Abstract
The effect of an arbuscular mycorrhizal fungus “AMF” (Glomus constrictum Trappe) on growth, pigments, and phosphorous content of marigold (Tagetes erecta) plant grown under different levels of drought stress was investigated. The applied drought stress levels reduced growth vigor (i.e. plant height, shoot dry weight, flower diameter as well as its fresh and dry weights) of mycorrhizal and non-mycorrhizal plant as compared to control plant (non-drought stressed plant). The presence of mycorrhizal fungus, however, stimulated all growth parameters of the treated plant comparing to non-mycorrhizal treated plant. The photosynthetic pigments (carotene in flowers and chlorophylls a and b in leaves) were also stimulated by the mycorrhizal fungi of well-watered as well as of water-stressed plants. The total pigments of mycorrhizal plants grown under well-watered conditions were higher than those of non-mycorrhizal ones by 60%. In most cases, drought-stressed mycorrhizal plants were significantly better than those of the non-mycorrhizal plants. So, the overall results suggest that mycorrhizal fungal colonization affects host plant positively on growth, pigments, and phosphorous content, flower quality and thereby alleviates the stress imposed by water with holding.
Keywords: Arbuscular mycorrhiza, Glomus constrictum, Fungus, Drought stress, Marigold, Tagetes erecta, Growth and flowering
1. Introduction
Marigold (Tagetes erecta L.) belongs to Asteraceae family and is a herbaceous plant with aromatic, pinnately divided leaves and is usually used as a bedding plant, cut flower, or as a coloring agent in poultry feed to obtain yellow egg yolks (Dole and Wilkins, 2005). T. erecta L. has smaller flowers and leaves than those of most other marigolds. The plants brighten up any sunny area in the landscape and attract attention. Moreover, marigold plants are considered a very valuable enter crop for controlling plant parasitic nematode as recorded by Basu and Roy (1975). The aerial parts of the plant contain high quality of essential oil that can be used for scenting soaps, perfumery, cosmetic, and pharmaceutical industries.
Arbuscular mycorrhizal (AM) fungal symbiosis is widely believed that it protects host plants from detrimental effects of drought (Augé, 2001; Abdel-Fattah et al., 2002; Ruiz-Lozano, 2003). Possible mechanisms for improving drought resistance of the mycorrhizal plants could be due to an increased in root hydraulic conductivity (Robert et al., 2008), stomatal regulation or transpiration rate (Allen and Boosalis, 1983), enhanced water uptake at low soil moisture levels as a result of extraradical hyphae (Fagbola et al., 2001), osmotic adjustment which promotes turgor maintenance even at low tissue water potential (Augé et al., 1986), increased photosynthetic activity, proline and carbohydrate accumulation, and increased nutritional status in mycorrhizal plants (Scheilenbaum et al., 1999). These mechanisms may be important in adaptation of the mycorrhizal plants to drought conditions. The symbiosis of plant roots with AM fungi is known to be one of the most ancient and widespread plant strategies to enhance nutrient acquisition which copes with the environmental stress (Brachmann and Parniske, 2006). The intra-radical mycelium of these soil fungi proliferates in root cortex of the host plant. Extraradical AM hyphae spread in the soil around the root and provide a surface area by which the AM fungus absorbs nutritional elements “such as phosphorus (P), nitrogen (N), zinc (Zn), or copper (Cu)” and transports and transfers them to the host plant (Smith and Read, 2008).
One of the increasing interest and economic importance is the variation of the mycorrhizal responsiveness of a specific fungus by the variations in cultivars of the same host plants. This concept has been reported for field-grown crops, including basil (Gupta et al., 2000), grapes (Karagiannidis et al., 1995), onions (Tawaraya et al., 2001), wheat (Zhu et al., 2001), marigold (Robert et al., 2003), Brodiaea laxa (Scagel, 2004), Coriander spp. (Aliabadi et al., 2008), Viola calaminaria (Fernández et al., 2008), Calendula spp. (Rahmani et al., 2008), and medicinal and aromatic plants (Hossein et al., 2009).
Marigolds are annual plants that generally respond to mycorrhizal infection, but they do not always exhibit significant responsiveness under P-limiting conditions (Koide et al., 1999). However, plants of this nature may still benefit from the symbiosis, if not by enhancing growth, and hence increasing disease resistance (Linderman, 2000), and environmental stress tolerance (Cantrell and Linderman, 2001), or other physiological changes (Koide, 2000).
