Abstract
The harvested eggs of Rhynchophorus ferrugineus are ovo-cylindrical shaped, averaged 1.09 mm in length and 0.43 mm in width, with ratio of 4.42. The chorionic layer of electron dense material is seen covering the exochorion structure of the eggs. The egg main body chorion exhibits a polygonal pattern and architecture surface of the egg is supported by a system of irregular interconnecting grooves. The micropylar apparatus of the eggs of the Red Palm Weevil, R. ferrugineus is described in the present study for the first time. Two micropylar openings are found closed to the center of the posterior wide pole of the egg. Each micropylar opening presents a single small orifice and its surrounding chorion is porous and densely set with tiny projections allowing the spermatozoa to penetrate the egg. Respiratory aeropyles are distributed on the borders of reticulations in the area chorionic surface of egg capsule. The hatching region is detected on the anterior part at the opposite side of the egg. Changes in the appearance and shape of R. ferrugineus eggs as well as the incidence of embryonic development are observed.
Keywords: Rhynchophorus ferrugineus, Egg shell, Chorionic, Architecture, Micropylar apparatus, Airopyle, Hatching region
1. Introduction
Egg shell of insect forms a barrier to protect the egg and the embryo from possible disadvantageous environmental influences like desiccation, water loss, bacterial infection and physical destruction. On the other hand, the egg shell enables gas exchange and maintenance of proper humidity. These diverse functions are usually performed by distinct and structurally specified regions of the egg shell (Chapman, 1998).
Egg shells are often species-specific, moreover their morphology, when considered comparatively, may reflect important evolutionary adaptations and characters of egg shells may be satisfactorily used for phylogenetic considerations (Howard and Kistner, 1978; Dominguez and Cuezzo, 2002). Basically, the egg shells consist of two major parts: vitelline envelope and chorion (Rosciszewska, 1996a,b; Simiczyjew, 1999; Poprawa and Rost, 2004; Kubrakiewicz et al., 2005; Sierr et al., 1995; Gaino et al., 2008).
The chorion is secreted by cells in the follicular epithelium when eggs are laid; it is viscous, allowing the egg to adhere to the substrate. When dry, the chorion assumes its characteristic patterns. According to Mendonca et al. (2008) the chorion of the egg shell in other insects bears the more or less hexagonal honey-comb impression of the follicle cells (epithelium) of the females ovaries.
The chorionic characteristics structures of the insects’ eggs introduce many variations. In many insect eggs this chorion has two distinct layers: the endo- and the exochorion. They are not homologous among different species (Rogol et al., 1992).
The insect ootaxonomy, based on egg chorionic sculpturing observed by scanning electron microscopy (SEM), is well advanced for a comparative morphological study of eggs of various families of the Dipteran insects (Hinton, 1981; Fousto et al., 1993), species of Coleopteran insects from Chrysomelidae (Rowley and Peters, 1972), and from Bostrichidae (Kucerova and Stejskal, 2008).
According to Sierr et al. (1995) chorionic structures can be grouped into three basic types (micropyles, attachment structures, and chorionic sculpturing) each of which can also be classified according to some characteristics of the chorion structures, such as like ultrastructure, single or collective arrangement, and position or distribution on egg shell surface.
Insect eggs have a gap-like structure in the chorion denominated micropyles. Some insects have a single terminal micropyle. But several species present a micropylar apparatus constituted by a set of openings, where the spermatozoa penetrate the eggs (Yamauchi and Yoshitake, 1984). Depending on the insect species, the number and the position of micropylar apertures may vary from two to one hundred and this apparatus is present in the Acrididae, which shows 30–40 openings disposed in a ring at the posterior region of the egg (Sarashina et al., 2005). However, Weesner (1969) stated that, the micropylar apparatus in Termitidae was present near the posterior region of the eggs and the number of its openings were concentrated in a single row or arc and ranged from 6 to 11, varied considerably among the species and even among eggs of the same species. The micropylar openings were reaching up to 40 in Cryptotermes brevis (Roonwal and Rathore, 1975).
