Abstract
The crude extract of the red seaweed, Asparagopsis sp. was evaluated for in vivo antibacterial activity against the shrimp vibrio pathogens. The algal extract was rationalized with commercial shrimp feed and orally administered for different duration of time followed by the artificial bacterial challenge experiment. In dose titration experiments, the oral administration of Asparagopsis sp. at a dosage of 850 mg kg–1 of biomass was highly efficacious in the treatment of natural infestations of Vibriosis in Penaeus monodon. The results of the confirmatory dose experiment revealed that the prophylactic treatment with moderate dose of 850 mg kg–1 of biomass day–1 for four weeks followed by 14 days of post infection therapy was highly effective in controlling Vibrio infection in shrimps. Moreover, results of the percent survival index and microbiological analysis clearly show that Asparagopsis extract incorporated medicated feed had broad therapeutic potential for managing shrimp Vibriosis. In addition, in vivo trials and results obtained in this work are based on the crude organic extract sourced from an unidentified Asparagopsis cryptic lineage, therefore further molecular analysis to identify the species will be required.
Keywords: Vibriosis, Asparagopsis extract, Penaeus monodon, Medicated feed, Algal extract
1. Introduction
Disease management in shrimp is complicated due to multifaceted problems such as emergence of new pathogenic strains, resistant pathogens, misidentification of species, short term efficacy of antibiotics, vaccines and environmental factors (Selvin et al., 2009; Cano-Gomez et al., 2011). The balance between health and disease is a delicate one and shrimps are vulnerable organisms in any but ideal conditions. Sustainability of the shrimp industry depends largely on efficient disease control and of the shrimp health (Selvin et al., 2009). Therefore, prevention of the disease and the management has been considered as a priority for this industry (Roch, 1999). Globally, Vibriosis is the significant cause of morbidity and mortality in shrimps. The major species causing Vibriosis in shrimp are Vibrio harveyi, V. fluvialis, V. parahaemolyticus, V. damsela and V. vulnificus (Chythanya et al., 2002). Accurate identification of Vibrio spp. is an important factor for the effective management and control of diseases in shrimp. Recent studies reported that multilocus sequence analysis (MLSA) is a successful molecular tool for the identification of Vibrio strains (Cano-Gomez et al., 2011).
In most of the commercial shrimp farms, Vibrio proliferation has been controlled by prophylaxis and chemotherapy (Gatesoupe et al., 1989). However the intensive and extensive use of antibiotics and chemotherapeutics is widely criticized. Recently, the emergence of antibiotic resistant Vibrio strains of shrimp triggered the withdrawal of synthetic antibiotics from aquaculture (Karunasagar et al., 1994; Hameed et al., 2003). The lack of effective disease control has turned out to be the cardinal limiting factor against the realization of highly stable shrimp production. As a result to this dilemma, new integrative disease management is therefore a critical consideration in suitable shrimp production.
Among alternatives to synthetic antibiotics in shrimp aquaculture, use of natural products as antimicrobials has been reported as a relevant strategy (Selvin et al., 2009). The use of bioactive compounds from natural sources that enhance disease resistance in animals may be an excellent tool against infection by pathogenic organisms. Extracts and natural products isolated from those plants exhibit a bewildering array of activities in bioassays and in animal models. The application of natural antimicrobial/immune-stimulant for disease prevention in aquaculture is becoming increasingly popular due to an increasing demand for eco-friendly aquaculture. Recently, the therapeutic effect of botanicals has been demonstrated in mammals (Kromna et al., 2006), birds (Mtambo et al., 1999) and fishes (Auro de Ocampo and Jimenez, 1993; Abutbul et al., 2004; Pachanawan et al., 2008). Many marine and terrestrial plant extracts have been investigated for antimicrobial activity against shrimp/fish pathogens (Das et al., 1999; Bansemir et al., 2006; Castro et al., 2008; Tukmechil et al., 2010).
