Summary
Background
The objective of this study was to establish the efficacy and safety of a new treatment regimen consisting of dose-dense cisplatin-based chemotherapy and radical surgery in children with high-risk hepatoblastoma.
Methods
SIOPEL-4 was a prospective single-arm feasibility study. Patients aged 18 years or younger with newly diagnosed hepatoblastoma with either metastatic disease, tumour in all liver segments, abdominal extrahepatic disease, major vascular invasion, low α fetoprotein, or tumour rupture were eligible. Treatment consisted of preoperative chemotherapy (cycles A1–A3: cisplatin 80 mg/m2 per day intravenous in 24 h on day 1; cisplatin 70 mg/m2 per day intravenous in 24 h on days 8, 15, 29, 36, 43, 57, and 64; and doxorubicin 30 mg/m2 per day intravenous in 24 h on days 8, 9, 36, 37, 57, and 58) followed by surgical removal of all remaining tumour lesions if feasible (including liver transplantation and metastasectomy, if needed). Patients whose tumour remained unresectable received additional preoperative chemotherapy (cycle B: doxorubicin 25 mg/m2 per day in 24 h on days 1–3 and 22–24, and carboplatin area under the curve [AUC] 10·6 mg/mL per min per day intravenous in 1 h on days 1 and 22) before surgery was attempted. After surgery, postoperative chemotherapy was given (cycle C: doxorubicin 20 mg/m2 per day in 24 h on days 1, 2, 22, 23, 43, and 44, and carboplatin AUC 6·6 mg/mL per min per day in 1 h on days 1, 22, and 43) to patients who did not receive cycle B. The primary endpoint was the proportion of patients with complete remission at the end of treatment. Analysis was by intention to treat. This trial is registered at ClinicalTrials.gov, NCT00077389.
Findings
We report the final analysis of the trial. 62 eligible patients (39 with lung metastases) were included and analysed. 60 (98%, 95% CI 91–100) of 61 evaluable patients (one child underwent primary hepatectomy) had a partial response to preoperative chemotherapy. Complete resection of all tumour lesions was achieved in 46 patients (74%). At the end of therapy, 49 (79%, 95% CI 67–88) of 62 patients were in complete remission. With a median follow-up of 52 months, 3-year event-free survival was 76% (95% CI 65–87) and 3-year overall survival was 83% (73–93). 60 (97%) patients had grade 3–4 haematological toxicity (anaemia, neutropenia, or thrombocytopenia) and 44 (71%) had at least one episode of febrile neutropenia. Other main grade 3 or 4 toxicities were documented infections (17 patients, 27%), anorexia (22, 35%), and mucositis (seven, 11%). One child died of fungal infection in neutropenia. Moderate-to-severe ototoxicity was documented in 31 (50%) patients. 18 serious adverse events (including two deaths) reflecting the observed side-effects were reported in the trial (the most common was ototoxicity in five patients).
Interpretation
The SIOPEL-4 treatment regimen is feasible and efficacious for complete remission at the end of treatment for patients with high-risk hepatoblastoma.
Funding
Cancer Research UK and Cancer Research Switzerland/Oncosuisse.
Introduction
Despite important progress in the cure of children with localised hepatoblastoma, the prognosis of patients with metastatic disease remains poor, with 5-year event-free survival of 21–28% and 5-year overall survival of 27–57%.1–14 Only the most recently published study of the International Childhood Liver Tumours Strategy Group (SIOPEL-3),15 which used intensive multiagent chemotherapy and aggressive surgery, managed to achieve a better outcome for these children (3-year event-free survival 56%, 3-year overall survival 62%). To further improve the number of children cured with high risk, and in particular metastatic hepatoblastoma, new therapeutic approaches are needed that can increase treatment efficacy without excessive toxicity.
Data from earlier studies suggest that cisplatin is the key chemotherapeutic drug in the treatment of hepatoblastoma, but its optimum schedule and dose in this setting is not yet established. As shown in studies in adults, the modulation of dose density—the frequency of effective drug dose administration—could be an important way to improve the antitumour efficacy of cisplatin.16 Chemotherapy regimens based on the administration of weekly cisplatin have been efficacious and feasible in different types of adult cancer without excessive severe toxicity.17–22 In view of the mild toxicity of cisplatin in the previous SIOPEL trials, such a regimen could perhaps also be safe in children with hepatoblastoma.
On the basis of these data and considerations SIOPEL launched the present study to establish the efficacy and safety of a new chemotherapy regimen based on dose-dense (weekly) preoperative administration of cisplatin in combination with monthly doxorubicin and radical surgery in children with high-risk hepatoblastoma.
Methods
Study design and patients
SIOPEL-4 was an international, multicentre, prospective, single-arm feasibility trial to study the efficacy and safety of an experimental treatment. The study was approved by local institutional review boards and undertaken in accordance with the principles of the Declaration of Helsinki and the rules of Good Clinical Practice. Informed written consent was obtained from all patients or parents.
The study was open to children with untreated high-risk hepatoblastoma (age ≤18 years). Risk stratification was based on the results of previous SIOPEL studies. According to these results, high risk was defined as tumour involving all four hepatic sections (PRE-Treatment EXTent of disease [PRETEXT]-IV23,24), or presence of distant metastases, or tumour extension into the vena cava or all three hepatic veins, or into the main or both branches of the portal vein, or extrahepatic intra-abdominal disease, or serum α fetoprotein (AFP) less than 100 μg/L at diagnosis, or tumour rupture. Patients with abnormal cardiac, renal, or liver function at diagnosis or known pre-existing hearing loss were excluded. Tumour extension was assessed by pretreatment imaging (ultrasound, CT, and MRI) according to the PRETEXT system.23,24 For the purposes of eligibility for this trial, pulmonary lesions were judged to be metastases if there was one nodule more than 10 mm or several nodules with at least one more than 5 mm. Biopsy and histological evaluation of the primary tumour was mandatory for all children.
Procedures
Treatment consisted of three cycles of preoperative chemotherapy (cycles A1–A3) followed by surgical removal of all remaining tumour lesions if feasible (including liver transplantation and metastasectomy if needed) followed by postoperative chemotherapy (cycle C). Patients whose tumour remained unresectable received additional chemotherapy (cycle B) before surgery was attempted. In these cases no postoperative chemotherapy was given (figure 1). No additional therapy was recommended for microscopic residual disease after liver surgery.