Also, the chorion of many insects’ eggs contains an air layer. The aeropyle is adopted to allow sufficient gas exchange, and formation of this layer which acts as an efficient distribution system of gases for the developing eggs, has been studied in details in some insects including Drosophila (King et al., 1956; Cummings and King, 1969) and the silkworm moth, Bombyx mori (Matsuzaki, 1968; Mazur et al., 1980).
The purpose of the present study is to investigate the eggs morphometric and morphological external characteristics of eggs surface. The details of chorion sculpturing, architecture, micropylar and airopyle apparatus style, the embryo hatching area as well as the gradual differentiation of the egg envelopes and chorionic changes in the eggs of the Red Palm Weevil, R. ferrugineus (Oliver).
This study may be considered a standard reference in the egg morphological descriptions of the insect, however, no structures or ultrastructures of egg capsule surface or egg shells were described before, for this species.
2. Materials and methods
The morphology and the formation of the egg shell in the Red Palm Weevil, Rhynchophorus ferrugineus (Oliver) were studied based on light and scanning electron microscope (SEM) techniques. The insects were obtained from infested wild date palm, in Riyadh and Al-Kharj during 2008 and 2009, maintained and followed up under laboratory conditions.
2.1. Source and insect rearing
Larvae, pupae and adults of the Red Palm Weevil, R. ferrugineus (Oliver) were obtained from infested wild date palm in Riyadh and Al-Kharj, and maintained on sugarcane in the laboratory. The pupae in the cocoon were incubated in the dark at 26 ± 2 °C. Upon emergence, the adults were fed with fresh sugar cane and later allowed to mate. Isolated females were supplied with pieces of split sugarcane to oviposit on it. The sugarcanes were replaced every day.
2.2. Eggs collecting and preparing for light and scanning electron microscope
The used sugarcanes from the females were carefully dissected and the eggs were removed by fine camel hair brush, gently cleaned in a drop of distilled water. The collected eggs were surface sterilized with 0.05% sodium hypochlorite and were transferred to 90 mm Petri dishes lined with moistened filter paper, sealed with parafilm and incubated at 26 ± 2 °C until the neonate emerged. The egg measurements included the following characters; length (L), width (W), and of egg shell were determined using the scanning electron microscope (SEM). Observations of the chorion surface as well as changes in egg appearance during the embryonic development were studied with a SEM (JEOL-JSM b36 OLV) in college of dentistry, King Saud University, Al-Rhyiadh at magnifications of 200× to 30,000× and a light microscope at magnifications of 400×.
2.3. Analysis in SEM and photography
The chorion of the eggs was viewed and photographed using scanning electron microscope JEOL-5600LV. Three-day-old R. ferrugineus eggs, were used for SCANNING microscopy. The eggs were washed for 2 min in 0.1 M 7.2 pH phosphate buffer at 4 °C. They were fixed for 2 h at 4 °C in a 2.5% solution of glutaraldehyde. After fixing, these were washed three times for 1 h in a 1% osmium tetroxide solution in a 7.2 pH phosphate buffer at 4 °C. The eggs were dehydrated in an acetone series (30%, 50%, 70%, 80%, 90%, 95% and 100%), and then exposed to the air to dry overnight. They were coated with gold (Au) in an SPI ion coater for 90 s at 18 mAmp and at a pressure of 0.3 mbar. The characteristic structure of the insect egg during the sequences of embryonic development had been examined and described using light microscope technique also .Hatchability was observed to detect the position of hatching area.
3. Results and discussion
The Red Palm Weevil, R. ferrugineus (Oliver) was found to lay their eggs, which are oblong with curved poles and 1.9 mm long and 0.43 mm wide with ratio of 4.42 (Fig. 1). Freshly laid eggs are soft, whitish and gloomy in color. It has ventral and dorsal surfaces with present bilateral symmetry (Fig. 1).
Figure 1.
