Abstract
The aim of this study was to identify the main weed communities in Al-Jouf province in northern Saudi Arabia. Moreover, the composition and diversity of these communities were studied in relation to soil variables and crop type. Some 54 stands representing olive orchards, date palm orchards, wheat crop and watermelon crop were studied, using ten quadrats (1 × 1 m) per stand. A total of 71 species belonging to 22 families and 61 genera were observed. The classification of vegetation using the Two Way Indicator Species Analysis (TWINSPAN) resulted in the recognition of four vegetation groups representing wheat crop, orchards in winter season, orchards in summer season and watermelon crop. These results suggested the importance of both crop and season for the formation of weed community. Detrended Correspondence Analysis (DCA) showed that these groups are clearly distinguished by the first two DCA axes. The species richness was higher in both olive and date palm orchards than in wheat and watermelon crops. This pattern of species richness could be related to farm management practices and habitat micro-heterogeneity. Soil electrical conductivity, organic carbon and soil texture showed significant correlations with species richness and the cover values of some dominant species, suggesting the significant role of soil characteristics in weed community structure and diversity.
Keywords: Diversity, Saudi Arabia, Soil characteristics, Weeds
1. Introduction
Weeds are plants which grow where they are not wanted. They differ from other plants in being more aggressive, having peculiar characteristics that make them more competitive. Weeds decrease the crop yield by competing for water, nutrients, space and light (Qasem and Hill, 1995; Wang et al., 2007). Some weeds are also allelopathic and adversely affect crops (Shah and Khan, 2006; Jabeen and Ahmed, 2009). Losses in crop yield and production caused by weeds are well documented in many studies (e.g., Aldrich, 1984; Akobundu, 1987; Swanton et al., 1993; Khedr and Hegazy, 1998; Fayed et al., 1999). Therefore, there is an urgent need for effective weed management programs. For such programs to be visible, accurate information on the weed flora and the distribution, abundance and phenology of weed species and weed communities are pre-requisite (Frick and Thomas, 1992; Ghersa and Holt, 1995). Such kind of data may also be valuable for understanding weed communities and for creating a higher biodiversity in arable land (Andreasen and Skovgaard, 2009).
Weed communities are affected by many factors as farm management practices (Derksen et al., 1994; Andersson and Milberg, 1998; Thomas and Frick, 1993), crop type (Andersson and Milberg, 1998; Andreasen and Skovgaard, 2009), season (El-Demerdash et al., 1997) and soil characteristics (Fried et al., 2008; Pinke et al., 2010). The many factors involved in the formation of the weed community make it difficult to evaluate the relative importance of each individual factor (Pysek and Leps, 1991).
The weed flora of Saudi Arabia was presented by Chaudhary and Akram (1987). The weed flora of date palm orchards in Al-Hassa Oasis in eastern Saudi Arabia was documented by El-Halawany and Shaltout (1992), while the weed communities of date palm orchards in the same area were identified and described by Shaltout and El-Halawany (1992). A check list of weeds in Al-kharj area in the central region of Saudi Arabia was made by Al-Yemeny (1999). Recently, Sher and Al-Yemeni (2011) prepared an ecotaxonomical inventory of weed flora in Al-kharj area of Saudi Arabia. Moreover, Gazer (2011) studied the floristic composition and diversity of the weed vegetation as well as the relationships between weed assemblages and soil characters in date palm orchards of Al-Qassim area in central Saudi Arabia.
Studies on weed flora and weed communities in the Kingdom of Saudi Arabia are still fragmentary and incomplete (Sher and Al-Yemeni, 2011). The lack of such information is more obvious for Al-Jouf province in northern Saudi Arabia. To the present author’s knowledge, the weed communities of Al-Jouf province have not been previously studied. The present work aims to recognize the major plant communities in Al-Jouf province (northern Saudi Arabia) and assess their structure, diversity and distribution in relation to crop type and soil characters.
2. Materials and methods
2.1. Study area
Al-Jouf province is located in the northern part of Saudi Arabia, where it is bounded from the north and east by the Northern Borders province and from the south by Hail and Tabuk provinces and delimited from the north and west by Jordan (Fig. 1). It is located between latitudes 29° and 32°N and longitudes 37° and 42°E. Its area is about 107,794 km2, representing 4.9% of the total area of Saudi Arabia. Al-Jouf province consists of the town of Sakaka and two governorates (Dawmat Al-Jandal and Al-Qurayat).
Figure 1.
Map of Saudi Arabia showing the study area and the sampling locations (1, Sakaka; 2, Dawmat Al-Jandal; 3, Abo Ajram; 4, Tabarjal; 5, Al-Qurayat).
Al-Jouf province is one of the important agricultural regions of Saudi Arabia. The cultivated area approximates 460,000 ha. The region is characterized by the cultivation of orchards, particularly olive and date palm in addition to other field crops as wheat, barley, alfalfa, sorghum, and watermelon.