Figure 1.
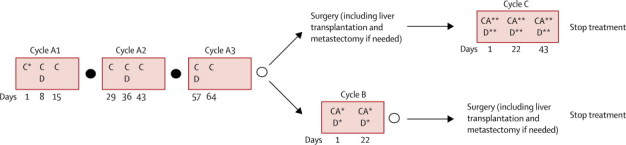
Treatment design of the SIOPEL-4 study
C*=cisplatin 80 mg/m2 per day intravenous infusion in 24 h; on day 1 in cycle A1. C=cisplatin 70 mg/m2 per day intravenous infusion in 24 h; on days 8, 15, 29, 36, 43, 57, and 64 in cycles A1–A3. D=doxorubicin 30 mg/m2 per day intravenous infusion in 24 h; on days 8, 9, 36, 37, 57, and 58 in cycles A1–A3. D*=doxorubicin 25 mg/m2 per day intravenous infusion in 24 h; on days 1, 2, 3, 22, 23, and 24 in cycle B. D**=doxorubicin 20 mg/m2 per day intravenous infusion in 24 h; on days 1, 2, 22, 23, 43, and 44 in cycle C. CA*=carboplatin area under the curve (AUC) 10·6 mg/mL per min per day intravenous infusion in 1 h; on days 1 and 22 in cycle B. CA**=carboplatin AUC 6·6 mg/mL per min per day intravenous infusion in 1 h; on days 1, 22, and 43 in cycle C. Filled circle=assessment of response. Empty circle=assessment of response and resectability.
Chemotherapy was given as follows: cisplatin, 70 mg/m2 per day intravenous infusion in 24 h on days 8, 15, 29, 36, 43, 57, and 64 in cycles A1–A3, 80 mg/m2 per day in 24 h on day 1 in cycle A1; doxorubicin, 30 mg/m2 per day intravenous infusion in 24 h on days 8, 9, 36, 37, 57, and 58 in cycles A1–A3, 25 mg/m2 per day in 24 h on days 1, 2, 3, 22, 23, and 24 in cycle B, 20 mg/m2 per day in 24 h on days 1, 2, 22, 23, 43, and 44 in cycle C; and carboplatin area under the curve (AUC) 10·6 mg/mL per min per day intravenous infusion in 1 h on days 1 and 22 in cycle B, AUC 6·6 mg/mL per min per day in 1 h on days 1, 22, and 43 in cycle C. Cycle A3 was modified to avoid delay in surgery due to bone marrow toxicity of this course. Before starting a cycle patients should have recovered clinically from the previous course and must not have signs of active infection. Absolute neutrophil count should have recovered to higher than 1 × 109 per L and platelet count to higher than 100 × 109 per L. For infants and children with bodyweight less than 10 kg and in case of delay in chemotherapy because of toxicity, dose reduction was recommended by the protocol (appendix). Delays in therapy due to toxicity were allowed according to protocol. To assist optimum treatment decisions by the treating centres, the protocol provided detailed guidelines for tumour assessment and surgery including the issue of difficult or extreme resection, liver transplantation, and metastasectomy (appendix). Details of post-transplant immunosuppressive regimen were not collected systematically.
The primary endpoint was the proportion of patients with complete remission at the end of trial treatment, which is a good surrogate for event-free survival and was thought to be an adequate outcome measure for this trial. Secondary endpoints were the proportion of patients who responded to preoperative chemotherapy, the proportion of patients with complete resection, overall survival, and event-free survival. Tumour response and resectability were assessed by imaging and serum AFP concentration after each chemotherapy cycle according to the following criteria: complete response, defined as no evidence of disease and normal AFP (for age); partial response, defined as any tumour volume shrinkage and a decrease of AFP greater than 1 log below the original measurement; stable disease, defined as no tumour volume change and no change or less than 1 log fall of AFP; progressive disease, defined as unequivocal increase of tumour size in one or more dimensions or any unequivocal increase of the AFP concentration, or both; complete surgical resection, defined as total macroscopic removal of all tumour lesions; and complete remission, defined as no evidence of tumour on imaging and normal (for age) serum AFP. Overall tumour response to chemotherapy was evaluated at the end of preoperative chemotherapy.
Acute toxicity was evaluated after each chemotherapy cycle. Evaluation of cardiac toxicity and ototoxicity was also required during follow-up. Establishment of normal baseline cardiac, renal, liver, and audiological functions were required before the start of treatment. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0 and the Brock grading for ototoxicity.25
The trial was monitored for excessive toxicity by regular evaluation of severe adverse event reports and toxicity data. These data were reported to an independent data monitoring committee that assessed and judged observed toxicity at least once a year and made recommendations for amendments or closing of the study if necessary.
Statistical analysis
We used Simon's two-stage optimal design with a probability of complete remission in 60% of patients or less (from the SIOPEL-3HR study) as the null hypothesis and a probability of 80% of patients or higher as the alternative hypothesis. The sample size was determined by testing of the null versus the alternative hypothesis at a significance level of 0·05 and a power of 90%. The sample size was n1=19 for stage 1 and n2=34 for stage 2, resulting in a maximum sample size of 53 if the trial was not stopped after stage 1 analysis. The stage 1 evaluation was done on the first 19 treated patients in February, 2007, and concluded that early stopping criteria were not met and the trial could be continued. The alternative hypothesis was to be accepted if the number of patients with complete remission was 38 or higher. A stopping rule for safety was devised on the basis of the number of patients in whom possible treatment-related severe adverse events developed, including death and grade 4 toxic effects. SAS version 9.1 was used for all analyses.
Role of the funding source
The funding sources had no role in the design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between Jan 1, 2005, and Aug 31, 2009, 67 patients were enrolled in the trial from 18 countries worldwide (figure 2). Five patients were excluded from the analysis because of misdiagnosis after central pathology review of the biopsy material (four hepatocellular carcinomas, one rhabdoid tumour). Table 1 shows the clinical characteristics of the remaining 62 eligible patients. No children were excluded because of abnormal cardiac, renal, or liver function or pre-existing hearing loss.
Figure 2.