Bioactive compounds derived from algae have been used as medicine to treat and control shrimp diseases, and successful results have been reported from various locales globally (Chotigeat et al., 2004; Hou and Chen, 2005; Yeh et al., 2006, 2010; Fu et al., 2007; Yeh and Chen, 2009). The efficacy of natural products from marine algae against various shrimp bacterial pathogens has been demonstrated in previous studies (Jose et al., 2008; Lipton et al., 2009; Kanjana et al., 2011). Efficacy has also been demonstrated against other shrimp pathogens including virus such as WSSV in Penaeus monodon (Witvrouw and De Clercq, 1997; Chotigeat et al., 2004; Manilal et al., 2009a), Vibriosis in Fenneropenaeus chinensis (Huang et al., 2006) and Litopenaeus vannamei (Yeh et al., 2006).
Oral administration of natural antimicrobials is the preferred route of chemotherapy in shrimp aquaculture owing to the ease of use and lack of any additional stress to the shrimp during treatment. Furthermore, it is impossible to isolate infected shrimp for the treatment purposes as done in mammals. The application of algal-based feed may be an effective means for increasing the immune-proficiency and disease resistance/control in shrimp. The disease resistance of shrimp has been found to be induced by feeding with an algal-based medicated feed that had been a successful strategy for disease management (Selvin et al., 2011). There has been only limited research effort in the development of therapeutics from natural products for shrimp disease management. Algal-based medicated feed is a valuable vehicle for oral collective antibiotic treatments in shrimps provided that an adequate amount of the active ingredient is available for the animals. As per the earlier in vitro studies the Asparagopsis sp. was found to be a highly active alga from the southwest littoral of India (Manilal et al., 2009b). Methanolic extract of Asparagopsis specimens showed 100% inhibition against pathogenic Vibrio strains isolated from moribund shrimps and MTCC culture of shrimp Vibrio pathogens. Considering the broad anti-Vibrio potency, less in vitro shrimp toxicity (Manilal et al., 2010a) and huge biomass availability, Asparagopsis sp. is well suited for the development of potential therapeutic agent. In this background, the aims of the present study are: to determine the in vivo efficacy of Asparagopsis extract incorporated feed for the treatment of disease due to experimental infection with respective pathogenic Vibrio spp. and to establish the effective dose level in the treatment of Vibriosis.
2. Materials and methods
2.1. Collection and extraction
Specimens of Asparagopsis sp. were sourced from the intertidal biotope of Kollam littoral (08°54′N and 76°38′E), India. It is one of the more common red algae in the Southwest littoral of Kollam prefecture. The extract of Asparagopsis sp. was prepared by standardized procedure as described by Manilal et al. (2009b).
2.2. Preparation of medicated feed and formulation
The doses of Asparagopsis extract for shrimp disease treatment was selected according to minimum inhibitory concentration values against pathogenic Vibrio and toxicological data (Manilal et al., 2009b, 2010a). Considering the facts that parenteral (intra-muscular injection) median lethal dose (480 mg kg–1 shrimp; Manilal, 2011) may not be lethal in oral intubation (Loomis, 1978) the following dosing regimens were preferred to attain effective therapeutic values. To prepare the medicated feed 250, 500, 750, 1000, 1250 and 1500 mg of ethyl acetate partitioned fraction of Asparagopsis sp. suspended in 100 mL of 6% gelatin water. The mixture was thoroughly sprayed on 1000 g of commercial pellet feed (Charoen Pokphand India Pvt. Ltd.) using a TLC sprayer (Xu and Rogers, 1994). Finally, the wet pellets were dried in an oven at 40 °C for about 12 h to achieve the final moisture content (about 8%). The control feed was prepared by coating feed pellets with gelatin in the same proportion as used for the medicated feed.
2.3. Determination of effective dose of medicated feed (in vivo dose titration study)
Test doses were calculated as intake of the active substance per kilogram biomass per time. Therefore, data on lower, moderate and higher regimen are necessary. In order to determine an appropriate dosage regimen, two dose titration trials were performed. In the first set of preliminary experiments, four dose regimens (285, 575, 850 and 1150 mg kg−1) of medicated feed were evaluated. In order to compensate the leaching losses, 15% of algae extract was incorporated as extra (Manilal, 2011). Based on the results from previous dose titration study, a second set of dose titration experiments was performed with three dose regimen (575, 850 and 1150 mg kg−1) of medicated feed. Two trials were identical except for a very slight difference in shrimp size and number of dose levels of medicated feed.