Therefore, the objective of this study was to investigate the effects of arbuscular mycorrhizal fungus (Glomus constrictum) on growth, phosphorus content, and flower quality of marigold plants subjected to various levels of drought stress conditions.
2. Materials and methods
2.1. Growth conditions
Seeds germination and transplanting of marigold (T. erecta) plants, cultivar ‘Jubilee’ were carried out in a greenhouse with a temperature of 27/18 °C day/night, and a supplemental light of 750 μmol m−2 s−1 (at a canopy level) provided by high-vapor pressure sodium lamps for 14 h day−1, and misted twice daily until transplanted, Plant Production Departments, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia. Seeds were germinated in 72-cell plug flats containing a seedling germination mix of 50% fine peat moss and 50% vermiculite. Seeds were sown on 16 September, 2009 and transplanted 2 weeks later into plastic pots of 25 cm diameter with one seedling per pot containing a mixture of one coarse sand:one low P sandy loam soil (v/v) media with a textural analysis of 77% sand, 9% clay, and 14% silt. The sandy loamy soil was collected from the experimental and agricultural research station at Dirab, Riyadh region, Saudi Arabia. Prior to the study, the mix was steam-pasteurized with AM fungal inoculants or non-inoculated controls and then cooled and stored for at least a week prior to use. Soil characteristics were as follow: the water pH 7.8; 23 mg phosphorus kg−1; 17 mg nitrogen kg−1; 28 mg potassium kg−1, and 34 mg magnesium kg−1 soil.
2.2. Mycorrhizal fungal inoculum preparation
The mycorrhizal fungus was originally isolated from the Dirab Experimental Station of College of Food and Agricultural Sciences, Riyadh region, Saudi Arabia. The fungus was propagated in pot culture on roots of bunching onion (Allium cepa L. ‘White Lisbon’) grown in loam:sand (1:1) medium for 5 months. The non-mycorrhizal control soil had a similar culture, but without AM fungi. Inocula consisted of a mixture of soil medium, extraradical hyphae, and spores, and colonized root segments (⩽2 mm in length). The inoculum (5 g of soil containing spores of mycorrhizal fungus) was placed 3 cm below the surface of the soil (before sowing) to produce mycorrhizal pants. The non-mycorrhizal treatment received an equal amount of sterilized soil inoculum to provide the same microflora without mycorrhizal fungi.
2.3. Experimental design
Pots were arranged on a greenhouse bench in a 2 × 4 factorial randomized block design included two mycorrhizal treatments (with mycorrhizal fungi “M+” and non-mycorrhizal fungi “M−”), and four levels of drought stress (no drought, mild drought, moderate drought, and severe drought). Plants were carefully watered as needed with tap water to maintain soil moisture near field capacity for 3 weeks, and then plants were subjected to eight treatments (five pots were used for each treatment). When seedlings were transplanted, irrigation was given uniformly to all pots. Marigold seedlings were exposed to four levels of drought stresses (100% (D0), 75% (D1), 50% (D2), and 25% (D3) according to water holding capacity of the soil. Plants were fertilized twice weekly with 13 N–0.9 P–10.8 K soluble fertilizer prepared to supply N and K at approximately 200 mg kg−1 N, and P at 16 mg kg−1. Each pot received approximately 100 ml of the fertilizer solution to ensure thought-out saturation of the medium. Growth measurements, pigment analysis, and determination of phosphorous content in both the leaves and the flowers were carried out after 6 weeks from transplantation.
2.4. Seedling measurements
The following parameters were examined at the end of the experiment (8 weeks old):
-
1.
Plant height (cm).
-
2.
Shoot fresh and dry weights per plant (g).
-
3.
Flower fresh and dry weights (g) and diameter (cm) per plant.
-
4.
Mineral analysis: oven-dried shoots (leaves and flowers) and samples were ground to pass through 0.5 mm sieve and then analyzed for P content according to method of Allen (1989).
-
5.
Estimation of photosynthetic pigments content: the photosynthetic pigments (chlorophylls a, b and carotenoids) were extracted and determined in fresh leaves of marigold plants according to the spectrophotometric method recommended by Metzner et al. (1965).
2.5. Statistical analysis
A randomized complete block design with three replicates was used. Data were subjected to statistical analysis according to (Steel and Torrie, 1980). The treatment means were compared using the least significant differences (LSD) test at 0.05% level.