The study area is characterized by dry climate with hot summer and cool winter. According to the records of Al-Jouf Airport meteorological station for the period 2000–2010, the mean monthly air temperature ranges between 9.8 °C during January and 33.8 °C during August. The mean monthly relative humidity varies between 16% during June and 53% during January. The average annual wind speed is 13 km/h. The rainfall in the region is erratic and irregular and the mean annual rainfall is 55 mm, with the rainy season stretching from October to May.
2.2. Vegetation sampling
The weed vegetation were sampled in 54 stands representing two kinds of orchards (olive and date palm) as well as a winter (wheat) and a summer crop (watermelon). The stands were distributed in five locations (Sakaka, Dawmat Al-Jandal, Abo Ajram, Tabarjal and Al-Qurayat, Fig. 1) in Al-Jouf province. Each type of orchards was represented by 15 stands, while 24 stands were sampled in wheat and watermelon crops (12 stands per crop). The area of the stand was 20 × 20 m. The orchards were sampled during both winter (March 2011) and summer (June 2011) seasons, while the wheat crop was sampled during March 2011 and watermelon crop during June 2011. In each stand, the present species were recorded and their cover was evaluated visually as percentage of the ground surface in 10 randomly sampled quadrats (1 × 1 m each). Species identification and nomenclature followed Chaudhary and Akram (1987), Chaudhary (1999, 2000, 2001) and Al-Hassan (2006). Species were categorized in terms of their life form according to Raunkiaer (1934) into therophytes, hemicryptophytes, geophytes, chamaephytes and phanerophytes.
2.3. Soil analysis
Three soil samples were taken per stand, from a depth of 0–50 cm. The samples were pooled together, forming one composite sample for each stand. The samples were air dried and sieved through a 2 mm sieve before analysis. For soil texture analysis, the soil fractions were separated by sieves. Hundred grams of each soil sample was passed through a series of sieves to separate gravels (>2 mm), coarse and medium sand (2–0.25 mm), fine and very fine sand (0.25–0.05 mm), and silt and clay (<0.05 mm). The percentage of CaCO3 was estimated using 1 N HCl (Jackson, 1967). Oxidizable organic carbon was determined by modified Walkley–Black method (Jackson, 1958). Soil–water extracts of 1:5 were prepared and used for determination of electrical conductivity (EC) and soil reaction (pH) using a conductivity and pH meter (Jenway 4330).
2.4. Data analysis
TWINSPAN, Two Way Indicator Species Analysis (Hill, 1979a), was applied for the classification of stands into groups based on the cover values of species. The Detrended Correspondence Analysis (DCA) (Hill, 1979b) was used to ordinate stands in two-dimensional space using the cover values of species. Data of the soil variables of the vegetation groups identified by TWINSPAN were compared by one-way ANOVA followed by Tukey’s post hoc test. The same analysis was used to compare between the diversity indices of the vegetation groups. Linear correlations of soil variables with diversity indices, DCA axes and cover values of the dominant species were made to relate the diversity, distribution and structure of weed communities to edaphic factors. The one-way ANOVA and correlation analyses were conducted using SPSS 12 for Windows.
Species richness and Shannon index were applied for measurement of diversity in each stand (Pielou, 1975):
where pi is the relative cover of species i.
3. Results
3.1. Floristic composition
In total, 71 plant species belonging to 22 families and 61 genera were observed. The largest family was Poaceae (21 species), followed by Asteraceae (9 species), Fabaceae (5 species), Chenopodiaceae and Solanaceae (4 species for each) (Table 1). The life form spectrum exhibited a wide range of variation (Table 1). Therophytes were the predominant life form and constituted 66.2% of the total flora, followed by chamaephytes (12.7%), hemicryptophytes (11.3%), geophytes (5.6%), and phanerophytes (4.2%).
Table 1.
A list of the species recorded in the study area with their families, life form and mean cover values in the four vegetation groups resulted from TWINSPAN classification. Th, therophytes; H, hemicryptophytes; G, geophytes; Ch, chamaephytes; Ph, phanerophytes; +, present; −, absent.