2.4. Shrimp experimental system and maintenance
P. monodon juveniles (PL-20; 500 Nos.) were obtained from the large-scale hatchery of Matsyafed, Kollam, Kerala, and acclimatized to standard conditions: 25 ± 2 °C, 15‰, and constant aeration. After three weeks of nursing phase, shrimps that had no signs of disease were selected for experiment. After the initially biometric analysis (3.1 ± 1.2 cm), shrimps were divided into five groups and randomly stocked at a density of 100 shrimps in each rectangular 2000 L capacity Fiber Reinforced Plastic (FRP) tanks. The results of preliminary animal feeding behavior to various dose levels indicated that shrimps exhibited a 60–70% of deterrent behavior in higher doses (1450 and 1725 mg kg−1) of medicated feed. Therefore, these dose levels were excluded for further titration trials. For the first dose titration studies, the experimental design consists of four nominal doses (285, 575, 850 and 1150 mg kg−1 biomass) of medicated feed. Shrimps in the control group were kept untreated and fed with normal commercial feed. The shrimps were fed with their allocated medicated feed of 3.5% of the body weight three times a day regularly until the end of experiment. Approximately 25% of the total water volume was replaced daily to maintain water quality. During the course of experiment, temperature (25 ± 2 °C), dissolved oxygen of water (6 mg L−1) and the salinity (15 ± 1.5‰) were measured daily using the Hach kit model (Hach Company, Loveland, CO). However, in the first dose titration study, higher percentage of mortalities (disease sensitive) occurred in the lower dose (285 mg kg−1) of medicated feed. For this reason, lower dose was occluded and a second dose titration study was performed. The treatments which achieved highest (disease resistant) survival rate were selected to be used for further dose titration study. The same experiment was repeated with three dose levels (575, 850 and 1150 mg kg−1 biomass) of medicated feed in the second dose titration.
2.5. Challenge experiments using Vibrio spp.
After three weeks of feeding trial, ten shrimps from each group (medicated and control groups) were individually challenged with four species of live Vibrio: V. harveyi, V. alginolyticus, V. parahaemolyticus and Photobacterium damsela (Manilal et al., 2010b). The in vivo challenge experiment was performed as per Manilal et al. (2012). The challenged animals were monitored for a period of two weeks for mortality and infections. During the challenge period, the shrimps were fed continuously with their respective feeds. The cause of death/infection was ascertained by re-isolating the respective organism from the shrimp body and subjecting the isolates to standard biochemical tests. The morbidity and mortality were recorded daily and final mortality was evaluated after two weeks of post challenge, percentage of mortality was calculated as per Manilal et al. (2012):
where “A” represents the number of dead shrimps on the first day after challenge. This mortality rate could be considered as a result of handling stress.The percent of survival index (therapeutic efficacy) was calculated as
2.6. Determination of optimum time of dosing (time titration study)
This study was conducted to ascertain whether the duration of pre-feeding (prophylactic) and post-feeding (therapeutic) of effective dosage had influence on the survival and infection of the shrimps. At the same time, in another experimental set up, time titration studies were also carried out to determine the efficacy of best formulation (850 mg kg−1) prophylactically and therapeutically in different dosing intervals (21 and 28 days). Medication was given for the recommended duration, with replicates, as follows: Group I: shrimps fed with medicated feed 21 days prior to challenge and subsequent 14 days feeding with normal feed. Group II: shrimps fed with medicated feed 21 days prior to challenge and subsequent 14 days feeding with medicated feed. Group III: shrimps fed with medicated feed 28 days prior to challenge and subsequent two weeks feeding with normal feed. Group IV: shrimps fed with medicated feed 28 days prior to challenge and subsequent two weeks feeding with medicated feed. The experimental facilities, source of the post-larvae, maintenance and challenge experiments were identical to those in the dose titration trials.