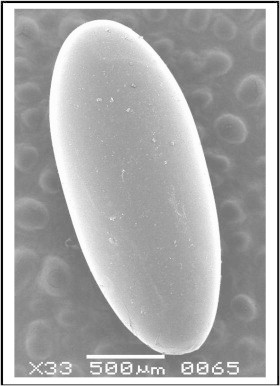
Scanning electron micrograph of general view of the eggs of R. ferrugineus (Oliver). The egg is oblong in shape and show rounded poles.
Few hours later, they begin to harden and become white, calm and transparent at the bilateral two external edges. The cytoplasm of the egg is filled with yolk and enclosed by the vitelline membrane and chorion (Fig. 2). A similar change in the chorion color and hardness was observed in numerous insect species, Thermobia domestica (Klag, 1971; Poprawa and Rost, 2004), Endoclyta signifer (Ando and Tanaka, 1980), Drosophila melanogaster (Margaritis, 1985) and R. cruentatus (Weissling and Davis, 1995). They suggested that these changes accompanied with the egg capsule proteins stabilization. Corley and Tinker (2003) described the oblong eggs of Rhynchophorus weevils, which were 2–3 mm long. However, Bong et al. (2008), described the eggs of the Red-Stripe Weevil Rhynchophorus schach Oliv., which were whitish-yellow, ovo-cylindrical in shape and measured 2.4 mm long and 0.9 mm wide.
Figure 2.

Light micrograph of 6–12 h-old R. ferrugineus (Oliver) egg (400×). The cytoplasm of the egg is filled with yolk and it is enclosed by the vitelline membrane and chorion.
In many butterflies and moths the eggs are spherical or oval and brightly colored, but in the majority of insects they are elongate, sausage-shaped, and dull in color (Chauvin and Barbier, 1979). Often, the eggs have ridges or grooves that may extend as wing like projections, as in the eggs of many flies. Insect eggs also vary greatly in color (Dorn, 1976).
The Red Palm Weevil egg shell surface is uniform, smooth or densely set with small, raised projections. The eggs showed chorionic sculpturing and the characteristic network of structures was seen on the egg capsule surface (small-mesh irregular reticule). The mainbody chorion exhibits a polygonal pattern (Fig. 3a). The respiratory aeropyles are seen to be distributed on the borders of reticulations in the area of egg capsule chorionic surface (Fig. 3b) (Hinton, 1969).
Figure 3.
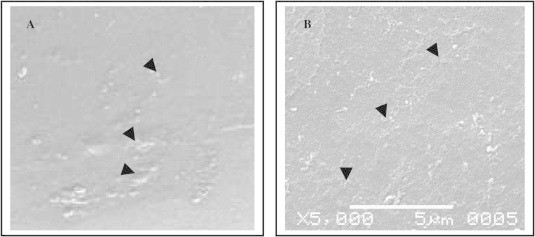
Scanning electron micrographs of the Red Palm Weevil, R. ferrugineus (Oliver) eggs showing. (A) Chorionic sculpturing and characteristic network of structures seen on the egg capsule surface (small-mesh irregular reticule). (B) Respiratory aeropyles distributed on the borders of reticulations in the area of egg capsule, chorionic surface.
The chorionic layer of electron dense material is seen covering the egg outer layer (Fig. 4). The surface of the micropyle and its surrounding chorion is porous and densely set with tiny projections. Egg shell is shown at higher magnification exhibits a polygonal pattern (Fig. 5a). The surface of the eggs is covered by a system of interconnecting grooves and the micropylar apparatus, which have been described for the first time in our study is presented in Fig. 5b. Two micropylar openings were observed on the egg located close to the center of the posterior wide pole of the egg capsule, however, the hatching region was observed on the anterior part at the opposite side of the egg. Each micropylar opening is presented in a single, small and a full circle orifice in penetration of spermatozoa. The reticulate exochorion around the micropylar orifices was absent (Fig. 5b). The hatching region was observed on the opposite side of the micropylar apparatus and appeared as a set of imprints separated by furrows and oriented along the longitudinal axis of the egg (Fig. 5c). Chorion morphology and the polygonal ornament of the outer chorionic surface shows broad apparent phylogenetic trends in various insect eggs (Weglarska, 1955; Margaritis, 1985; Margaritis and Mazzini, 1998), reveal the imprints of the follicle cells that have participated in the egg shell formation (Hinton, 1981). This region (hatching region) was not clearly visualized on the eggs of other species, Mazzini (1976).