| Species | Family | Life form | Vegetation group |
|||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| Alhagi graecorum Boiss. | Fabaceae | H | – | 0.2 | 0.4 | – |
| Amaranthus graecizans L. | Amaranthaceae | Th | – | – | 0.2 | 0.5 |
| Amaranthus lividus L. | Amaranthaceae | Th | – | – | 0.2 | 0.3 |
| Anagallis arvensis L. | Primulaceae | Th | 0.5 | 0.3 | – | – |
| Avena fatua L. | Poaceae | Th | – | 0.2 | – | – |
| Brachiaria reptans (L.) C.A. Gardner and C.E. Hubb. | Poaceae | Th | – | – | 0.1 | 1.8 |
| Brassica tournefortii Gouan | Brassicaceae | Th | 0.1 | – | – | – |
| Cenchrus biflorus Roxb. | Poaceae | Th | – | – | 0.7 | 0.4 |
| Chenopodium album L. | Chenopodiaceae | Th | – | 0.3 | – | – |
| Chenopodium murale L. | Chenopodiaceae | Th | 12.1 | 0.2 | – | – |
| Cichorium endivia L. | Asteraceae | Th | 0.1 | 0.1 | – | – |
| Citrullus colocynthis (L.) Schrad. | Cucurbitaceae | H | – | 0.1 | – | – |
| Convolvulus arvensis L. | Convolvulaceae | G | 1.0 | 5.7 | 4.7 | 1.4 |
| Conyza bonariensis (L.) Cronquist. | Asteraceae | Th | – | – | 6.4 | – |
| Corchorus olitorius L. | Tiliaceae | Th | – | – | 0.2 | 0.1 |
| Cynodon dactylon (L.) Pers. | Poaceae | G | 0.1 | 4.7 | 15.7 | 1.0 |
| Cyperus rotundus L. | Cyperaceae | G | – | 0.3 | 0.7 | 0.1 |
| Dactyloctenium aegyptium (L.) Willd. | Poaceae | Th | – | – | 2.7 | 1.6 |
| Datura stramonium L. | Solanaceae | Th | – | – | 0.1 | – |
| Desmostachya bipinnata (L.) Stapf | Poaceae | H | – | – | 0.1 | – |
| Dichanthium annulatum (Forssk.) Stapf | Poaceae | H | – | – | 0.1 | – |
| Digitaria sanguinalis (L.) Scop. | Poaceae | Th | – | – | 1.6 | 1.5 |
| Echinochloa colona (L.) Link. | Poaceae | Th | – | – | 0.4 | 10.9 |
| Emex spinosa (L.) Campd. | Polygonaceae | Th | 4.2 | 0.2 | – | – |
| Eragrostis cilianensis (All.) F. T. Hubb. | Poaceae | Th | – | – | 6.3 | 3.8 |
| Erodium malacoides (L.) L’Her. | Geraniaceae | Th | 0.1 | – | – | – |
| Euphorbia helioscopia L. | Euphorbiaceae | Th | – | 0.2 | – | – |
| Euphorbia peplus L. | Euphorbiaceae | Th | – | 1.8 | – | – |
| Halocnemum strobilaceum (Pall.) M. Bieb | Chenopodiaceae | Ch | – | – | 0.1 | – |
| Haloxylon salicornicum (Moq.) Bunge ex Boiss. | Chenopodiaceae | Ch | – | – | 0.1 | – |
| Hibiscus trionum L. | Malvaceae | Th | – | – | 0.1 | 0.1 |
| Hyoscyamus muticus L. | Solanaceae | Ch | – | 0.1 | – | – |
| Imperata cylindrica (L.) Raeusch | Poaceae | H | – | 24.4 | 1.5 | – |
| Juncus rigidus Desf. | Juncaceae | H | – | 0.1 | – | – |
| Lactuca serriola L. | Asteraceae | Th | 1.8 | 0.1 | – | – |
| Launaea capitata (Spreng.) Dandy | Asteraceae | Th | 0.1 | – | – | – |
| Launaea nudicaulis (L.) Hook. F. | Asteraceae | H | 1.1 | 0.1 | – | – |
| Lolium temulentum L. | Poaceae | Th | 0.1 | – | – | – |
| Malva parviflora L. | Malvaceae | Th | 0.5 | 2.6 | – | – |
| Melilotus indicus (L.) All. | Fabaceae | Th | 6.8 | 1.3 | – | – |
| Panicum turgidum Forssk. | Poaceae | Ch | – | 0.1 | – | – |
| Paspalum distichum L. | Poaceae | H | – | – | 0.1 | – |
| Phalaris minor Retz. | Poaceae | Th | 0.1 | 0.2 | – | – |
| Phragmites australis (Cav.) Trin.ex Steud. | Poaceae | G | – | 1.5 | 3.6 | – |
| Plantago amplexicaulis Cav. | Plantaginaceae | Th | 0.1 | – | – | – |
| Plantago major L. | Plantaginaceae | Th | – | 0.1 | – | – |
| Plantago lagopus L. | Plantaginaceae | Th | – | 6.6 | – | – |
| Pluchea dioscoridis (L.) DC. | Asteraceae | Ph | – | – | 0.1 | – |
| Poa annua L. | Poaceae | Th | – | 0.2 | – | – |
| Polypogon monspeliensis (L.) Desf. | Poaceae | Th | – | 0.3 | – | – |
| Portulaca oleracea L. | Portulacaceae | Th | – | – | 0.4 | 5.8 |
| Pulicaria undulata (L.) C.A. Mey. | Asteraceae | Ch | – | 0.1 | – | – |
| Reichardia tingitana (L.) Roth. | Asteraceae | Th | 0.7 | – | – | – |
| Ricinus communis L. | Euphorbiaceae | Ph | – | 0.1 | – | – |
| Rumex dentatus L. | Polygonaceae | Th | 0.3 | 0.3 | – | – |
| Rumex vesicarius L. | Polygonaceae | Th | 0.1 | – | – | – |
| Schismus barbatus (L.) Thell. | Poaceae | Th | 0.2 | – | – | – |
| Setaria pumila (Poir.) Roem. and Schult. | Poaceae | Th | – | – | 2.2 | 0.1 |
| Setaria verticillata (L.) P. Beauv. | Poaceae | Th | – | – | 0.1 | 0.1 |
| Sisymbrium irio L. | Brassicaceae | Th | 0.3 | 0.2 | – | – |
| Solanum nigrum L. | Solanaceae | Th | – | 0.1 | 0.1 | – |
| Sonchus oleraceus L. | Asteraceae | Th | 1.5 | 1.2 | 0.1 | – |
| Spergularia marina (L.) Griseb. | Caryophyllaceae | Th | – | 0.1 | – | – |
| Tamarix nilotica (Ehrenb.) Bunge | Tamaricaceae | Ph | – | – | 0.5 | – |
| Trigonella hamosa L. | Fabaceae | Th | – | 0.2 | – | – |
| Trigonella stellata Forssk. | Fabaceae | Th | 0.1 | – | – | – |
| Vicia sativa L. | Fabaceae | Th | – | 0.1 | – | – |
| Withania somnifera (L.) Dunal. | Solanaceae | Ch | – | 0.1 | – | – |
| Zilla spinosa (L.) Prantl | Brassicaceae | Ch | – | 0.1 | – | – |
| Zygophyllum album L. F. | Zygophyllaceae | Ch | – | 0.1 | – | – |
| Zygophyllum coccineum L. | Zygophyllaceae | Ch | – | 0.1 | 0.1 | – |
3.2. Vegetation classification
The application of TWINSPAN classification technique on the cover values of the recorded species in the 54 stands leads to the separation of four vegetation groups (A–D, Fig. 2). Each vegetation group comprises a set of stands which are similar in their vegetation.
