/mg prot.) | Control | 54.00 | 60.50 | 56.33 ± 2.48 | 0.000 |
| CCl4-treated | 35.57 | 43.40 | 39.22 ± 2.88a | ||
| CCl4-treated + then Mushroom dose | 54.84 | 60.95 | 57.99 ± 2.34 | ||
| Protected by Mushroom + then CCl4-treated | 50.52 | 58.77 | 54.86 ± 3.00 | ||
| Protected by Mushroom | 52.80 | 60.60 | 56.58 ± 2.92 | ||
Groups and Dunnett test as multiple comparisons. Significant levels between the four groups are illustrated as superscripts letters when P < 0.05.
Table 2.
Describes the one-way ANOVA test between the Control, CCl4-treated, CCl4-treated + then Mushroom dose, Protected by Mushroom + then CCl4-treated and Protected by Mushroom for Vitamin C, Vitamin E, MDA and GSH.
| Parameters | Groups | Min. | Max. | Mean ± S.D. | P value |
|---|---|---|---|---|---|
| Vit. C (mg/g) | Control | 312.00 | 382.00 | 347.33 ± 28.58 | 0.000 |
| CCl4-treated | 204.00 | 248.50 | 228.12 ± 16.06a | ||
| CCl4-treated + then Mushroom dose | 310.00 | 376.00 | 350.38 ± 24.52 | ||
| Protected by Mushroom + then CCl4-treated | 285.00 | 330.00 | 305.63 ± 16.24a | ||
| Protected by Mushroom | 318.00 | 332.00 | 324.68 ± 05.10 | ||
| Vit. E (mg/g) | Control | 0.69 | 0.79 | 0.74 ± 0.03 | 0.000 |
| CCl4-treated | 0.29 | 0.43 | 0.35 ± 0.05a | ||
| CCl4-treated + then Mushroom dose | 0.67 | 0.72 | 0.70 ± 0.02 | ||
| Protected by Mushroom + then CCl4-treated | 0.74 | 0.78 | 0.76 ± 0.01 | ||
| Protected by Mushroom | 0.71 | 0.78 | 0.75 ± 0.02 | ||
| MDA (nmol/mg prot.) | Control | 3.49 | 4.20 | 03.92 ± 0.29 | 0.000 |
| CCl4-treated | 10.53 | 12.55 | 11.36 ± 0.77a | ||
| CCl4-treated + then Mushroom dose | 4.76 | 5.25 | 04.99 ± 0.18a | ||
| Protected by Mushroom + then CCl4-treated | 4.24 | 4.89 | 04.56 ± 0.24 | ||
| Protected by Mushroom | 3.15 | 4.24 | 03.73 ± 0.42 | ||
| GSH (nmol/mg prot.) | Control | 22.80 | 25.90 | 24.32 ± 1.13 | 0.000 |
| CCl4-treated | 13.30 | 15.90 | 14.65 ± 1.02a | ||
| CCl4-treated + then Mushroom dose | 20.10 | 23.40 | 22.42 ± 1.36 | ||
| Protected by Mushroom + then CCl4-treated | 21.90 | 25.51 | 23.19 ± 1.43 | ||
| Protected by Mushroom | 25.11 | 27.69 | 26.58 ± 0.99a | ||
Groups and Dunnett test as multiple comparisons. Significant levels between the four groups are illustrated as superscripts letters when P < 0.05.
Figure 1.
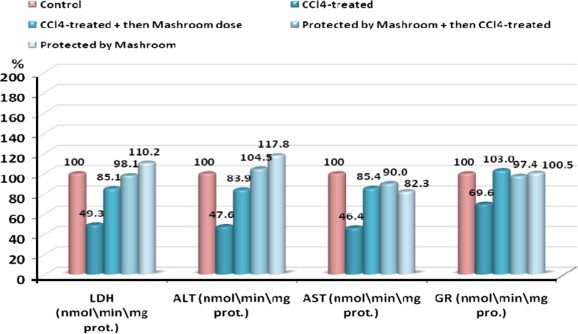
Percentage change of LDH, ALT, AST and GR in CCl4-treated, CCl4-treated + then Mushroom dose, Protected by Mushroom + then CCl4-treated and Protected by Mushroom groups compared to control.