Figure 4.

Scanning electron micrograph of the Red Palm Weevil (Oliver) egg showing chorionic layer of electron dense material covering the exochorion structure.
Figure 5.
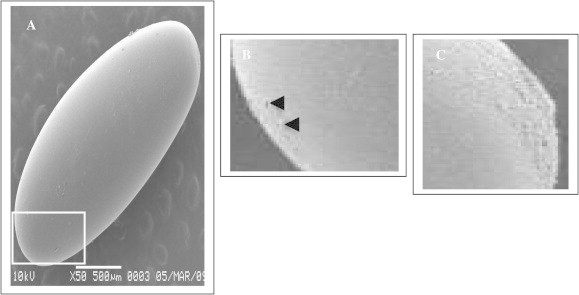
Scanning electron micrographs of the eggs of the Red Palm Weevil, R. ferrugineus (Oliver) showing. (A) The surface of the eggs covered by a system of interconnecting grooves. (B) Magnification of (A) showing the micropylar opening is located close to the center of the posterior wide pole of the egg. Each micropylar opening presents a single small orifice. (C) The hatching region is observed on the anterior part of the egg capsule at the opposite side of the micropylar apparatus and appeared as a set of imprints separated by furrows and oriented along the longitudinal axis of the egg.
Eggs of R. ferrugineus (Oliver), present lines of weakness along which the larva eclodes (Figs. 6 and 7). These lines were detected in the eggs of some Diptera (Endris et al., 1987), they suggested the function of these lines to facilitate hatching.
Figure 6.
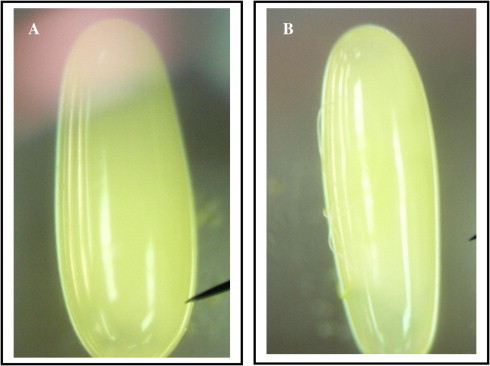
(A and B) Light micrographs of 24–36 h-old R. ferrugineus (Oliver) eggs, present lines of weakness along which the larva eclodes (400×).
Figure 7.
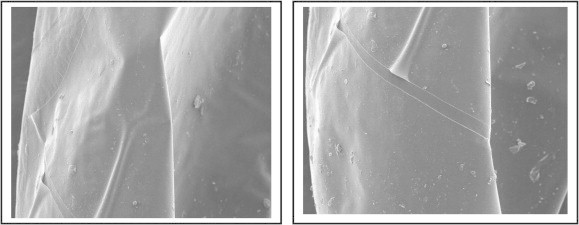
Scanning electron micrograph of the 24–36 h-old R. ferrugineus (Oliver) eggs, present lines of weakness along which the larva eclodes (400×).
According to Rowley and Peters (1972), Regier et al. (1980) and Mouzaki and Margaritis (1987, 1994), two layers of cuticle were found to be secreted by the embryos of endopterygotes orders (Coleoptera, Hymenoptera, Diptera and Lepidoptera). They are outer and inner epicuticles and a more or less reduced procuticle (Fig. 8).
Figure 8.
Scanning electron micrograph of the Red Palm Weevil, R. ferrugineus (Oliver) egg showing details of inter-ridge areas on the outer chorion. Exochorion material finely arranged covering rough basal layers. (A) Fragment of the longitudinal section of the chorion: vitelline envelope (Ve), external layer of the chorion (Ec), internal layer of the chorion (Ic).