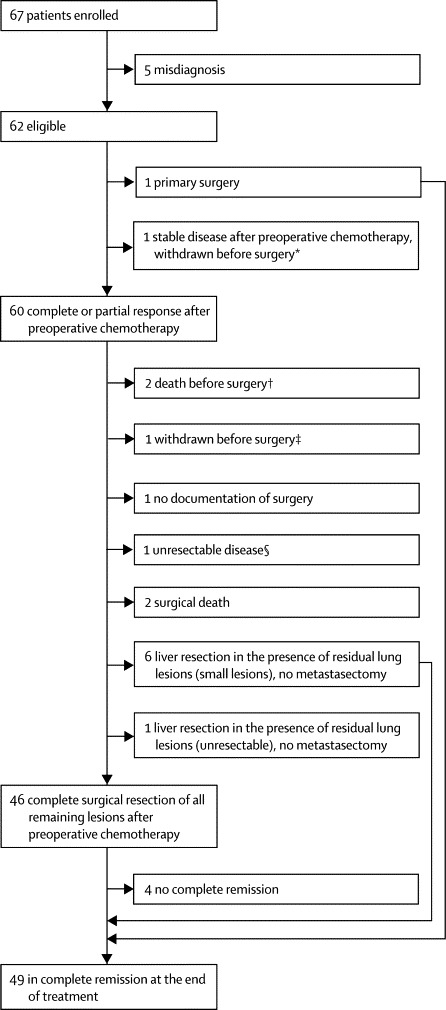
Enrolment, treatment, and outcome of patients
*Withdrawn after second preoperative chemotherapy cycle (A2) because of grade 3 ototoxicity. † One toxic death, one death due to tumour rupture. ‡Withdrawn after third preoperative chemotherapy cycle (A3) with partial response. §Unresectable because of multiple lung lesions, no surgery attempted.
Table 1.
Patient characteristics at diagnosis and chemotherapy cycles given
| Patients (n=62) | |
|---|---|
| Age | |
| Median (months) | 19·1 (2·6–15·9) |
| 0 to <12 months | 14 (23%) |
| 1 to <3 years | 32 (52%) |
| ≥3 years | 16 (26%) |
| Sex | |
| Female | 23 (37%) |
| Male | 39 (63%) |
| AFP | |
| Median (μg/L) | 297 768·0 (42·0–3 315 400·0) |
| <100 μg/L | 2 (3%) |
| Tumour extension according to PRETEXT system | |
| PRETEXT I | 2 (3%) |
| PRETEXT II | 17 (27%) |
| PRETEXT III | 27 (44%) |
| PRETEXT IV | 16 (26%) |
| Lung metastases* | 39 (63%) |
| Vascular invasion | 24 (39%) |
| Abdominal extrahepatic disease† | 6 (10%) |
| Tumour rupture at diagnosis | 10 (16%) |
| SCUD histology‡ | 5 (8%) |
| Multifocal primary tumour | 20 (32%) |
| Chemotherapy cycles administered | |
| Cycle A1 | 62 (100%) |
| Cycle A2 | 61 (98%) |
| Cycle A3 | 59 (95%) |
| Cycle B | 13 (21%) |
| Cycle C | 37 (60%) |
| Number of cycles received by patient | |
| 1 cycle (A1) | 1 (2%) |
| 2 cycles (A1+A2) | 2 (3%) |
| 3 cycles (A1+A2+A3) | 9 (15%) |
| 4 cycles (A1–A3+B or C) | 50 (81%) |
Data are n (%) or median (range). AFP=serum α fetoprotein. PRETEXT=PRE-Treatment EXTent of disease.23,24 SCUD=small cell undifferentiated hepatoblastoma.
Multiple lesions in 38, single lesion in one patient.
Established on imaging, not confirmed by histology.
Established locally in two (no review available), locally and in central review in two, and in central review only in one patient.
Table 1 shows the number of chemotherapy cycles given in total and per patient. In 50 (81%) patients all four cycles were given and documented. Six patients received fewer than the prescribed four cycles because of death during therapy (four patients) or withdrawal (two). In six patients postoperative chemotherapy was not documented. The cumulative delay in the administration of the second and third cycles of preoperative therapy (A2 and A3) because of toxicity ranged from −4 to 63 days (median 7 days, mean 10 days); reasons for early start of the next cycle of chemotherapy in some patients were good haematological recovery and clinical condition. Dose reduction was applied in four of the 61 second cycles (A2), seven of the 59 third cycles (A3), and eight of the 37 postoperative (C) cycles all because of previous haematological toxicity (no dose reductions were observed in the additional preoperative [B] cycle). Two patients who withdrew from the study received some non-protocol chemotherapy after withdrawal.
60 (98%, 95% CI 91–100) of 61 evaluable patients (one child underwent primary hepatectomy) had a partial response to preoperative chemotherapy. The remaining patient had a decrease in tumour volume and a decrease in AFP less than 1 log and was classified as having stable disease. This patient later achieved complete remission and has been cured. In most patients, partial response was reached early during preoperative treatment. No patients could achieve complete response because the evaluation was done before surgery. In the 13 patients who received additional preoperative chemotherapy (cycle B), the overall response status was not changed. However, one patient who had partial response in the lungs after cycles A1–A3 had a complete response of the lung lesions after additional preoperative chemotherapy. Of the 39 patients with initial lung metastases, a complete response of the lung lesions was achieved in 20 patients and partial response in 18 patients, with preoperative chemotherapy alone (97% patients responded). In one patient no evaluation of the lung lesions was done.
In 53 (85%) of the 62 patients, complete macroscopic resection of the primary tumour was achieved with partial hepatectomy (n=37) or liver transplantation (n=16). Two children died of postoperative complications. In five patients, no surgery was attempted because of unresectable disease (one patient), early death (two), or withdrawal before surgery (two). One patient underwent primary surgery because of tumour rupture, and for one patient data were missing. Microscopic residuals were seen in five of the 37 patients who underwent partial hepatectomy. All achieved complete remission and none relapsed.
Of the 53 patients with complete resection of the primary tumour, seven also underwent pulmonary metastasectomy. Complete resection of all tumour lesions was achieved in 46 patients (74%, 95% CI 62–84). In seven patients with residual lung lesions after chemotherapy, the liver tumour was resected but no metastasectomy was done (figure 2).