3. Results and discussion
3.1. Growth parameters and plant productivity
Under well-watered conditions, mycorrhizal fungus significantly increased all the growth attributes such as plant height, shoot dry weight, flower diameter, flower fresh, and dry weights of marigold plants comparing to non-mycorrhizal plants (Table 1). The drought stress treatments significantly reduced the height, shoot dry weight, flower diameter, flower fresh, and dry weights of both the mycorrhizal and the non-mycorrhizal plants. This reduction was greatly offset by the G. constrictum stimulation of growth response of the treated plants comparing to the non-mycorrhizal treated plants (Fig. 1). Generally, under drought stress, mycorrhizal fungus stimulated greater growth criteria and flower parameters of treated plants than those of the non-mycorrhizal plants (Fig. 2). The enhanced dry weights of AM inoculated plants were 5.29, 4.12, 3.06, and 2.62 g, while of non-AM inoculated plants were 4.44, 3.28, 2.89, and 2 g at different levels of water-stressed conditions (well-watered, mild, moderate and severe drought-stressed treatments, respectively). Such pronounced growth response to mycorrhizal colonization was observed by Wu and Xia (2006) and Wu et al. (2008) who noticed that the mycorrhizal (AM) seedlings of Citrus tangerine and Poncirus trifoliate had significantly higher shoot and root dry weights, plant height, leaf area, leaf number per plant, and stem diameter under well-watered and water-stressed conditions than the corresponding non-AM seedlings. Similar results have been reported for other plant species (Kaya et al., 2003; Wu and Xia, 2006). The positive effect was likely attributed to the improvement of phosphorus nutrition (Bethlenfalvay et al., 1988) and uptake of water by hyphae (Faber et al., 1991). Dell-Amico et al. (2002) also found that the inoculation of tomato plants with G. clarum encouraged higher growth rates of plants in both well-watered and stressed conditions. Drought-stressed non-mycorrhizal plants produced lower products than mycorrhizal ones at all drought treatments (Table 1). These results are in good agreement with Al-Karaki et al. (2004), who observed that the inoculation with AM fungi provided an important enhancement to yield of two wheat cultivars. Sorial (2001) also observed increases in straw and grain yield of wheat plants subjected to different levels of water stress and inoculated with arbuscular mycorrhiza when compared to non-mycorrhizal plants. The enhancement in marigold growth and biomass yields due to AM fungi might be attributed to higher mineral and water uptake. Al-Karaki and Clark (1998) stated that the enhanced plant growth as well as yield following AM inoculation were due to the improved uptake of P and Cu, especially under water-stressed conditions. Whereas, M+ plants were taller than M− plants regardless of levels of drought stress, and the mycorrhizal response was more pronounced in severe drought treatment (D4).
Table 1.
Growth parameters of marigold plants in response to mycorrhizal inoculation grown under different levels of drought stress (D0 no drought, D1 mild, D2 moderate and D3 severe drought).
| Treatments |
Plant height (cm) | Shoot dry weight (g) | Flower diameter (cm) | Flowers fresh weight (g) | Flowers dry weight (g) | |
|---|---|---|---|---|---|---|
| Drought | Mycorrhizal status | |||||
| D0 | M+ | 58.6 | 5.29 | 5.22 | 9.01 | 1.64 |
| M− | 54.4 | 4.44 | 4.62 | 8.14 | 1.15 | |
| D1 | M+ | 53.3 | 4.12 | 4.23 | 7.14 | 1.02 |
| M− | 50.8 | 3.28 | 4.01 | 6.91 | 0.93 | |
| D2 | M+ | 47.6 | 3.06 | 4.02 | 6.33 | 0.83 |
| M− | 45.1 | 2.89 | 3.79 | 5.75 | 0.75 | |
| D3 | M+ | 36.5 | 2.62 | 3.34 | 5.24 | 0.67 |
| M− | 31.4 | 2.01 | 2.86 | 4.84 | 0.55 | |
| LSD 5% | 1.5⁎ | 0.31⁎ | 0.22⁎ | 0.19⁎ | 0.11⁎ | |
Where M+ = mycorrhizal treatment and M− = non-mycorrhizal treatment.
Significant difference at p ⩽ 0.05 (Steel and Torrie, 1980).
Figure 1.

Effects of mycorrhizal fungus (Glomus constrictum) on the vegetative growth of marigold plants grown either under well-watered (left) or under drought stress condition (right), where M = treated and NM = non-treated mycorrhizal plants.