2.7. In vivo confirmatory experiments on shrimp disease control using effective dose of algal extracts
Confirmatory titration trial was designed to determine whether the proposed dose level is effective against single and mixed challenge experiment. A batch of healthy P. monodon was divided into two subgroups were fed with 850 mg kg−1 medicated feed while the control group was fed on the normal feed (feed free from Asparagopsis extract). The experimental design, feeding pattern, water management system, facilities and the size of shrimp used in confirmatory experiment were analogous those used in the dose titration studies. At the conclusion of fourth week of the feeding period, 10 shrimps from each group were challenged with appropriate single and mixed culture of bacterial suspension (Manilal et al., 2012). The inoculums were prepared by deliberate mixing of different species (V. harveyi + V. alginolyticus; V. harveyi + V. parahaemolyticus; V. harveyi + Ph. damsela) of bacteria of known doses (ca. 5 × 102 + 5 × 102). Each challenge experiment was carried out in triplicate. For microbiological analysis, 20% of the animals from each group were analyzed.
2.8. Data analysis
All the data are presented as mean ± standard deviation (S.D.). Mean values were compared among treatments and the control using one way analysis of variance (ANOVA) using SPSS for Windows version 11.2 (SPSS, Chigago, IL, USA).
3. Results
3.1. In vivo dose titration trials
The overall objective of the dose titration trial is to evaluate the ability of shrimp fed with medicated feed of different dose levels to overcome the challenge experiment. In the initial dose titration trial, shrimps were orally administered at the rate of 285, 575, 850 and 1150 mg kg−1 biomass for three weeks and subsequently challenged with respective Vibrio spp. The results of the dose titration profiles were summarized in the Table 1. After infection with challenge inocula of respective Vibrio spp. (ca. 105 CFU animal−1), medicated feed treated groups proved to be effective in increasing the survival rate of shrimp. The efficiency of the medicated feed varied with different dosage rates, type of Vibrio spp. challenged and treatment schedule. There was an apparent correlation between percent survival index of the shrimp and dose levels of medicated feed. At the end of the post challenge treatment period, cumulative mortalities and morbidities of the shrimp treated with moderate and higher dose of medicated feed was significantly lower than that of non-medicated group (control group). Non-medicated shrimps had severe infection and mortality. In contrast, all of the unchallenged control shrimps survived throughout the experimental period and was microbiologically negative for Vibriosis. This observation clearly confirmed that shrimps were not carrying the bacteria prior the start of the experimentation. The percent survival index of shrimp fed with 850 and 1150 mg kg−1 was significantly superior to any of the medicated group of shrimp and ranged between 53 and 67 after the challenge experiment (Table 2). Only two dose levels of medicated feed (850 and 1150 mg kg−1) significantly inhibited the Vibrio infection. The degree of morbidity in the challenged shrimps was significantly lower in these dose levels. However, the lower doses (575 mg kg–1) of medicated feed were also found to increase the percent survival index of shrimp but the percentage of infection was higher. At the end of the experiment, the percent survival index of 575 mg kg–1 treated shrimp ranged between 29 and 43 (Table 2). At this dose, the shrimp challenged with lethal dose of V. harveyi, caused morbidity to the extent of 74% while shrimps that injected with V. alginolyticus, V. parahaemolyticus and Ph. damsela caused 63%, 65% and 60%, respectively. The reason may be due to the insufficient administration and bioavailability of the medication in this treatment. The rate of mortality and morbidity in 285 mg kg−1 treated group was higher than in other treatments. The shrimps treated with 285 mg kg−1 resulted in average 61% mortality. The severity of infection was higher in this group and majority of the treated shrimps at this dose levels exhibited pathological signs such as feeble movement, dark body coloration, necrosis of legs, antenna and tail. By contrast, challenged control specimens fed with normal feed experienced 100% of infection and mortality was ranging between 60% and 100%. The lower doses of medicated feed were found to be unpromising candidates and therefore eliminated in the further experiment.