16 patients were transplanted, eight with PRETEXT-IV, five with PRETEXT-III, and three with PRETEXT-II tumour. Seven of these patients had initial metastases, which were cleared with chemotherapy in six patients. In one patient, metastasectomy was done with no viable tumour cells in the specimen. None of these seven patients had a (pulmonary) relapse.
Of the 46 patients with complete resection of all tumour lesions, one developed recurrent lung lesions before the end of treatment. In three patients, AFP remained raised. Conversely, six patients with small residual pulmonary lesions after liver surgery who did not undergo metastasectomy achieved complete remission with postsurgical chemotherapy given according to protocol. In total 49 (79%, 95% CI 67–88) of 62 patients were in complete remission at the end of therapy, including the one who underwent primary hepatectomy, close to the prespecified goal of 80%. Six patients were alive with evidence of disease (three with tumour visible on imaging studies, three with slightly raised AFP), four died (one of tumour bleeding, one of toxicity, two of postoperative complications), two were withdrawn (one after two cycles with stable disease for grade 3 ototoxicity; one after cycles A1–A3 in partial response), and one was not documented (figure 2).
Median follow-up for surviving patients was 52 (range 3–83, IQR 27·9) months. At last follow-up 15 events were registered: five relapses, six progressions from residual disease, and four deaths for other reasons. 12 patients died of either tumour progression or relapse (eight), toxicity (one, fungal infection in profound neutropenia), surgical complications (two, one intraoperative bleeding and shock; one, lethal postoperative wound infection), or tumour bleeding (one, spontaneous intratumour bleeding with irreversible haemorrhagic shock before the second cycle of chemotherapy). The Kaplan-Meier estimate of 3-year event-free survival was 76% (95% CI 65–87) and of overall survival was 83% (95% CI 73–93) for the whole group (figure 3). The relapses occurred 5–48 months after the end of treatment at the following sites: locoregional (three), lungs (one), unknown (one). Of the five patients, two had initial metastases, two PRETEXT-IV tumours, and three initial tumour rupture. Two of the five patients died of their cancer, the other three had a second complete remission.
Figure 3.
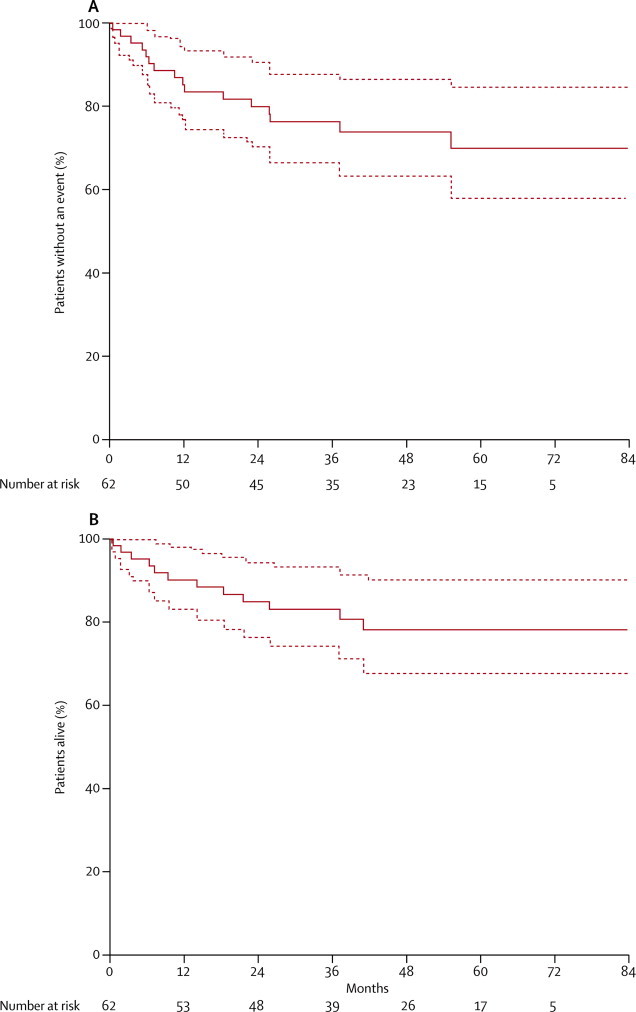
Kaplan-Meier estimates of (A) event-free and (B) overall survival for the whole study population
Dotted lines show 95% CIs.
19 of the 20 patients with metastatic disease with complete response in the lungs were in complete remission at the end of treatment. In one patient lung lesions recurred before the final evaluation. Of the 18 patients with partial response, seven underwent metastasectomy (six complete, one incomplete but no viable tumour). Four of these seven had a complete remission. In the other three patients no visible tumour was detected, but AFP was still raised at the end of therapy. Six of the 18 patients had some residual lesions in the lungs at the end of preoperative chemotherapy but did not undergo pulmonary metastasectomy. All achieved complete remission with further chemotherapy per protocol. In one of these patients, lung disease recurred 5 months after the end of therapy. In two of the 18 children the lung lesions remained unresectable; in one the liver tumour was resected but no metastasectomy was done, and in the other no surgery was attempted. Three of the 18 children died before surgery.
27 (69%) of 39 patients with metastatic disease had complete resection of all tumour lesions. 29 patients (74%) were in complete remission at the end of therapy. Of the remaining ten patients, six did not achieve complete remission because of residual pulmonary disease: in one patient lung lesions disappeared with preoperative chemotherapy but recurred after liver surgery; in two patients pulmonary lesions were diminished, but remained unresectable; and in three patients no visible tumour was left, but AFP remained slightly raised at the end of treatment. Two of these six patients had low initial AFP and small cell undifferentiated (SCUD) tumour. 3-year event-free survival was 77% (95% CI 63–90) and 3-year overall survival was 79% (95% CI 66–92) for the 39 patients with metastasis. In an exploratory analysis, 27 (78%) of 37 patients with metastatic disease and AFP higher than 100 μg/L had achieved continuous complete remission at the end of therapy.