Figure 2.
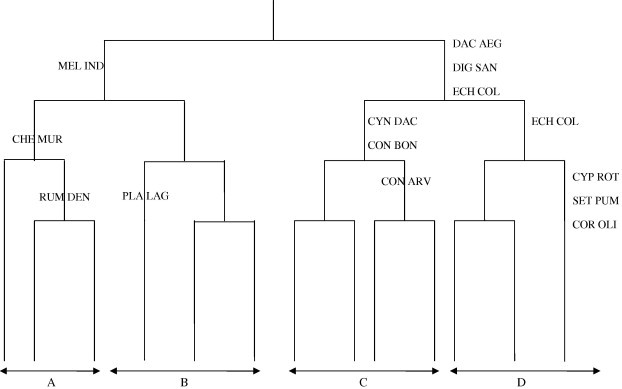
TWINSPAN dendrogram of the 54 stands based on the cover values of species. Indicator species names are abbreviated to the first three letters of both species and genus names. For complete names, see Table 1.
Group A represents the stands of wheat. The dominant species of this vegetation group are Chenopodium murale and Melilotus indicus. The common associated species are Emex spinosa, Lactuca serriola and Sonchus oleraceus. C. murale is the indicator species of this group.
Group B includes the stands of olive and date palm orchards in winter season. Imperata cylindrica, Plantago lagopus and Convolvulus arvensis are the dominant species in this group, while the common species are Cynodon dactylon, Malva parviflora, Euphorbia peplus and Phragmites australis.
Stands of Group C represent the olive and date palm orchards in summer season and are dominated by C. dactylon, Conyza bonariensis and Eragrostis cilianensis. The common species are C. arvensis, Dactyloctenium aegyptium, Digitaria sanguinalis, I. cylindrica, P. australis and Setaria pumila, while the indicator species of this group are C. dactylon and C. bonariensis.
Group D includes the stands sampled in watermelon crop and are dominated by Echinochloa colona and Portulaca oleracea. E. colona is the indicator species of the group, whereas Brachiaria reptans, D. aegyptium, D. sanguinalis and E. cilianensis are the important common species.
3.3. DCA ordination
Ordination of the 54 stands given by DCA (Fig. 3) indicates that the vegetation groups produced by TWINSPAN classification are markedly distinguishable and show a clear pattern of segregation on the ordination planes. The vegetation groups are clearly distinguished and distributed mainly along axis 1 from left to right in the order: groups D, C, B and A. The eigenvalues for the first two DCA axes are 0.851 and 0.345, respectively. The high eigenvalue for DCA axis 1 indicates that it explains the major variation in species composition of the vegetation groups.
Figure 3.
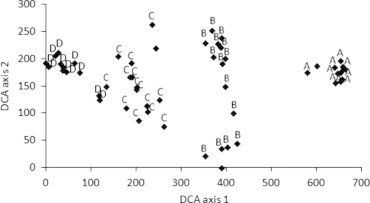
DCA ordination of the 54 stands based on the cover values of species with the vegetation groups resulted from TWINSPAN superimposed.
3.4. Species diversity
Species richness varies significantly among vegetation groups (P < 0.05). Vegetation group B has the highest species richness (13.5 species/stand), followed by vegetation group C (11.7 species/stand), vegetation group A (9.8 species/stand) and vegetation group D (8.1 species/stand). On the other hand, the vegetation groups do not show significant differences in the values of Shannon index (Table 2).
Table 2.