Figure 2.
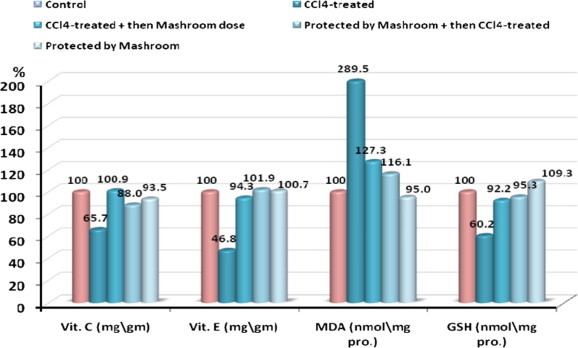
Percentage change of Vit. C, Vit. E, MDA and GSH in CCl4-treated, CCl4-treated + then Mushroom dose, Protected by Mushroom + then CCl4-treated and Protected by Mushroom groups compared to control.
Carbon tetrachloride is a well-known hepatotoxic agent. It causes significant increases in serum levels of ALT, AST and LDH5 and centrilobular hepatocellular vacuolar degeneration and necrosis (Trivedi and Mowat, 1983; Berman et al., 1992). However, serum levels fall to near normal levels as hepatic damage became more severe (Trivedi and Mowat, 1983; Al-Ghamdi, 2003). Furthermore, its effect on rats is age dependent and much less severe in old rats than in young adult rats (Rikans et al., 1994). In the present study, the dose of CCl4 was very high which may explain the contrary trend of decreased serum transaminases (ALT and AST) and LDH5 (LDH isoenzymes which synthesized by liver) which are probably due to decreased synthesis resulting from extensive damage of the hepatic cells. This could be supported by the previous study by Al-Ghamdi (2003). This damage appeared to inhibit or modulate by administration of AbM indicating restoration of hepatic cell function.
Liver damage is always associated with cellular necrosis, and depletion of reduced liver glutathione. In our study oxidative stress was evidenced by decreased GSH, GR which is identical to as reported by Khan et al. (2011) and with the increased production of malondialdehyde in all CCl4-treated animals which were opposite, what was found in animals treated with AbM alone or AbM in treatment with CCl4 (therapeutic group, protective group). It is well known that GSH is a major non-enzymatic antioxidant and plays an important role in cellular defense, which is a crucial determinant of tissue susceptibility to oxidative damage (Meister and Anderson, 1983; Prescott, 1982. GSH depletion occurs as a consequence of CCl4-induced toxicity. Non-enzymic antioxidants such as reduced glutathione, vitamin C and vitamin E play an excellent role in protecting the cells from oxidative damage (Aldrige, 1981; Pesh-Imam and Reckuagel, 1977). It is well established that GSH in blood keeps up the cellular levels of the active forms of vitamin C and vitamin E by neutralizing the free radicals. When there is a reduction in the GSH content the cellular levels of vitamin C is also lowered, indicating that GSH, vitamin C, and vitamin E are closely interlinked to each other (Winkler, 1992). Lipid per oxidation is one of the major characteristic of oxidative damage in accordance with the data obtained from our study CCl4 administration results in a significant elevation of malondialdehyde production. Malondialdehyde is commonly used as marker of free radical mediated lipid peroxidation injury (Amin and Ghoneim, 2009). Malondialdehyde level in serum of CCl4-treated group was significantly elevated compared to that in normal group while in all the groups treated with mushroom extracted resulted in significant decrease in malondialdehyde levels. Izawa and Inoue (2004) suggested that AbM extracts contain a complex mixture of antioxidants and other substances that may act synergically for reducing free radicals. Huang and Mau (2006) reported naturally antioxidant composites in methanolic extracts of AbM such as ascorbic acid, tocopherol and total phenols. Administration of AbM extract in all the experimental animals may have increased the levels of these antioxidants in the serum. In the present study AbM muchrooms showed protective effect against liver damage by CCl4-induced toxicity which is purported by the result of Abdulmajeed (2007).