In several insect species representing various orders, Rosciszewska (1996a,b), Simiczyjew (1999), Ma et al. (2002) and Pyka-Fos´ciak et al. (2003) concluded that the stratification of chorionic layers reflects the temporal changes in the synthetic activity of the follicular epithelium, while regional specialization of the eggshells provides evidence that during oogenesis follicular cells become subdivided (diversified) into distinct, developmentally specialized populations, each of which is responsible for the formation of particular egg shell region.
On the other hand, the characteristic structure of the insect egg at the beginning of its development had been described by many authors (Storch and Krysan, 1980; Roth, 2004; van der Zee et al., 2005; Panfilio, 2008). Changes in the appearance and shape of the R. ferrugineus eggs as well as the incidence of embryonic development were detected through the light microscope examination and photographs (Fig 9). Total observation of the egg is useful for grasping the localization of the embryo in ovo.
Figure 9.
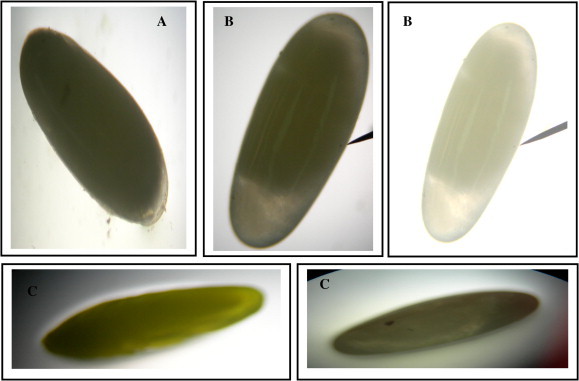
(A–C) Light micrographs of eggs of the Red Palm Weevil, R. ferrugineus (Oliver) showing the embryonic developmental sequences, extension and shortening of the germ band (400×). (A) 12–18 h-old egg showing the maximum extension of the germ band. (B) 18–24 h-old egg showing the beginning of shortening of the germ band. The contraction of the yolk plasmodium at this time, leaving a spacious haemocoele between itself and the body wall. (C) 36–42 h-old egg showing the short germ band when being nearing completion. The lateral body walls begin to grow up and eventually fuse in the mid-dorsal line.
Germ band formation, gastrulation and the extension of the germ band and formation of extra-embryonic membranes were detected at the eggs of 12–18 h-old (Fig. 9A). After remaining for a short period in its extended state, the germ band reverses its movement and shortens (Fig. 9b). The beginning of shortening of the germ band, the contraction of the yolk plasmodium at this time, leaving a spacious haemocoele between itself and the body wall, characterize the eggs of 18–24 h–old. As shortening of the germ band is nearing completion in the eggs of 36–42 h-old, the lateral body walls begin to grow up and eventually fuse in the mid-dorsal line (Fig. 9). Organs formation and segmental divisions begin to appear in the embryos epidermis of the eggs of 48–72 h-old (Fig. 10). The embryological studies of insect orders are the most important for understanding the ground plan of insecta as well as for clarifying insect evolution (Weissling and Davis, 1995; Stanley and Grundmann, 1970; Handel et al., 2000; Kobayashi et al., 2002; Tojo, 2003).
Figure 10.
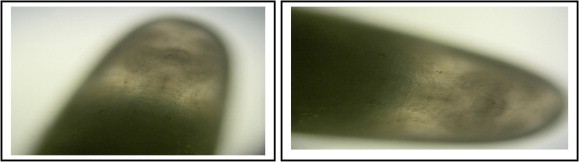
Light micrographs of the 48–72 h-old R. ferrugineus (Oliver) eggs showing the development of the normal embryos (400×). Organs formation begin and segmental divisions begin to appear in the epidermis.
Generating data for further comparison between the examined species, R. ferrugineus (Oliver) and its relation with different other species and genera of other tropical Asian, African and European Rhynchophorus species which feed on palms, would be of economic value and need assessment of the prospects for improving current management techniques through the development and integration of control programmes.