Means ± SD of diversity and edaphic variables of the different vegetation groups.
| Variable | Vegetation group |
|||
|---|---|---|---|---|
| A | B | C | D | |
| Species richness | 9.8a ± 1.5 | 13.5b ± 1.4 | 11.7c ± 1.0 | 8.1d ± 1.3 |
| Shannon index | 1.61a ± 0.12 | 1.41a ± 0.24 | 1.63a ± 0.22 | 1.50a ± 0.28 |
| CaCO3 (%) | 3.0a ± 1.0 | 3.3a ± 0.9 | 3.3a ± 0.9 | 3.2a ± 0.9 |
| 0.07a ± 0.01 | 0.07a ± 0.01 | 0.07a ± 0.02 | 0.06a ± 0.01 | |
| pH | 7.7a ± 0.2 | 7.6a ± 0.1 | 7.7a ± 0.2 | 7.7a ± 0.2 |
| Electrical conductivity (mS/cm) | 0.68a ± 0.06 | 0.87b ± 0.14 | 0.90b ± 0.19 | 0.74a ± 0.07 |
| Organic carbon (%) | 0.52a ± 0.14 | 0.95b ± 0.11 | 0.92b ± 0.12 | 0.60a ± 0.13 |
| Gravels (%) | 10.0a ± 2.3 | 9.2a ± 2.7 | 9.4a ± 2.6 | 10.2a ± 2.1 |
| Coarse and medium sand (%) | 35.8a ± 3.9 | 32.5a ± 3.0 | 33.0a ± 3.0 | 35.7a ± 4.1 |
| Fine and very fine sand (%) | 37.8a ± 4.0 | 39.4a ± 3.4 | 38.0a ± 3.9 | 37.3a ± 4.1 |
| Silt and clay (%) | 16.4a ± 1.6 | 18.9b ± 1.4 | 19.6b ± 1.3 | 16.9a ± 1.7 |
Values in a row sharing the same letter are not significantly different at the 0.05 level of probability.
3.5. Vegetation–soil relationships
Edaphic characteristics of the four vegetation groups are summarized in Table 2. Of the measured soil parameters, electrical conductivity, organic carbon, silt and clay show significant differences (P < 0.05) among vegetation groups. Electrical conductivity is significantly higher in groups B and C (0.87 and 0.90 mS/cm, respectively) than in groups A and D (0.68 and 0.74 mS/cm, respectively). Vegetation groups B and C show values of organic carbon (0.95 and 0.92%, respectively) which are significantly higher than in groups A and D (0.52 and 0.60%, respectively). Likewise, the percentages of silt and clay are significantly higher in groups B and C (18.9 and 19.6%, respectively) compared to groups A and D (16.4 and 16.9%, respectively).
Species richness shows significant correlations with electrical conductivity (r = 0.367, P < 0.01), organic carbon (r = 0.615, P < 0.01), coarse and medium sand (r = −0.395, P < 0.01) and silt and clay (r = 0.526, P < 0.01). Shannon index and DCA axis 1 do not show any significant correlations with the measured soil parameters. DCA axis 2 is significantly correlated with only soil organic carbon (r = −0.297, P < 0.05) (Table 3).
Table 3.
Linear correlation coefficients (r) of edaphic factors with diversity indices and the first two DCA axes.
| Edaphic parameter | Species richness | Shannon index | DCA axis 1 | DCA axis 2 |
|---|---|---|---|---|
| CaCO3 (%) | 0.057 | −0.240 | −0.092 | 0.175 |
| 0.174 | −0.017 | 0.073 | −0.125 | |
| pH | −0.064 | −0.012 | 0.006 | −0.024 |
| Electrical conductivity (mS/cm) | 0.367⁎⁎ | 0.038 | −0.049 | 0.045 |
| Organic carbon (%) | 0.615⁎⁎ | −0.031 | −0.206 | −0.297⁎ |
| Gravels (%) | −0.173 | −0.007 | −0.010 | −0.090 |
| Coarse and medium sand (%) | −0.395⁎⁎ | 0.038 | 0.081 | −0.004 |
| Fine and very fine sand (%) | 0.223 | −0.066 | 0.028 | 0.106 |
| Silt and clay (%) | 0.526⁎⁎ | 0.064 | −0.195 | −0.085 |
P < 0.05.
P < 0.01.
Correlations of edaphic variables with the cover values of the dominant species are shown in Table 4. Electrical conductivity exhibits significant correlations with C. murale (r = −0.293, P < 0.05) and E. colona (r = −0.285, P < 0.05). With the exception of E. cilianensis and P. oleracea, all the tested dominant species show significant correlations with organic carbon. Coarse and medium sand are correlated significantly with C. dactylon (r = −0.354, P < 0.01) and I. cylindrica (r = −0.272, P < 0.05). Silt and clay correlate significantly with all the tested dominant species except C. arvensis, E. cilianensis, I. cylindrica and P. oleracea.
Table 4.