In 11 of the 16 patients with initial PRETEXT-IV tumour, chemotherapy led to downstaging of the liver mass to category III in six, category II in four, and category I in one patient as assessed preoperatively. In four patients the tumour extent remained category IV (information was missing for one patient). In four children the tumour was resected with partial hepatectomy, and eight patients underwent liver transplantation. Pulmonary metastasectomy was done in one patient. Complete resection of all tumour lesions was achieved in 12 patients (75%). One patient died of postoperative complications, in one no surgery was attempted because of unresectable lung disease, and two patients were withdrawn before surgery. All 12 children with complete tumour resection had complete remission. 3-year event-free survival was 73% (95% CI 51–96) and overall survival was 80% (95% CI 60–100) for the 16 patients with PRETEXT-IV tumours, including the seven with lung metastases. Of the nine patients who did not have metastatic disease at diagnosis, eight (89%) had a complete resection and eight (89%) achieved complete remission.
60 (97%) patients had grade 3–4 haematological toxicity after at least one of the cycles. The highest frequency of neutropenia and thrombocytopenia was after additional preoperative chemotherapy (cycle B; 12 [92%] and 11 [85%], respectively), which was given only to 13 patients. 44 (71%) patients had at least one episode of febrile neutropenia. Grade 3–4 infection or mucositis affected only a few patients. Table 2 lists all reported short-term toxicity (other than ototoxicity). One patient died of fungal infection in profound neutropenia.
Table 2.
Short-term toxicity in the SIOPEL-4 trial
|
Reported toxicity in cycles A1–A3 |
Number of patients with reported toxicity in cycle B | Number of patients with reported toxicity in cycle C | ||
|---|---|---|---|---|
| Number of patients | Number of cycles | |||
| Haemoglobin | ||||
| Grade 1 | 6 (10%) | 8 (4%) | .. | 1 (3%) |
| Grade 2 | 27 (44%) | 40 (22%) | 3 (23%) | 7 (19%) |
| Grade 3 | 53 (85%) | 105 (58%) | 7 (54%) | 18 (49%) |
| Grade 4 | 11 (18%) | 12 (7%) | 2 (15%) | 6 (16%) |
| Neutrophils | ||||
| Grade 1 | 2 (3%) | 3 (2%) | .. | 1 (3%) |
| Grade 2 | 6 (10%) | 7 (4%) | .. | 2 (5%) |
| Grade 3 | 20 (32%) | 27 (15%) | .. | 5 (14%) |
| Grade 4 | 53 (85%) | 105 (58%) | 12 (92%) | 25 (68%) |
| Platelets | ||||
| Grade 1 | 21 (34%) | 25 (14%) | 1 (8%) | 3 (8%) |
| Grade 2 | 9 (15%) | 9 (5%) | .. | 2 (5%) |
| Grade 3 | 29 (47%) | 38 (21%) | 2 (15%) | 6 (16%) |
| Grade 4 | 39 (63%) | 66 (36%) | 9 (69%) | 19 (51%) |
| Febrile neutropenia | ||||
| Grade 1 | 1 (2%) | 1 (1%) | .. | 1 (3%) |
| Grade 2 | 2 (3%) | 4 (2%) | .. | 1 (3%) |
| Grade 3 | 41 (66%) | 7 (4%) | 8 (62%) | 16 (43%) |
| Grade 4 | 3 (5%) | 3 (2%) | .. | 2 (5%) |
| Infection (documented) | ||||
| Grade 2 | 7 (11%) | 8 (4%) | 1 (8%) | .. |
| Grade 3 | 17 (27%) | 18 (10%) | 2 (15%) | 5 (14%) |
| Grade 4 | .. | .. | .. | 1 (3%) |
| Fever | ||||
| Grade 1 | 15 (24%) | 19 (10%) | 1 (8%) | 3 (8%) |
| Grade 2 | 13 (21%) | 15 (8%) | 1 (8%) | 1 (3%) |
| Grade 3 | 2 (3%) | 3 (2%) | .. | .. |
| Vomiting | ||||
| Grade 1 | 20 (32%) | 21 (12%) | 1 (8%) | 3 (8%) |
| Grade 2 | 29 (47%) | 46 (25%) | 1 (8%) | 5 (14%) |
| Grade 3 | 4 (6%) | 4 (2%) | .. | 1 (3%) |
| Anorexia | ||||
| Grade 1 | 11 (18%) | 12 (7%) | 2 (15%) | 3 (8%) |
| Grade 2 | 10 (16%) | 12 (7%) | 2 (15%) | 3 (8%) |
| Grade 3 | 22 (35%) | 36 (20%) | 2 (15%) | 5 (14%) |
| Mucositis | ||||
| Grade 1 | 13 (21%) | 16 (9%) | 1 (8%) | 3 (8%) |
| Grade 2 | 7 (11%) | 9 (5%) | 3 (23%) | 1 (3%) |
| Grade 3 | 7 (11%) | 11 (6%) | 1 (8%) | .. |
| Diarrhoea | ||||
| Grade 3 | 2 (3%) | 2 (1%) | .. | 2 (5%) |
| Hypertension | ||||
| Grade 4 | 1 (2%) | 1 (1%) | .. | .. |
| Haemorrhage or bleeding | ||||
| Grade 4 | 1 (2%) | 1 (1%) | 1 (8%) | .. |
| Allergy | ||||
| Grade 3 | 1 (2%) | 1 (1%) | .. | .. |
| Left ventricular systolic dysfunction | ||||
| Grade 3 | 1 (2%) | 1 (1%) | .. | .. |
| Glomerular filtration rate | ||||
| Grade 1 | 7 (11%) | 7 (4%) | .. | 3 (8%) |
| Grade 2 | 2 (3%) | 2 (1%) | 1 (8%) | 2 (5%) |
| Grade 3 | 1 (2%) | 1 (1%) | ||
| Aspartate aminotransferase | ||||
| Grade 1 | 31 (50%) | 47 (26%) | 3 (23%) | 9 (24%) |
| Grade 2 | 13 (21%) | 13 (7%) | .. | 2 (5%) |
| Grade 3 | 7 (11%) | 7 (4%) | .. | 2 (5%) |
| Grade 4 | 1 (2%) | 1 (1%) | ||
| Alanine aminotransferase | ||||
| Grade 1 | 25 (40%) | 36 (20%) | 2 (15%) | 7 (19%) |
| Grade 2 | 6 (10%) | 6 (3%) | 1 (8%) | 2 (5%) |
| Grade 3 | 5 (8%) | 5 (3%) | .. | 3 (8%) |
| Grade 4 | .. | 1 (3%) | ||
| Hypomagnesaemia | ||||
| Grade 1 | 23 (37%) | 34 (19%) | 2 (15%) | 5 (14%) |
| Grade 2 | 10 (16%) | 16 (9%) | .. | 1 (3%) |
| Grade 3 | 4 (6%) | 4 (2%) | .. | 1 (3%) |
| Hypophosphataemia | ||||
| Grade 3 | 2 (3%) | 2 (1%) | .. | .. |
| Grade 4 | 1 (2%) | |||
| Hypokalaemia | ||||
| Grade 4 | 1 (2%) | .. | .. | .. |
| Hyperbilirubinaemia | ||||
| Grade 1 | 11 (18%) | 11 (6%) | .. | 2 (5%) |
| Grade 2 | 2 (3%) | 2 (1%) | .. | 2 (5%) |
| Grade 3 | 3 (5%) | 3 (2%) | .. | .. |
Data are n (%). Cycles A1–A3 were given to 62 patients (in total 182 cycles), additional preoperative chemotherapy (cycle B) to 13 patients, and postoperative chemotherapy (cycle C) to 37 patients.