References
- Ando H., Tanaka M. Early embryonic development of the primitive moths, Endoclyta signifer Walker and E. excrescens Butler (Lepidoptera: Hepialidae) Int. J. Insect Morphol. Embryol. 1980;9:67–77. [Google Scholar]
- Bong J. Choon-Fah, Chin-Chin E.R., Yiu Pang-Hung, Rajan A. Growth performance of the Red-Stripe Weevil Rhynchophorus schach Oliv. (Coleoptera: Curculionidae) on meridic diets. Am. J. Agr. Biol. Sci. 2008;3(1):403–409. [Google Scholar]
- Chapman R.F. Cambridge University Press; London: 1998. The Insects: Structure and Function. [Google Scholar]
- Chauvin G., Barbier R. Morphogenese de l’enveloppe vitelline, ultrastructure du chorion et de la cuticule serosale chez Korscheltellus lupulinus L. (Lepidoptera: Hepialidae) Int. J. Insect Morphol. Embryol. 1979;8:375–386. [Google Scholar]
- Corley R.H.V., Tinker P.B. 4th ed. Blackwell Science Ltd.; 2003. World Agriculture Series: The Oil Palm. pp. 422–440. [Google Scholar]
- Cummings M.R., King R.C. The cytology of the vitellogenic stages during oogenesis in Drosophila melanogaster. I. General staging characteristics. J. Morphol. 1969;128:427–442. [Google Scholar]
- Dominguez E., Cuezzo M.G. Ephemeroptera egg chorion characters: a test of their importance in assessing phylogenetic relationships. J. Morphol. 2002;253:148–165. doi: 10.1002/jmor.1117. [DOI] [PubMed] [Google Scholar]
- Dorn A. Ultrastructure of embryonic envelopes and integument of Oncopeltus fasciatus Dallas (Insecta Heteroptera) I Chorion, amnion, serosa, integument. Zoomorphologie. 1976;85:111–132. [Google Scholar]
- Endris R.G., Young D.G., Perkins P.V. Ultrastructural comparison of eggs surface morphology of five Lutzomyia species (Diptera: Psychodidae) J. Med. Entomol. 1987;24:412–415. [Google Scholar]
- Fousto A.M., Mazzini M., Marlo M., Mutinga M.J. Scanning electron microscopical study of the eggshell of three species of Sergentomyia (Diptera: Psychodidae) Insect. Sci. Appl. 1993;14:483–488. [Google Scholar]
- Gaino E., Piersanti M., Rebora M. Egg envelope synthesis and chorion modification after ovipostion in the Dragonfly depressa (Odonata, Libellulidae) Tissue Cell. 2008;44:317–324. doi: 10.1016/j.tice.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Handel K., Grünfelder C.G., Roth S., Sander K. Tribolium embryogenesis: a SEM study of cell shapes and movements from blastoderm to serosal closure. Dev. Genes Evol. 2000;210:167–179. doi: 10.1007/s004270050301. [DOI] [PubMed] [Google Scholar]
- Hinton H.E. Pergamon Press; Oxford: 1981. Vol. I–III Biology of Insect Egg. [Google Scholar]
- Hinton H.E. Respiratory systems of insect egg shells. Annu. Rev. Entomol. 1969;14:343–368. doi: 10.1146/annurev.en.14.010169.002015. [DOI] [PubMed] [Google Scholar]
- Howard R.W., Kistner D.H. The eggs of Trichopsenius depressus and T. frosti (Coleoptera: Staphylinidae, Trichopseniinae) with a comparison to those of their host termites Reticulitermes virginicus and R. flavipes (Isoptera: Rhinotermitidae, Heterotermitinae) Sociobiology. 1978;3:99–106. [Google Scholar]
- King R.C., Rubinson A.C., Smith R.F. Oogenesis in adult Drosophila melanogaster. Growth. 1956;20:121–157. [PubMed] [Google Scholar]
- Klag J. The biology of Thermobia domestica (Pack) (Thysanura) in the laboratory culture. Zeszyty Naukowe UJ. 1971;17:7–28. [Google Scholar]