Linear correlation coefficients (r) between edaphic factors and the cover values of the dominant species.
| Species | Edaphic variable |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaCO3 | pH | Electrical conductivity | Organic carbon | Gravels | Coarse and medium sand | Fine and very fine sand | Silt and clay | ||
| Chenopodium murale | −0.014 | −0.044 | 0.139 | −0.293⁎ | −0.541⁎⁎ | 0.139 | 0.143 | −0.044 | −0.356⁎⁎ |
| Convolvulus arvensis | −0.230 | 0.179 | −0.111 | 0.055 | 0.377⁎⁎ | 0.081 | 0.003 | −0.093 | 0.075 |
| Conyza bonariensis | 0.238 | −0.081 | 0.187 | 0.164 | 0.327⁎ | 0.031 | −0.257 | −0.002 | 0.452⁎⁎ |
| Cynodon dactylon | 0.063 | 0.001 | 0.266 | 0.202 | 0.390⁎⁎ | 0.048 | −0.354⁎⁎ | 0.122 | 0.375⁎⁎ |
| Echinochloa colona | −0.034 | −0.186 | 0.088 | −0.285⁎ | −0.304⁎ | 0.122 | 0.142 | −0.065 | −0.292⁎ |
| Eragrostis cilianensis | 0.036 | 0.133 | −0.270⁎ | −0.039 | 0.121 | −0.049 | 0.073 | −0.082 | 0.079 |
| Imperata cylindrica | 0.242 | 0.124 | −0.189 | 0.154 | 0.369⁎⁎ | −0.173 | −0.272⁎ | 0.264 | 0.213 |
| Melilotus indicus | −0.290⁎ | −0.083 | 0.008 | −0.155 | −0.297⁎ | 0.089 | 0.214 | −0.100 | −0.320⁎ |
| Plantago lagopus | 0.060 | −0.033 | 0.052 | 0.211 | 0.356⁎⁎ | −0.125 | −0.069 | −0.006 | 0.294⁎ |
| Portulaca oleracea | 0.118 | −0.048 | −0.010 | −0.247 | −0.240 | 0.095 | 0.110 | −0.071 | −0.186 |
P < 0.05.
P < 0.01.
4. Discussion
The weed vegetation in the study area comprises 71 plant species, including 47 annuals (66.2%) and 24 perennials (33.8%). The high contribution of annuals can be attributed to their short life cycle that enables them to resist the instability of the agro-ecosystem. Moreover, they are generally characterized by high allocation of resources to the reproductive organs (Harper, 1977) and the production of flowers early in their lifespan to ensure some seed production even in a year when the growing season is cut short (Sans and Masalles, 1995).
The application of TWINSPAN resulted in the classification of weed vegetation in the study area into four vegetation groups, representing wheat crop, orchards in winter season, orchards in summer season and watermelon crop. Such classification indicates the significant effects of both crop and season on the weed community composition and structure. The vegetation groups, resulted from TWINSPAN classification, are clearly distinguished by the first two DCA axes. Thus, the DCA analysis also strengthens the importance of crop and season for the formation of weed community. These results agree with those of El-Demerdash et al. (1997) and Andersson and Milberg (1998), who pointed out that season and crop type, contribute to the composition of weed community. The effect of crop may be indirect. For example, fertilization regimes, soil management practices, application of herbicides and weed management may vary depending on the crop type, and these factors influence weed community composition (Hume, 1982; Légere and Samson, 1999; Leeson et al., 2000).
The weed vegetation in wheat crop is dominated by C. murale and M. indicus. These two species were reported by Shaltout and El-Halawany (1992) to dominate some weed communities of date palm orchards in eastern Saudi Arabia. Moreover, they also dominate weed communities of winter crops in Egypt (Hegazy et al., 2004). The dominant species of weed vegetation of olive and date palm orchards in the study area during winter season are I. cylindrica, P. lagopus and C. arvensis. These species were recorded by Shaltout and El-Halawany (1992) as dominant weeds in eastern Saudi Arabia. Moreover, C. arvensis was listed as a co-dominant species in the date palm orchards of Central Saudi Arabia (Gazer, 2011). The weed vegetation in olive and date palm orchards in summer season is dominated by C. dactylon, C. bonariensis and E. cilianensis. C. dactylon was reported as a dominant or co-dominant weed in orchards and field crops in Saudi Arabia and the surrounding countries (Chaudhary et al., 1981; Gazer, 2011). C. bonariensis was reported by Al-Yemeny (1999) as a serious weed causing very severe infestations in field crops and orchards in Saudi Arabia. E. colona and P. oleracea are the dominant weeds in watermelon crop in the study area. These two species were listed by Chaudhary et al. (1981) among weeds that cause severe infestation in agricultural areas in the central, southern and eastern Arabian Peninsula.
The weed vegetation in the study area includes, in addition to arable weeds, some desert species that grow in the surrounding natural habitats as Citrullus colocynthis, Haloxylon salicornicum, Panicum turgidum, Zilla spinosa and Zygophyllum coccineum. Similar observations were documented by Gazer (2011).