Of the 61 children who received at least two preoperative cycles, ototoxicity was evaluated and documented in 54, with at least two measurements, both done after at least two cycles or during follow-up. The number and percentage of patients with ototoxicity according to the Brock criteria were: grade 0: 18 (30%), grade 1: five (8%), grade 2: 15 (25%), grade 3: 12 (20%), grade 4: four (7%). In seven patients (10%) ototoxicity was not sufficiently documented.
18 serious adverse events, including two deaths, were reported (appendix). The most common serious adverse event was ototoxicity in five patients. One child was withdrawn by the local physician after two cycles (A1+A2) because of Brock grade 3 ototoxicity. On the basis of the observed toxicity, the independent data monitoring committee did not recommend any changes or amendment to the protocol during the trial.
Discussion
The SIOPEL-4 trial addressed the question of whether increased dose density of cisplatin in the preoperative phase can improve the prognosis of children with high-risk, in particular metastatic, hepatoblastoma (panel). Our results provide firm evidence for the efficacy of the applied regimen, with close to 80% of patients achieving complete remission at the end of treatment. The proportion of patients with complete remission and the 3-year event-free and overall survival compare favourably with results of previous studies and suggest an improvement in the prognosis of children with high-risk hepatoblastoma.
Panel. Research in context.
Systematic review
We searched PubMed on Jan 20, 2004, for clinical trials published in English before Jan 1, 2004, using the keywords “hepatoblastoma”, “metastatic”, or “high risk”, or “locally advanced or “unresectable hepatoblastoma” and identified 11 articles that provided prospective survival data for at least one of these patient groups. No randomised trials were found in these specific subgroups. The results showed 5-year event-free survival of 21–28% and 5-year overall survival of 27–57% in patients with metastatic hepatoblastoma. Published 5-year event-free survival was 48% and overall survival was 53% for patients with high-risk hepatoblastoma. When preparing the report we did a further PubMed search on Oct 1, 2012, using the same search terms and conditions. Four more relevant articles were identified, one of which showed improved results—compared with the previous data—in high-risk hepatoblastoma (3 year event-free survival 65%, overall survival 69%; 56% and 62% for the metastatic subgroup). We cited the most relevant articles in this Article. Systematic search for publications on the use of “(high) dose-dense” or “weekly” cisplatin in childhood cancer retrieved no articles.
Interpretation
Our study used a novel and unique design of dose-dense (weekly) cisplatin in the treatment of patients with high-risk hepatoblastoma and provides firm evidence for the feasibility and efficacy of this approach. To our knowledge, our paper is the first report on the use of dose-dense cisplatin in the treatment of paediatric patients with cancer. The reported outcome, in particular for those with metastatic disease, is higher than ever before achieved for children with high-risk hepatoblastoma and denotes an important improvement in the prognosis of these children. Although further evaluation of the long-term toxicity is needed, the presented treatment strategy could be now regarded as first choice in the treatment of patients with metastatic or very high-risk hepatoblastoma.
In view of the rarity of high-risk hepatoblastoma, a randomised controlled study to directly prove the superiority of the study treatment was not deemed feasible within an acceptable timeframe. Instead, a single-arm study was designed with complete remission at the end of trial treatment as the primary endpoint, which is an objective and relevant outcome measure and allows analysis shortly after the study is closed. The efficacy of this regimen was primarily compared with the results of SIOPEL-3HR, the previous high-risk study of the SIOPEL group. SIOPEL-4 aimed to achieve an improvement in proportion of patients with complete remission of 20% or more (from ≤60% [in SIOPEL-3HR] to ≥80%), which is regarded as a clinically relevant and significant improvement.
Since tumour-free status at the end of treatment is a prerequisite for cure and the relapse rate in high-risk hepatoblastoma is low, complete remission is thought to be a good surrogate for overall and event-free survival. Additionally, to produce robust survival data that can be adequately compared with previously published results, patients were followed up for a median of 52 months (table 3). Data from previous studies suggest that almost all relapses after hepatoblastoma treatment occur in the first 2 years after the end of treatment. On the basis of these data the current follow-up seems largely sufficient for a good estimate of the long-term efficacy of the regimen and gives a good basis for comparison with other studies.
Table 3.