- Kobayashi Y., Suzuki H., Ohba N. Embryogenesis of the glowworm Rhagophthalmus ohbai wittmer (Insecta: Coleoptera, Rhagophthalmidae), with emphasis on the germ rudiment formation. J. Morphol. 2002;253:1–9. doi: 10.1002/jmor.1109. [DOI] [PubMed] [Google Scholar]
- Kubrakiewicz J., Jedrzejowska I., Szymanska B., Bilinski M. Szczepan. Micropyle in neuropterid insects. Structure and late stages of morphogenesis. Arthropod Struct. Dev. 2005;34:179–188. [Google Scholar]
- Kucerova Z., Stejskal V. Differences in egg morphology of the stored- grain pests Rhyzopertha dominica and Prostephanus truncates (Coleoptera: Bostrichidae) J. Stored Prod. Res. 2008;44:103–105. [Google Scholar]
- Ma P.W.K., Baird S., Ramaswamy S.B. Morphology and formation of the eggshell in the tarnished plant bug, Lygus lineloaris (Palisot de Beauvois) (Hemiptera: Miridae) Arthropod Struct. Dev. 2002;31:131–146. doi: 10.1016/s1467-8039(02)00019-1. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M. Electron microscopic observations on the chorion formation of the silkworm, Bombyx mori. J. Sericult. Sci. Jpn. 1968;37:483–490. [Google Scholar]
- Margaritis L.H. Structure and physiology of the egg shell. In: Kerkut G.A., Gilbert L.I., editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Plenum Press; New York: 1985. [Google Scholar]
- Margaritis L.H., Mazzini M. Structure of the egg. In: Harrison F.E., Locke M., editors. vol. 11C. Wiley-Liss; New York: 1998. pp. 995–1037. (Microscopic Anatomy of Invertebrates Insecta). [Google Scholar]
- Mazur G.D., Regier J.C., Kafatos F.C. The silk worm chorion: morphogenesis of surface structures and its relation to synthesis of specific proteins. Dev. Biol. 1980;76:305–321. doi: 10.1016/0012-1606(80)90381-4. [DOI] [PubMed] [Google Scholar]
- Mazzini M. Fine structure of the insect micropyle—III. Ultrastructure of the egg of Chrysopa carnea Steph. (Neuroptera: Chrysopidae) Int. J. Insect Morphol. Embryol. 1976;5:273–278. [Google Scholar]
- Mendonca P.M., Santos-Mallet J.R., Mello R.P., Gomes L., Carvalho Queiroz M.M. Identification of fly eggs using scanning electron microscopy for forensic investigation. Micron. 2008;39:802–807. doi: 10.1016/j.micron.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Mouzaki, D.G., Margaritis, L.H., 1987. Comparative structural study of the egg-shell (chorion) in Dacus oleae, Rhagoletis cerasi, Ceratitis capitata, and Eurytoma amygdali. Fruit flies procs. In: II International Symposium, Crete, September 1986, pp. 79–87.
- Mouzaki D.G., Margaritis L.H. The egg shell of the almond wasp Eurytoma amygdali (Hymenoptera, Eurytomidae)—1. Morphogenesis and fine structure of the eggshell layers. Tissue Cell. 1994;26:559–568. doi: 10.1016/0040-8166(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Panfilio Kristen A. Extraembryonic development in insects and the acrobatics of blastokinesis. Dev. Biol. 2008;313:471–491. doi: 10.1016/j.ydbio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Poprawa I., Rost M.M. Structure and ultrastructure of the egg capsule of Thermobia domestica (PACKARD) (Insecta: Zygentoma) Folia Biol. 2004;52(3–4):185–190. doi: 10.3409/1734916044527511. [DOI] [PubMed] [Google Scholar]
- Pyka-Fos´ciak G., Jankowska W., Szklarzewicz T. Ultrastructural studies on the formation of egg envelopes in Fulgoromorphans (Insecta, Hemiptera, Fulgoromorpha: Dictyopharidae) Folia Biologica (Krako´w) 2003;51:171–179. [PubMed] [Google Scholar]