The species richness is higher in both olive and date palm orchards than in wheat and watermelon crops. The environment of weeds in orchards is influenced by the protection given by the tree foliage. Two kinds of light conditions occur in orchards, the shaded microhabitat present below the crowns of trees and the relatively sunny microhabitat present between trees. The high species richness in orchards may be related to this environmental micro-heterogeneity that promotes diversity (Palmer and Maurer, 1997). The difference in field management practices may also be a factor that explains differences in weed species richness (Stevenson et al., 1997; Sher and Al-Yemeni, 2011). The low species richness in wheat and watermelon crops compared to orchards can be attributed to the fact that the land of field crops is generally plowed each season before the sowing of crops, a practice that reduces the richness of weeds compared to the orchards that are rarely plowed.
With the exception of organic carbon, none of the measured soil variables show significant correlations with DCA axes. This indicates that soil characters are not the major factors that contribute to the distribution of communities along DCA axes. Other factors as crop type and season may contribute more importantly to the distribution of weed communities along DCA axes.
Soil electrical conductivity, organic carbon, coarse and medium sand, silt and clay showed significant correlations with species richness and the cover values of some dominant species. Moreover, electrical conductivity, organic carbon, silt and clay exhibited significant differences between orchards and field crops. These results suggest the effective role of these soil parameters in the weed community structure and diversity. The present findings agree with those of Fried et al. (2008), Andreasen and Skovgaard (2009) and Pinke et al. (2010) that indicated the importance of soil texture, salinity and organic carbon for the composition and species richness of weed communities. Organic matter content as a pivotal soil fertility factor can affect phytodiversity (Zhang et al., 2010). Moreover, soil texture may affect soil or productivity via influence on the soil water holding capacity, infiltration rate, moisture availability for plants and consequently plant nutrition (Sperry and Hacke, 2002).
References
- Akobundu I.O. Wiley; Chichester: 1987. Weed Science in the Tropics. Principles and Practices. [Google Scholar]
- Aldrich R.J. Breton Publishers; Belmont, California: 1984. Weed–Crop Ecology, Principles in Weed Management. [Google Scholar]
- Al-Hassan H.O. Ministry of Agriculture, Camel and Range Research Center; Al-Jouf, Saudi Arabia: 2006. Wild Plants of the Northern Region of the Kingdom of Saudi Arabia (field guide with photographs) [Google Scholar]
- Al-Yemeny M.N. A check list of weeds in Al-kharj area of Saudi Arabia. Pak. J. Biol. Sci. 1999;2:7–13. [Google Scholar]
- Andersson T.N., Milberg P. Weed flora and the relative importance of site, crop, crop rotation, and nitrogen. Weed Sci. 1998;46:30–38. [Google Scholar]
- Andreasen C., Skovgaard I.M. Crop and soil factors of importance for the distribution of plant species on arable fields in Denmark. Agr. Ecosyst. Environ. 2009;133:61–67. [Google Scholar]
- Chaudhary S.A. vol. I. Ministry of Agriculture and Water; Riyadh, Saudi Arabia: 1999. (Flora of the Kingdom of the Saudi Arabia). [Google Scholar]
- Chaudhary S.A. vol. II. Ministry of Agriculture and Water; Riyadh, Saudi Arabia.: 2000. (Flora of the Kingdom of the Saudi Arabia). [Google Scholar]
- Chaudhary S.A. vol. III. Ministry of Agriculture and Water; Riyadh, Saudi Arabia: 2001. (Flora of the Kingdom of the Saudi Arabia). [Google Scholar]
- Chaudhary S.A., Akram M. Weeds of Saudi Arabia and the Arabian Peninsula. Regional Agriculture and Water Research Center, Ministry of Agriculture and Water; Riyadh, Saudi Arabia: 1987. [Google Scholar]
- Chaudhary S.A., Parker C., Kasasian L. Weeds of central, southern and eastern Arabian Peninsula. Trop. Pest Manag. 1981;27:181–190. [Google Scholar]
- Derksen D.A., Thomas A.G., Lafond G.P., Loeppky H.A., Swanton C.J. Impact of agronomic practices on weed communities: fallow within tillage systems. Weed Sci. 1994;42:184–194. [Google Scholar]
- El-Demerdash M.A., Hosni H.A., Al-Ashri N. Distribution of the weed communities in the north east Nile Delta, Egypt. Feddes Repert. 1997;108:219–232. [Google Scholar]
- El-Halawany E.F., Shaltout K.H. Weed flora of date palm orchards in Eastern Saudi Arabia. J. King Saud Univ. 1992;5(1):25–37. [Google Scholar]
- Fayed M.T.B., El-Geddawy I.H., El-Zeny M.M. Influence of weed interference on growth, yield and quality of sugar beet. Egypt. J. Agric. Res. 1999;77:1239–1249. [Google Scholar]
- Frick B., Thomas A.G. Weed surveys in different tillage systems in south western Ontario field crops. Can. J. Plant Sci. 1992;72:1337–1347. [Google Scholar]