4. Conclusions
In conclusion, the data achieved by this study revealed that AbM is a natural source of antioxidant compounds and have hepatoprotective activities against CCl4-induced liver damage. However, further studies may still be needed to clarify its effects on ALT and AST and LDH5.
Acknowledgment
We extend our appreciation to Deanship of Scientific Research at King Saud University for funding the work through the Research Group Project No. RGP-VPP-063.
References
- Abou Gabal A.A., Essawy A.E., Abdel-Moneim A.M., Hamed S.S., Elzergy A.A. The protective effect of black seed (Nigella sativa) against carbon tetrachloride-induced chromosomal aberrations and ultrastructural changes of bone marrow cells. Arab J. Biotechnol. 2007;10(2):275–288. [Google Scholar]
- Abdulmajeed N.A. Influence of Agaricus blazei on rate liver toxicity induced by carbon tetrachloride. J. Egypt. Soc. Biotechnol. Environ. Sci. 2007;9:63–79. [Google Scholar]
- Aldrige W.N. Mechanism of toxicity: new concept are required in toxicology. Trends Pharmacol. Sci. 1981;2:228–231. [Google Scholar]
- Aletor V.A., Aladetimi O.O. Compositional studies on edible tropical species of mushrooms. Food Chem. 1995;54(3):265–268. [Google Scholar]
- Al-Ghamdi M.S. Protective effect of Nigella sativa seeds against carbon tetrachloride-induced liver damage. Am. J. Chin. Med. 2003;31(5):721–728. doi: 10.1142/S0192415X03001399. [DOI] [PubMed] [Google Scholar]
- Amin Amr., Ghoneim M.Doaa. Zizyphus spina-christi protects against carbon tetrachloride-induced liver fibrosis in rats. Food Chem. Toxicol. 2009;47:2111–2119. doi: 10.1016/j.fct.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Appiah I., Milovanovic S., Radojicic A., Nikolic-Kokic A., Orescanin-Dusic Z., Slavic M., Trbojevic S., Skrbic R., Spasic M.B., Blagojevic D. Hydrogen peroxide affects contractile activity and anti-oxidant enzymes in rat uterus. Br. J. Pharmacol. 2009;158:1932–1941. doi: 10.1111/j.1476-5381.2009.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardanaz N., Pagano P.J. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp. Biol. Med. 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- Bentler E., Duran O., mikus K.B. Improved method for determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882. [PubMed] [Google Scholar]
- Berman E., House D.E., Allis J.W., Simmons J.E. Hepatotoxic interactions of ethanol with allyl alcohol or carbon tetrachloride in rats. J. Toxicol. Environ. Health. 1992;37(1):161–176. doi: 10.1080/15287399209531663. [DOI] [PubMed] [Google Scholar]
- Borchers A.T. Mushrooms, tumors and immunity. Proc. Soc. Exp. Biol. Med. 1999;221(4):281–293. doi: 10.1046/j.1525-1373.1999.d01-86.x. [DOI] [PubMed] [Google Scholar]
- Breene W.M. Nutritional and medicinal value of specialty mushrooms. J. Food Prot. 1990;53:883–894. doi: 10.4315/0362-028X-53.10.883. [DOI] [PubMed] [Google Scholar]
- Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:306–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Desai I.D., Machlin L.J. Vitamin E. In: Augustin J., Klein B.P., Beker D., Venugopal P.B., editors. Methods of Vitamin Assay. fourth ed. A Wiley-Interscience Publication, John Wiley and Sons; New York: 1985. 255. [Google Scholar]
- Girish C., Pradhan S.C. Drug development for liver disease; foeus on picroliv, ellagic acid and curcumin. Fundam. Clin. Pharmacol. 2008:22–623. doi: 10.1111/j.1472-8206.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Henry J.B. W.B. Saunders; Philadelphia: 1974. Clinical Diagnosis and Management by Laboratory Methods. 332-35. [Google Scholar]