Figure 2.

Effects of mycorrhizal fungus (Glomus constrictum) on the flowering growth of marigold plants grown either under well-watered (left) or under drought stress condition (right), where M = treated, NM = non-treated mycorrhizal plants.
3.2. Photosynthetic pigments
The content of photosynthetic pigments (chlorophylls a, b in leaves and carotenoids in flowers) of mycorrhizal and non-mycorrhizal marigold plants are presented in Table 2 and Fig. 3. In general, with all treatments, the contents of chlorophylls a, b and carotenoids in mycorrhizal plants were significantly greater than those of non-mycorrhizal ones at all stages of plant growth. The total photosynthetic pigments increased due to mycorrhizal colonization by 60% at well-watered conditions. These results also indicated that the deleterious effect of drought treatment on total pigments was at the severe drought stress of the plants.
Table 2.
Chlorophylls a and b for mycorrhizal (M+) and non-mycorrhizal (M−) marigold plants exposed to different levels of drought stress (D0 no drought, D1 mild, D2 moderate and D3 severe).
| Treatments |
Chlorophylls (mg/g fresh weight) |
||
|---|---|---|---|
| Drought | Mycorrhizal | Chlorophyll a | Chlorophyll b |
| D0 | M+ | 0.343 | 0.145 |
| M− | 0.335 | 0.136 | |
| D1 | M+ | 0.323 | 0.127 |
| M− | 0.313 | 0.117 | |
| D2 | M+ | 0.303 | 0.112 |
| M− | 0.295 | 0.104 | |
| D3 | M+ | 0.284 | 0.101 |
| M− | 0.270 | 0.095 | |
| LSD 5% | 0.004⁎ | 0.004⁎ | |
Where M+ = mycorrhizal treatment and M− = non-mycorrhizal treatment.
Significant difference at p ⩽ 0.05 (Steel and Torrie, 1980).
Figure 3.
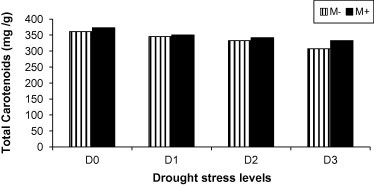
Total carotenoids in flowers of mycorrhizal M+ (filled bars) and non-mycorrhizal M− (empty bars) marigold plants grown under different levels of drought stress.
The decrease of chlorophyll content in marigold plants as a result of water deficit has also been reported by several authors (Dhanda et al., 2004; Shao et al., 2007) and citrus (Wu and Xia, 2006). Shao et al. (2007) reported that chlorophyll was the substantial basis for wheat photosynthesis, so the content of chlorophyll could be one of the indexes for evaluating photosynthesis. Many reports showed that drought could lead to lower photosynthesis and efficiency (Dhanda et al., 2004). However, Moran et al. (1994) stated that the decrease in chlorophyll or protein concentrations would be a typical symptom of oxidative stress that had been observed in drought-stressed plants. The increase of photosynthetic pigments as a result of mycorrhizal colonization was also supported by Aboul-Nasr (1996) and Wu and Xia (2006). The present results indicate that AM application assist plants to counter photoinhibition and photodestruction of pigments under stressed conditions by increasing the content of carotenoids. It is well known that carotenoids are involved in protecting photosynthetic apparatus against the photoinhibitory damage by the single oxygen. Therefore, carotenoids can directly deactivate, and can also quench the excited triple state of chlorophyll (Foyer and Harbinson, 1994). Moreover, it has been mentioned that the higher chlorophyll content in AM than in non-AM plants has sometimes been associated with a higher rate of photosynthesis, or with the increase in nitrogen and magnesium contents (major components of chlorophyll molecules) of mycorrhizal plants (Mathur and Vyas, 1995).
3.3. Phosphorus content
Drought stress levels significantly reduced the phosphorus content of mycorrhizal and non-mycorrhizal marigold plants comparing to well-watered plants (Fig. 4). However, the rate of reduction was higher in non-mycorrhizal than in mycorrhizal plants. These results agreed with the findings of Al-Karaki et al. (2004) on wheat plants; Augé et al. (2007) on sorghum and squash; Wu and Xia (2006) on citrus. Moreover, Subramanian et al. (2006) also observed that the mycorrhizal tomato plants had significantly higher phosphorus uptake in both roots and shoots of the plants regardless of the drought stress intensities.