Response and outcome results of SIOPEL-4 compared with previous studies
| Stage | Number of patients | Patients who responded to treatment (95% CI) | Patients who underwent complete resection (95% CI) | Event-free or progression-free survival | Overall survival | |
|---|---|---|---|---|---|---|
| All patients with high-risk hepatoblastoma | ||||||
| SIOPEL-4 | High risk | 62 | 98% (91–100) | 74% (62–84) | 3-year 76% (95% CI 65–87) | 3-year 83% (95% CI 73–93) |
| SIOPEL-315 | High risk | 151 | 79% (71–85) | 70% (62–77) | 3-year 65% (95% CI 57–73) | 3-year 69% (95% CI 62–77) |
| SIOPEL-23 | High risk | 58 | 78% (65–87) | 67% (54–79) | 3-year 48% (SE 13) | 3-year 53% (SE 13) |
| Patients with distant metastases at diagnosis | ||||||
| SIOPEL-4 | Metastatic | 39 | 97% | 70% | 3-year 77% (95% CI 63–90) | 3-year 79% (95% CI 66–92) |
| SIOPEL-315 | Metastatic | 70 | 71% | .. | 3-year 56% (95% CI 44–68) | 3-year 62% (95% CI 50–73) |
| SIOPEL-23 | Metastatic | 25 | 72% | 60% | .. | 3-year 44% |
| SIOPEL-11,2 | Metastatic | 31 | 84% | .. | 5-year 28% (95% CI 12–44) | 5-year 57% (95% CI 39–75) |
| INT-009810 | Metastatic | 40 | .. | .. | 5-year 25% (SE 7) | 5-year 37% (SE 8) |
| POG-934511 | Metastatic | 11 | .. | 36% | 5-year 27% (SE 16) | 5-year 27% (SE 16) |
| JPLT-214 | Metastatic | 35 | .. | .. | 5-year 21% | 5-year 44% |
| Patients with PRETEXT-IV tumour at diagnosis | ||||||
| SIOPEL-4 | PRETEXT IV (no metastases)* | 9 | 89% | 89% | 3-year 75% | 3-year 88% |
| SIOPEL-315 | PRETEXT IV (no metastases)* | 49 | 94% | 88% | 3-year 75% | 3-year 77% |
| SIOPEL-23 | PRETEXT IV (no metastases)* | 21 | 81% | 76% | .. | 3-year 61% |
PRETEXT=PRE-Treatment EXTent of disease.23,24
Patients with α fetoprotein less than 100 μg/L or metastasis were excluded.
Although we acknowledge that comparison with historical controls does not provide the highest level of evidence, we are convinced that in this context the use of historical controls is the best available option to indicate improvement. We believe that the high similarities in patient populations, inclusion and exclusion criteria, and trial design, conduct, and analysis make the comparison with previous SIOPEL studies appropriate.
The most important progress has been achieved in the cure of children with metastases. All evaluable patients showed a remarkable response to chemotherapy and in more than half, the lung lesions were entirely cleared. The complete response in the lungs achieved in this way appears stable, since 19 (95%) of the 20 patients remained in continuous complete remission, including the six who underwent liver transplantation. 25 of the 39 patients had complete remission without pulmonary surgery and two additional patients had no viable tumour cells in their metastasectomy specimens, emphasising the efficacy of the applied chemotherapy. The low number of patients who had lung relapse (one, 5%) also shows the stability of the lung responses achieved in this way. Additionally, two of the six children who did not have complete remission because of pulmonary disease had low initial AFP and SCUD histology, both features known as bad prognostic factors.26,27 The outcome of patients with metastatic disease achieved in this study appears to be better than the results of all previously published studies, including SIOPEL-3HR, and denotes a major improvement in the cure of these children (table 3).
The excellent response of lung metastases to preoperative chemotherapy in this study is in line with the promising results of the previous SIOPEL study (3HR), which seem to be further improved by the highly effective SIOPEL-4 regimen. Our results emphasise the importance of chemotherapy responsiveness of lung metastases and the role of chemotherapy in the cure of these patients. Surgery remains important in patients whose lung lesions are not cleared by chemotherapy alone.
The presented results for patients with PRETEXT-IV tumour confirm the fairly good prognosis of this subgroup and might suggest a slight improvement in overall survival compared with previous SIOPEL studies (table 3). Our data underline the importance of both chemotherapy and liver transplantation in the management of these tumours. Because of an excellent response to chemotherapy, the tumour extent decreased significantly in most patients and partial hepatectomy became feasible in four children who otherwise would have needed liver transplantation. This study confirms that transplantation is a safe and potentially curative option for patients whose primary tumour remains unresectable with partial hepatectomy.28–30 These results also emphasise that the presence of lung metastases at diagnosis is not a contraindication to liver transplantation, provided that effective preoperative chemotherapy is given, the lung lesions respond to chemotherapy, and are completely cleared before transplantation by chemotherapy and metastasectomy (if needed).
We believe that the most important explanation for the improved results is the increased preoperative dose density of cisplatin used in this treatment regimen (47·5 mg/m2 per week vs 22·9 mg/m2 per week in SIOPEL-3HR), in the context of the previously established multidisciplinary approach. To our knowledge this study is the first time this approach has been applied in a paediatric treatment protocol. Our results encourage exploration of the role of dose-dense cisplatin-based regimens in the treatment of other paediatric tumours.
As expected, the main toxicity was haematological, leading to profound neutropenia in most children. Extensive experience in management of chemotherapy-related side-effects, neutropenia, infections, bleeding, and adequate supportive treatment, including the possibility of intensive care support, are therefore needed when this protocol is used. Despite the increased dose density of cisplatin, no serious renal toxicity has been observed. The incidence and severity of documented ototoxicity seems acceptable in view of the previously unsatisfactory prognosis. However, because of the young age of most patients, follow-up audiology investigations will be needed to establish the incidence and severity of long-term ototoxicity after this regimen.
In conclusion, the SIOPEL-4 treatment regimen with dose-dense administration of cisplatin and radical surgery is a feasible and effective treatment and improves the prognosis of children with high-risk hepatoblastoma, in particular of those with metastatic disease.
Acknowledgments
Acknowledgments
This study was partly supported by Cancer Research UK and Cancer Research Switzerland/Oncosuisse grants to the SIOPEL group. We thank the participating SIOPEL members and institutions (appendix).