- Regier J.C., Mazur G.D., Kafatos F.C. The silkmoth chorion: morphological and biochemical characterization of four surface regions. Dev. Biol. 1980;76:286–304. doi: 10.1016/0012-1606(80)90380-2. [DOI] [PubMed] [Google Scholar]
- Rogol R., Kokwaro E.D., Mutinga M.J., Khamala C.P.M. Diferentiation of vector species of phlebotominae (Diptera: Psychodidae) in Kenya by chorionic sculpturing of their eggs. J. Med. Entomol. 1992;29:1042–1044. doi: 10.1093/jmedent/29.6.1042. [DOI] [PubMed] [Google Scholar]
- Roonwal M.L., Rathore N.S. Egg-wall sculpturing and micropylar apparatus in some termites and their evolution in the Isoptera. J. Zool. Soc. India. 1975;27:1–17. [Google Scholar]
- Rosciszewska E. Egg capsule structure of the stonefly Protonemura intricate (RIS, 1902) (Plecoptera: Nemuridae) Acta Biol. Cracov., Ser. Zool. 1996;38:41–49. [Google Scholar]
- Rosciszewska E. Diversification of the follicular cells in the panoistic ovary of the stone fly Perlodes intricata (Pictet, 1841) (Plecoptera: Perlodidae) during choriogenesis. Zool. Poloniae. 1996;41:89–102. [Google Scholar]
- Roth S. Gastrulation in other insects. In: Stern C.D., editor. Gastrulation: From Cells to Embryo. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2004. pp. 105–121. [Google Scholar]
- Rowley V.A., Peters D.C. Scanning electron microscopy of the eggshell of four species of Diabrotica (Coleoplera: Chrysomclidae) Ann. Entomol. Soc. Am. 1972;65:1188–1191. [Google Scholar]
- Sarashina I., Mito T., Saito M., Uneme H., Miyawaki K., Shinmyo Y., Ohuchi H., Noji S. Location of micropyles and early embryonic development of the two-spotted cricket Gryllus bimaculatus (Insecta, Orthoptera) Dev. Growth Differ. 2005;47:99–108. doi: 10.1111/j.1440-169x.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- Sierr A.D., Veiez I.D., Uribe S.I. Electronic microscopy of eggs as a taxonomic parameter. Biol. Dir. Mal. San Amb. 1995;30:327–336. [Google Scholar]
- Simiczyjew B. The ovary structure and oogenesis in Hydrometra stagnorum (Heteroptera: Hydrometridae) Acta Soc. Zool. Bohem. 1999;63:187–197. [Google Scholar]
- Stanley M.S.M., Grundmann A.W. The embryonic development of Tribolim confusum. Ann. Entomol. Soc. Am. 1970;63:1248–1256. [Google Scholar]
- Storch R.H., Krysan J.L. Embryology of Diabrotica undecimpuncata howardi (Coleoptera: Chrysomelidae) from germ band formation to hatching. Ann. Entomol. Soc. Am. 1980;73:362–366. [Google Scholar]
- Tojo K. Techniques in embryological studies of mayflies (Insecta: Ephemeroptera) Life History Reprod. 2003:205–209. [Google Scholar]
- Yamauchi H., Yoshitake N. Formation and ultrastructure of the micropylar apparatus in Bombyx mori ovarian follicles. J. Morphol. 1984;179:47–58. doi: 10.1002/jmor.1051790106. [DOI] [PubMed] [Google Scholar]
- Van der Zee M., Berns N., Roth S. Distinct function of the Tribolium zerknüllt genes in serosa specification and dorsal closure. Curr. Biol. 2005;15:624–636. doi: 10.1016/j.cub.2005.02.057. [DOI] [PubMed] [Google Scholar]
- Weesner F.M. The reproductive system. In: Krishna K., Weesner F.M., editors. Biology of Termites. Academic Press; New York: 1969. pp. 125–157. [Google Scholar]
- Weglarska B. The formation of the blastoderm and embryonic membranes in Polydrosus impar gozis (Coleoptera, Curculionidae) Bull. Entomol. Pol., Wrocaw. 1955;25(13):193–211. [Google Scholar]
- Weissling J., Davis G.M. Oligidic diets for culture of Rhynchophorus cruentatus (Coleoptera: Curculionidae) J. Rhynchophorus Cruentatus Diets. 1995:225. [Google Scholar]