Figure 4.
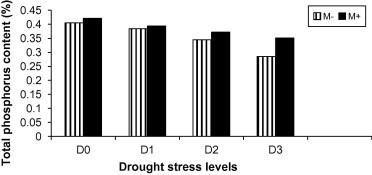
Phosphorus content in leaves of mycorrhizal M+ (filled bars) and non-mycorrhizal M− (empty bars) marigold plants grown under different levels of drought stress.
Phosphorus concentration may affect host water balance. For instance, stomatal conductance can be influenced by P starvation. Koide (2000) suggested that the increased stomatal conductance and transpiration rate in AM plants could be due to P-mediated improvement in photosynthetic capacity. Phosphorous concentrations in leaves may affect stomatal response to environmental perturbations, perhaps by affecting the energetic involved in guard cell osmotic potential or wall stiffening governing stomatal movements (Weyers and Meidner, 1990). Wu and Xia (2006) reported that under water stress conditions, higher plants accumulate some small molecules including organic solutes and inorganic ions. The accumulation of these molecules in AM seedlings resulted in a greater osmotic adjustment, and allowed AM seedlings to accumulate more carbohydrate and thus higher plant biomass. Davies et al. (1992) found that external hyphal development and soil aggregation of mycorrhizal plants were enhanced by drought acclimation. However, O’Keefe and Sylvia (1993) observed that external hyphae adhere to soil particles, and improve contact with soil solution. Furthermore, they mentioned that hyphae access smaller pore spaces better than plant roots and root hairs.
4. Conclusion
The results of this study indicated that marigold plants grown under semiarid habitats were affected greatly by water-stressed conditions. The data showed that the mycorrhizal colonization improved drought resistance of the marigold plants as a consequence of enhancing nutritional status, especially P and water status of the plants. This enhances plant growth, phosphorous uptake, and plant productivity. Therefore, this study recommends farmers in new reclaimed lands to not withhold irrigation during heading stage of marigold plants, and suggests adding arbuscular mycorrhizal fungi to arid farms, or to farms suffering from withholding irrigation water at critical growth stages.
References
- Abdel-Fattah G.M., Mighed F., Ibrahim A.H. Interactive effects of endomycorrhizal fungus Glomus etunicatum and phosphorous fertilization on growth and metabolic activities of board bean plants under drought stress conditions. Pak. J. Biol. Sci. 2002;5(8):835–841. [Google Scholar]
- Aboul-Nasr A. Effects of vesicular–arbuscular mycorrhiza on Tageteserecta and Zinnia elegans. Mycorrhiza. 1996;6:61–64. [Google Scholar]
- Aliabadi F., Lebaschi H., Hussein M., Hussein S.A., Reza1 V.A., Jahanfar D. Effects of arbuscular mycorrhizal fungi, different levels of phosphorus and drought stress on water use efficiency, relative water content and proline accumulation rate of Coriander (Coriandrum sativum L.) J. Med. Plants Res. 2008;2(6):125–131. [Google Scholar]
- Al-Karaki G.N., Clark R.B. Growth, mineral acquisition, and water use by mycorrhizal wheat grown under water stress. J. Plant Nutr. 1998;21(2):263–276. [Google Scholar]
- Al-Karaki G.N., McMichael B., Zah J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 2004;14:263–269. doi: 10.1007/s00572-003-0265-2. [DOI] [PubMed] [Google Scholar]
- Allen S.E. second ed. Black Well Sci. Pub. Osney; Oxford, London: 1989. Chemical Analysis of Ecological Materials. [Google Scholar]
- Allen M.F., Boosalis M.G. Effect of two species of VA mycorrhizal fungi on drought tolerance of winter wheat. New Phytol. 1983;93:67–76. [Google Scholar]
- Augé R.M. Water relations, drought and vesicular arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. [Google Scholar]
- Augé R.M., Scheikel K.A., Warmple R.L. Osmotic adjustment in leaves of VA-mycorrhiza and non-mycorrhizal rose plants in response to drought stress. Plant Physiol. 1986;82:765–770. doi: 10.1104/pp.82.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé R.M., Toler H.D., Moore J.L., Cho K., Saxton A.M. Comparing contributions of soil versus root colonization to variations in stomatal behavior and soil drying in mycorrhizal Sorghum bicolor and Cucurbita pepo. J. Plant Physiol. 2007;164:1289–1299. doi: 10.1016/j.jplph.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Basu, S.D., Roy, S.K., 1975. Rotylenchulus sp. a new ecto parasitic nematode in ted soil. Two and Bud (22(1), (17) Em). In: Abst, C.F.H., Tocklia Experimental Station Horhat, Aaaaem, India, vol. 46. Breeding for Resistance to Fungal Pathogens. Canadian Journal of Botany 68, 1039–1044 (1976).