Contributors
JZ, LB, PB, DR, RM, AZ, MCh, J-BO, DA, MS, PC, and GP were involved in the design and concept of the study and in writing of the protocol. JZ, LB, PB, RM, MCh, and PC were responsible for the running of the trial. JZ, LB, PB, RM, MCh, VL, SB, AR, MR, MCa, MS, BM, PC, and GP participated in data collection and enrolment of patients. AZ was responsible for pathology review. DR and DP were responsible for radiology review. RM was responsible for statistical analysis. JZ, LB, PB, DR, RM, MCh, VL, J-BO, SB, AR, MR, MCa, MS, BM, PC, and GP participated in data analysis and interpretation of results. JZ was responsible for preparing and writing the report. All authors have participated in drafting and finalising the report and have approved the final draft.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Pritchard J, Brown J, Shafford E. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach—results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–3828. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 2.Perilongo G, Brown J, Shafford E. Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer. 2000;89:1845–1853. doi: 10.1002/1097-0142(20001015)89:8<1845::aid-cncr27>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Perilongo G, Shafford E, Maibach R. Risk-adapted treatment for childhood hepatoblastoma. Final report of the second study of the International Society of Paediatric Oncology—SIOPEL 2. Eur J Cancer. 2004;40:411–421. doi: 10.1016/j.ejca.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Perilongo G, Maibach R, Shafford E. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009;361:1662–1670. doi: 10.1056/NEJMoa0810613. [DOI] [PubMed] [Google Scholar]
- 5.Von Schweinitz D, Byrd DJ, Hecker H. Efficiency and toxicity of ifosfamide, cisplatin and doxorubicin in the treatment of childhood hepatoblastoma. Study Committee of the Cooperative Paediatric Liver Tumour Study HB89 of the German Society for Paediatric Oncology and Haematology. Eur J Cancer. 1997;33:1243–1249. doi: 10.1016/s0959-8049(97)00095-6. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs J, Bode U, Von Schweinitz D. Analysis of treatment efficiency of carboplatin and etoposide in combination with radical surgery in advanced and recurrent childhood hepatoblastoma: a report of the German Cooperative Pediatric Liver Tumor Study HB 89 and HB 94. Klin Padiatr. 1999;211:305–309. doi: 10.1055/s-2008-1043805. (in German). [DOI] [PubMed] [Google Scholar]
- 7.Fuchs J, Rydzynski J, Von Schweinitz D. Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer. 2002;95:172–182. doi: 10.1002/cncr.10632. [DOI] [PubMed] [Google Scholar]
- 8.Haberle B, Bode U, von Schweinitz D. Differentiated treatment protocols for high- and standard-risk hepatoblastoma—an interim report of the German Liver Tumor Study HB99. Klin Padiatr. 2003;215:159–165. doi: 10.1055/s-2003-39375. (in German). [DOI] [PubMed] [Google Scholar]
- 9.Ortega JA, Krailo MD, Haas JE. Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol. 1991;9:2167–2176. doi: 10.1200/JCO.1991.9.12.2167. [DOI] [PubMed] [Google Scholar]
- 10.Ortega JA, Douglass EC, Feusner JH. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: a report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665–2675. doi: 10.1200/JCO.2000.18.14.2665. [DOI] [PubMed] [Google Scholar]
- 11.Katzenstein HM, London WB, Douglass EC. Treatment of unresectable and metastatic hepatoblastoma: a pediatric oncology group phase II study. J Clin Oncol. 2002;20:3438–3444. doi: 10.1200/JCO.2002.07.400. [DOI] [PubMed] [Google Scholar]
- 12.Malogolowkin MH, Katzenstein H, Krailo MD. Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol. 2006;24:2879–2884. doi: 10.1200/JCO.2005.02.6013. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga T, Sasaki F, Ohira M. Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr Surg Int. 2003;19:142–146. doi: 10.1007/s00383-002-0906-0. [DOI] [PubMed] [Google Scholar]
- 14.Hishiki T, Matsunaga T, Sasaki F. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol-2: report from the JPLT. Pediatr Surg Int. 2011;27:1–8. doi: 10.1007/s00383-010-2708-0. [DOI] [PubMed] [Google Scholar]
- 15.Zsiros J, Maibach R, Shafford E. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010;28:2584–2590. doi: 10.1200/JCO.2009.22.4857. [DOI] [PubMed] [Google Scholar]
- 16.Citron ML. Dose-dense chemotherapy: principles, clinical results and future perspectives. Breast Care (Basel) 2008;3:251–255. doi: 10.1159/000148914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citron ML, Berry DA, Cirrincione C. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 18.Blayney DW, LeBlanc ML, Grogan T. Dose-intense chemotherapy every 2 weeks with dose-intense cyclophosphamide, doxorubicin, vincristine, and prednisone may improve survival in intermediate- and high-grade lymphoma: a phase II study of the Southwest Oncology Group (SWOG 9349) J Clin Oncol. 2003;21:2466–2473. doi: 10.1200/JCO.2003.06.137. [DOI] [PubMed] [Google Scholar]
- 19.Blayney DW, McGuire BW, Cruickshank SE, Johnson DH. Increasing chemotherapy dose density and intensity: phase I trials in non-small cell lung cancer and non-Hodgkin's lymphoma. Oncologist. 2005;10:138–149. doi: 10.1634/theoncologist.10-2-138. [DOI] [PubMed] [Google Scholar]
- 20.Katsumata N, Yasuda M, Takahashi F. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 21.Bonilla L, Ben-Aharon I, Vidal L. Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst. 2010;102:1845–1854. doi: 10.1093/jnci/djq409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitschke R, Fagundo R, Berry DH, Falletta JM. Weekly administration of cis-dichlorodiammineplatinum(II) in childhood solid tumors: a Southwest Oncology Group study. Cancer Treat Rep. 1979;63:497–499. [PubMed] [Google Scholar]
- 23.Roebuck DJ, Aronson D, Clapuyt P. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–132. doi: 10.1007/s00247-006-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roebuck DJ, Olsen O, Pariente D. Radiological staging in children with hepatoblastoma. Pediatr Radiol. 2006;36:176–182. doi: 10.1007/s00247-005-0029-6. [DOI] [PubMed] [Google Scholar]
- 25.Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 26.Haas JE, Feusner JH, Finegold MJ. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer. 2001;92:3130–3134. doi: 10.1002/1097-0142(20011215)92:12<3130::aid-cncr10115>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 27.De Ioris M, Brugieres L, Zimmermann A. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer. 2008;44:545–550. doi: 10.1016/j.ejca.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Czauderna P, Otte JB, Aronson DC. Guidelines for surgical treatment of hepatoblastoma in the modern era—recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL) Eur J Cancer. 2005;41:1031–1036. doi: 10.1016/j.ejca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Otte JB, de Ville de GJ, Reding R. Liver transplantation for hepatoblastoma: indications and contraindications in the modern era. Pediatr Transplant. 2005;9:557–565. doi: 10.1111/j.1399-3046.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 30.Otte JB. Progress in the surgical treatment of malignant liver tumors in children. Cancer Treat Rev. 2010;36:360–371. doi: 10.1016/j.ctrv.2010.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