- Huang S.J., Mau J.L. Antioxidant properties of methanolic extracts from Agaricus blazei with various doses of irradiation. Lebensm. Wiss. Technol. 2006;39(7):707–716. [Google Scholar]
- Itoh H., Ito H., Amano H., Noda H. Inhibitory action of a (1→6)-beta-d-glucan-protein complex (F III-2-b) isolated from Agaricus blazei Murill (‘himematsutake’) on Meth A fibrosarcoma bearing mice and its antitumor mechanism. Jpn. J. Pharmacol. 1994;66:265–271. doi: 10.1254/jjp.66.265. [DOI] [PubMed] [Google Scholar]
- Izawa S., Inoue Y.A. A screening system for antioxidants using thioredoxin-deficient yeast: discovery of thermostable antioxidant activity from Agaricus blazei Murill. Appl. Microbiol. Biotechnol. 2004;64(4):537–542. doi: 10.1007/s00253-003-1467-4. [DOI] [PubMed] [Google Scholar]
- Jagota S.K., Dani H.M. A new calorimetric technique for estimation of vitamin C using folin phenol reagent. Anal. Biochem. 1982;127:178. doi: 10.1016/0003-2697(82)90162-2. [DOI] [PubMed] [Google Scholar]
- Jeong S.C., Jeong Y.T., Yang B.K. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 2010;30(1):49–56. doi: 10.1016/j.nutres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Khan M.R., Khan G.N., Ahmed D. Evaluation of antioxidant and fertility effects of Digera muricata in male rat. Afr. J. Pharm. Pharmacol. 2011;5(6):688–699. [Google Scholar]
- Lee C.H., Park S.W., Kim Y.S., Kang S.S., Kim J.A., Lee S.H., Lee S.M. Protective mechanism of glycyrrhizin on acute live injury induced by carbon tetrachloride in mice. Biol. Pharm. Bull. 2007;30:1898–1904. doi: 10.1248/bpb.30.1898. [DOI] [PubMed] [Google Scholar]
- Lin H.M., Tseng H.C., Wang C.J., Lin J.J., Lo C.W., Chou F.P. Hepatoprotective effects of Solanum nigrum Linn. extract against CCl4-induced oxidative damage in rats. Chem. Biol. Interact. 2008;171:283–293. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Lin S., Zhu P., Liao H. Bloking effect of mushroom beverages on carbon tetra chloride induced rat liver peroxidation. Zhongguo Gonggong Welsheng Xuebao. 1998;17 15–15. [Google Scholar]
- Manzi P., Pizzoferrato L. Beta glucans in edible mushrooms. Food Chem. 2000;68:315–318. [Google Scholar]
- Mattila P., Salo-Vaananen P., Konko K., Aro H., Jalava T. Basic composition and amino acid contents of mushrooms cultivated in Finlands. J. Agric. Food Chem. 2002;50:6419–6422. doi: 10.1021/jf020608m. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D. Differential distribution of glutathione and glutathione related enzymes in rabbit kidney: possible interactions in analgesic neuropathy. Cancer Res. 1984;44:5086–5091. [Google Scholar]
- Muhtaseb M.S., El Talwar D., Duncan A., St J., O’reilly D., Mckee R.F., Anderson J.H., Foulisa F.I.G. Free radical activity and lipid soluble anti-oxidant vitamin status in patients with long-term ileal pouch-anal anastomosis. Colorectal Dis. 2008;11:67–72. doi: 10.1111/j.1463-1318.2008.01517.x. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Tazuma S., Kanno K., Hyogo H., Iwai M., Horiuchi M. Antifibrogenic function of angiotensin II type 2 receptor in CCl4-induced liver fibrosis. Biochem. Biophys. Res. Commun. 2006;346:658–664. doi: 10.1016/j.bbrc.2006.05.183. [DOI] [PubMed] [Google Scholar]