- Bethlenfalvay G.J., Brown M.S., Ames R.N., Thomas R.E. Effects of drought on host and endophyte development in mycorrhizal soybeans in relation to water use and phosphate uptake. Physiol. Plant. 1988;72:565–571. [Google Scholar]
- Brachmann A., Parniske M. The most important symbiosis on earth. Soil Biol. 2006;4:239. [Google Scholar]
- Cantrell I.S., Linderman R.G. Preinoculation of lettuce and onion with VAmycorrhizal fungi reduces deleterious effects of soil salinitx. Plant Soil. 2001;223:269–281. [Google Scholar]
- Davies F.T., Potter J.R., Linderman R.G. Mycorrhiza and repeated drought exposure affect drought resistance and extraradical hyphae development of pepper plants independent of plant size and nutrient content. J. Plant Physiol. 1992;139:289–294. [Google Scholar]
- Dell-Amico J., Torrecillos A., Rodriguez P., Morte A., Sanchez-Blanco M.J. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. J. Agric. Sci. 2002;138:387–393. [Google Scholar]
- Dhanda S.S., Sethi G.S., Behl R.K. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2004;190(1):6–12. [Google Scholar]
- Dole J.M., Wilkins H.F. Prentice-Hall Inc.; USA: 2005. Floriculture Principles and Species. p. 1023. [Google Scholar]
- Faber B.A., Zasoki R.J., Munns D.N., Shackel K. A method for measuring hyphal nutrient and water uptake in mycorrhizal plants. Can. J. Bot. 1991;69:87–94. [Google Scholar]
- Fagbola O., Osonubi O., Mulongox K., Odunfa S.A. Effects of drought stress and arbuscular mycorrhiza on the growth of Gliricdia sepium (Jacq). Walp, Leucaenal leucocephala (Lam). De wit. In simulated eroded soil conditions. Mycorrhiza. 2001;11:215–223. [Google Scholar]
- Fernández O., Carrillo R.G., Vangrosveld J., González M.C. Arbuscular mycorrhizal fungi and Zn accumulation in the metallophytic plant Viola calaminaria (Gingins.) Lej. Serie Horticultura. 2008;14(3):355–360. [Google Scholar]
- Foyer C.H., Harbinson J. Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer C.H., Mullineausx P.M., editors. Causes of Photooxidative Stress and Amelioration of Defense System in Plants. CRS Press; Boca Raton: 1994. pp. 1–4. [Google Scholar]
- Gupta M.L., Khaliq A., Pandey R., Shukla R.S., Singh H.K., Kumar S. Vesicular–arbuscular mycorrhizal fungi associated with Ocimum spp. J. Herbs Spices Med. Plant. 2000;7:57–63. [Google Scholar]
- Hossein A.F., Valadabadi S.A., Daneshian J., Shiranirad A.H., Khalvati M.A. Medicinal and aromatic plants farming under drought conditions. J. Horti. Forestry. 2009;6:082–092. [Google Scholar]
- Karagiannidis N., Nikolaou N., Matteou A. Influence of three VA-mycorrhiza species on the growth and nutrient uptake of three grapevine rootstocks and one table grape cultivar. Vitis. 1995;34:85–89. [Google Scholar]
- Kaya C., Higgs D., Kirnak H., Tas I. Mycorrhizal colonization importoves fruit yield and water use efficiency in water-melon (Citrullus Lanatus Thunb.) grown under well watered and water-stressed conditions. Plant Soil. 2003;253:287–292. [Google Scholar]
- Koide R.T. Mycorrhizal symbiosis and plant reproduction. In: Kapulnik Y., Douds D.D., editors. Arbuscular Mycorrhizas: Physiology and Function. Kluwer Academic Publishers; Dordrecht: 2000. pp. 19–46. [Google Scholar]
- Koide R.T., Landherr L.L., Besmer Y.L., Detweiler D.M., Holcomb E.J. Strategies for mycorrhizal inoculation of six annual bedding plant species. HortScience. 1999;34:1217–1220. [Google Scholar]
- Linderman R.G. Effects of mycorrhizas on plant tolerance to diseases. In: Kapulnk Y., Douds D.D., editors. Arbuscular Mycorrhizas: Physiology and Function. Kluwer Academic Publishers; Dordrecht: 2000. pp. 345–365. [Google Scholar]