- Fried G., Norton L.R., Reboud X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008;128:68–76. [Google Scholar]
- Gazer M.H. Vegetation composition and floristical diversity in date palm orchards of Central Saudi Arabia. Acta Bot. Hung. 2011;53(1–2):111–126. [Google Scholar]
- Ghersa C.M., Holt J.S. Using phenology prediction on weed management: a review. Weed Res. 1995;35:461–470. [Google Scholar]
- Harper J.L. Academic Press; London: 1977. Population Biology of Plants. [Google Scholar]
- Hegazy A.K., Fahmy G.M., Ali M.I., Gomaa N.H. Vegetation diversity in natural and agro-ecosystems of arid lands. Community Ecol. 2004;5:163–176. [Google Scholar]
- Hill M.O. Cornell University; Ithaca, NY: 1979. TWINSPAN – A FORTRAN Program for Arranging Multivariate Data in an Ordered Two-way Table by Classification of the Individuals and Attributes. [Google Scholar]
- Hill M.O. Cornell University; Ithaca, NY: 1979. DECORANA – A FORTRAN Program for Detrended Correspondence Analysis and Reciprocal Averaging. [Google Scholar]
- Hume L. The long-term effects of fertilizer application and three rotations on weed communities in wheat (after 21–22 years at Indian Head, Saskatchewan) Can. J. Plant Sci. 1982;62:741–750. [Google Scholar]
- Jabeen N., Ahmed M. Possible allelopathic effects of three different weeds on germination and growth of maize (zea mays) cultivars. Pak. J. Bot. 2009;41(4):1677–1683. [Google Scholar]
- Jackson M.L. Constable and Co. Ltd.; London: 1958. Soil Chemical Analysis. [Google Scholar]
- Jackson M.L. Washington Department of Soil Sciences; USA: 1967. Soil Chemical Analysis-advanced Course. [Google Scholar]
- Khedr A.A., Hegazy A.K. Ecology of the rampant weed Nymphaea lotus L. Willdenow in natural and ricefield habitats of the Nile Delta. Egypt. Hydrobiol. 1998;386:119–129. [Google Scholar]
- Leeson J.Y., Sheard J.W., Thomas A.G. Weed communities associated with arable Saskatchewan farm management systems. Can. J. Plant Sci. 2000;80:177–185. [Google Scholar]
- Légere A., Samson N. Relative influence of crop rotation, tillage, and weed management on weed associations in spring barley cropping systems. Weed Sci. 1999;47:112–122. [Google Scholar]
- Palmer M.W., Maurer T.A. Does diversity beget diversity? A case study of crops and weeds. J. Veg. Sci. 1997;8:235–240. [Google Scholar]
- Pielou E.C. Wiley; London: 1975. Ecological Diversity. [Google Scholar]
- Pinke G., Pál R., Botta-Dukát Z. Effects of environmental factors on weed species composition of cereal and stubble fields in western Hungary. Cent. Eur. J. Biol. 2010;5(2):283–292. [Google Scholar]
- Pysek P., Leps J. Response of a weed community to nitrogen fertilization: a multivariate analysis. J. Veg. Sci. 1991;2:237–244. [Google Scholar]
- Qasem J.R., Hill T.A. Growth, development and nutrient accumulation in Senecio vulgaris L. and Chenopodium album L. Weed Res. 1995;35:187–196. [Google Scholar]
- Raunkiaer C. Oxford University Press; Oxford: 1934. The Life Forms of Plants and Statistical Plant Geography. [Google Scholar]
- Sans F.X., Masalles R.M. Phenological patterns in an arable land weed community related to disturbance. Weed Res. 1995;35:321–332. [Google Scholar]
- Shah G.M., Khan M.A. Checklist of noxious weeds of district Mansehra, Pakistan. Pak. J. Weed Sci. Res. 2006;12(3):213–219. [Google Scholar]
- Shaltout K.H., El-Halawany E.F. Weed communities of date palm orchards in eastern Arabia. Qatar Univ. Sci. J. 1992;12:105–111. [Google Scholar]
- Sher H., Al-Yemeni M.N. Ecological investigation of the weed flora in arable and non arable lands of Al-kharj area, Saudi Arabia. Afr. J. Agric. Res. 2011;6(4):901–906. [Google Scholar]
- Sperry J.S., Hacke U.G. Desert shrub water relations with respect to soil characteristics and plant functional type. Funct. Ecol. 2002;16:367–378. [Google Scholar]
- Stevenson F.C., Légere A., Simard R.R., Angers D.A., Pageau D., Lafond J. Weed species diversity in spring barley varies with crop rotation and tillage, but not with nutrient source. Weed Sci. 1997;45:798–806. [Google Scholar]
- Swanton C.J., Harker K.N., Anderson R.L. Crop losses due to weeds in Canada. Weed Technol. 1993;7:537–542. [Google Scholar]
- Thomas A.G., Frick B.L. Influence of tillage Systems on weed abundance in south western Ontario. Weed Technol. 1993;7:699–705. [Google Scholar]
- Wang S., Duan L., Li J., Tian X., Li Z. UV-B radiation increases paraquat tolerance of two broad leaved and two grass weeds in relation to changes in herbicide absorption and photosynthesis. Weed Res. 2007;47(2):122–128. [Google Scholar]
- Zhang K., Dang H., Tan Sh., Wang Zh., Zhang Q. Vegetation community and soil characteristics of abandoned agricultural land and pine plantation in the Qinling Mountains, China. Forest Ecol. Manag. 2010;259:2036–2047. [Google Scholar]