- Onori P., Morini S., Franchitto A., Sferra R., Alvaro D., Gaudio E. Hepatic microvascular features in experimental cirrhosis: a structural and morphometrical study in CCl4-treated rats. J. Hepatol. 2000;33:555–563. doi: 10.1034/j.1600-0641.2000.033004555.x. [DOI] [PubMed] [Google Scholar]
- Pawa S., Ali S. Liver necrosis and fulminant hepatic failure in rats: protection by oxyanionic form of tungsten. Biochim. Biophys. Acta. 2004;1688:210–222. doi: 10.1016/j.bbadis.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Pesh-Imam M., Reckuagel R.O. Lipid peroxidation and the concept of anti-oxygenic potential; vitamin E change in acute experimental CCl4, and ethanol induced liver injury. Toxicol. Appl. Pharmacol. 1977;42:463–475. doi: 10.1016/s0041-008x(77)80031-8. [DOI] [PubMed] [Google Scholar]
- Prescott L.F. Glutathione: a protective mechanism against hepatotoxicity. Biochem. Soc. Trans. 1982;10:84–85. doi: 10.1042/bst0100084. [DOI] [PubMed] [Google Scholar]
- Rikans L.E., Hornbrook K.R., Cai Y. Carbon tetrachloride hepatotoxicity as a function of age in female Fischer 344 rats. Mech. Ageing. 1994;76(2–3):89–99. doi: 10.1016/0047-6374(94)91584-9. 20. [DOI] [PubMed] [Google Scholar]
- Rosa L.H., Machado K.N., Jacob C.C., Capelari M., Rosa C.A., Zani C.L. Screening of Brazilian basidiomycetes for antimicrobial activity. Mem. Inst. Oswaldo Cruz. 2003;98:967–974. doi: 10.1590/s0074-02762003000700019. [DOI] [PubMed] [Google Scholar]
- Shen Xiangchun, Tang Yuping, Yang Ruihui, Yu Li, Fang Taihui, Duan Jin-ao. The protective effect of Zizyphus jujuba fruit on carbon tetrachloride-induced hepatic injury in mice by anti-oxidative activities. J. Ethnopharmacol. 2009;122:555–560. doi: 10.1016/j.jep.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Subramoniam A., Pushpangadan P. Development of phytomedicine for liver diseases. Ind. J. Pharmacol. 1999;31:166–175. [Google Scholar]
- Takaku T., Kimura Y., Okuda H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J. Nutr. 2001;131:1409–1413. doi: 10.1093/jn/131.5.1409. [DOI] [PubMed] [Google Scholar]
- Trivedi P., Mowat A.P. Carbon tetrachloride-induced hepatic fibrosis and cirrhosis in the developing rat: an experimental model of cirrhosis in childhood. Br. J. Exp. Pathol. 1983;64(1):25–33. [PMC free article] [PubMed] [Google Scholar]
- Vogel F.S., McGarry S.J., Kemper L.A., Graham D.G. Bacteriocidal properties of a class of quinoid compounds related to sporulation in the mushroom, Agaricus bisporus. Am. J. Pathol. 1974;76:165–174. [PMC free article] [PubMed] [Google Scholar]
- Wasser S.P., Weis A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit. Rev. Immunol. 1999;19(1):65–96. [PubMed] [Google Scholar]
- Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model Crit. Rev. Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Winkler B.S. Unequivocal evidence in support of the nonenzymatic redox coupling between glutathione/glutathione disulfide and ascorbic acid/dehydroascorbic acid. Biochim. Biophys. Acta. 1992;1117:287–290. doi: 10.1016/0304-4165(92)90026-q. [DOI] [PubMed] [Google Scholar]
- Wooten L.D.P., Freeman H. sixth ed. Churchill Livingstone; New York and London: 1982. Microanalysis in Medical Biochemistry. p. 109. [Google Scholar]
- Yuan L.P., Chen F.H., Ling L., Dou P.F., Bo H., Zhong M.M., Xia L.J. Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. J. Ethnopharmacol. 2008;16:539–546. doi: 10.1016/j.jep.2008.01.010. [DOI] [PubMed] [Google Scholar]