- Mathur N., Vyas A. Influence of VA mycorrhiza on net photosynthesis and transpiration of Ziziphus mauritiana. J. Plant Physiol. 1995;147:328–330. [Google Scholar]
- Metzner H., Rau H., Senger H. Untersuchungen zur Synchronnisierbarkeit einzelner pigmentmangel Mutanten von Chlorella. Planta. 1965;65:186. [Google Scholar]
- Moran J.F., Becana M., Iturbe-Ormaetre I., Frechilla S., Klucas R.V., Aparicio-Tejo P. Drought induces oxidative stress in pea plants. Planta. 1994;194:346–352. [Google Scholar]
- O’Keefe D.M., Sylvia D.M. Chronology and mechanisms of mycorrhizal mediated P uptake in sweet potato plants. New Phytol. 1993;122:651–659. [Google Scholar]
- Rahmani N., Aliabadi F.H., Valadabadi S.A.R. Abstracts Book of the World Congress on Medicinal and Aromatic Plants; South Africa: 2008. Effects of nitrogen on oil yield and its component of Calendula (Calendula officinalis L.) in drought stress conditions. p. 364. [Google Scholar]
- Robert G., Linderman A., Davis E.A. Varied response of marigold (Tagetes spp.) genotypes to inoculation with different arbuscular mycorrhizal fungi. Sci. Hortic. 2003;22:1–14. [Google Scholar]
- Robert M., Augé R.M., Heather D., Carl F., Sams E.A., Ghazala N. Hydraulic conductance and water potential gradients in squash leaves showing mycorrhiza-induced increases in stomatal conductance. Mycorrhiza. 2008;18:115–121. doi: 10.1007/s00572-008-0162-9. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano, J.M., 2003. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13 Online first published on April 11, doi:10.1007/s00572-003-0237-6. [DOI] [PubMed]
- Scagel C.F. Soil pasteurization and mycorrhizal inoculation alter flower production and corm composiyion of Brodiaea laxa “Queen Fabiola”. HortiScience. 2004;39(6):1432–1437. [Google Scholar]
- Scheilenbaum L., Sprenger N., Schuepp H., Wiemken A., Boller E. Effect of drought transgenic expression of a fructan synthesizing enzyme and of mycorrhizal symbiosis on growth and soluble carbohydrate pools in tobacco plants. New Phytol. 1999;142:67–77. [Google Scholar]
- Shao H.B., Chu L.Y., Wu G., Zhang J.H., Lu Z.H., Hu Y.C. Changes of some antioxidative physiological indices under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at tillering stage. Colloids Surf. B. 2007;54:143–149. doi: 10.1016/j.colsurfb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Smith S.E., Read D.J. third ed. Academic Press; UK: 2008. Mycorrhizal Symbioses. [Google Scholar]
- Sorial M.E. Growth, phosphorus uptake and water relations of wheat infected with an arbuscular mycorrhizal fungus under water stress. Ann. Agric. Sci.-Moshtohor. 2001;39(2):909–931. [Google Scholar]
- Steel R.C.D., Torrie J.M. Mc Graw-Hill Book Company; NY, USA: 1980. Principals and Procedures of Statistics. A Biometrical Approach. [Google Scholar]
- Subramanian K.S., Santhanakrishnan P., Balasubramanian P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hort. 2006;107:245–253. [Google Scholar]
- Tawaraya K., Tokairin K., Wagatsuma T. Dependence of Allium fistulosum cultivars on the arbuscular three pea cultivars. Plant Soil. 2001;103:296–298. [Google Scholar]
- Weyers J.D.B., Meidner H. Longman; London: 1990. Methods in Research. pp. 9–12. [Google Scholar]
- Wu Q.S., Xia R.X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well watered and water stress conditions. J. Plant Physiol. 2006;163:417–425. doi: 10.1016/j.jplph.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Wu Q.S., Xia R.X., Zou Y.N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 2008;44:122–128. [Google Scholar]
- Zhu Y.G., Smith S.E., Barritt A.R., Smith F.A. Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil. 2001;237:249–255. [Google Scholar]