Table 1.
First dose titration trial of control and medicated shrimps received different dose levels for 21 days and challenged with respective Vibrio spp.
| Treatment regimens (mg kg−1) | Mean % infection and mortality |
|||||||
|---|---|---|---|---|---|---|---|---|
| VH |
VA |
VP |
PD |
|||||
| %I | %M | %I | %M | %I | %M | %I | %M | |
| 285 | 85 ± 5.2 | 82 ± 1.6 | 83 ± 5.5 | 53 ± 2.5 | 81 ± 6.2 | 55 ± 1.3 | 82 ± 3.2 | 54 ± 1.5 |
| 575 | 74 ± 4.2 | 57 ± 1.8 | 63 ± 4.3 | 41 ± 2.3 | 65 ± 2.8 | 44 ± 2.7 | 60 ± 2.5 | 43 ± 1.9 |
| 850 | 37 ± 3.5 | 35 ± 2.5 | 21 ± 4.7 | 24 ± 1.1 | 18 ± 5.4 | 22 ± 1.1 | 20 ± 2.8 | 24 ± 2.0 |
| 1150 | 55 ± 4.7 | 35 ± 2.2 | 46 ± 5.2 | 30 ± 2.1 | 41 ± 5.2 | 29 ± 2.3 | 43 ± 3.2 | 25 ± 2.3 |
| +Control | 100 | 100 | 100 | 64 ± 2.4 | 100 | 67 ± 2.1 | 100 | 61 ± 1.5 |
| –Control | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 |
Mean ± SD; VH – V. harveyi; VA – V. alginolyticus; VP – V. parahaemolyticus; PD – Ph. damsela.
Table 2.
Percent survival index of medicated shrimps in first dose titration trial.
| Treatment regimens (mg kg−1) | Percent survival index |
|||
|---|---|---|---|---|
| VH | VA | VP | PD | |
| 285 | 18 | 17 | 17 | 11 |
| 575 | 43 | 35 | 34 | 29 |
| 850 | 65 | 62 | 67 | 60 |
| 1150 | 65 | 53 | 56 | 59 |
VH – V. harveyi; VA – V. alginolyticus; VP – V. parahaemolyticus; PD – Ph. damesla.
In the second dose titration study, the preferred dose levels of medicated feed were administered at 850 and 1150 mg kg−1 biomass for three weeks. The results indicate that treatment with both dose levels of medicated feed was an effective means of reducing the prevalence of infection due to Vibriosis. The oral administration of Asparagopsis extract incorporated medicated feed at 850 and 1150 mg kg−1 dose levels evidently increased the percent survival index of shrimp against the Vibrio infection suggesting that medicated feed had efficient and desirable influence on resistance of P. monodon to Vibriosis. Both dosage regimens are slightly different in terms of percent survival index pattern (Table 4). Regarding the percentage of infection, the moderate dose level of 850 mg kg−1 day−1 was significantly more efficacious than 1150 mg kg−1 day−1 treated group (Table 3). The shrimps treated with 850 mg kg−1 of Asparagopsis extract and following challenge with V. alginolyticus, V. parahaemolyticus and Ph. damsela displayed less than 20% of mild infection, whereas V. harveyi caused 31% infection. The severity of infection in the 850 mg kg−1 treated group was mild. The infected shrimp exhibited feeble movement and anorexia during the first three to four days of infection and recovered after ecdysis. It was notable that the proliferation of shrimp Vibrios to cause mass mortality in the host was considerably prevented in the shrimp treated with 850 mg kg−1 Asparagopsis extract. However, the degree of morbidity in 1150 mg kg−1 treated groups was in the range of 39–45% against V. alginolyticus, V. parahaemolyticus and Ph. damsela and 51% against V. harveyi. The infected shrimps were lethargic and anorectic during the post challenge period. In infected shrimps under medication, the molting process was regained within 15 days of treatment.
Table 4.
Percent survival index of medicated shrimps in second dose titration trial.
| Treatment regimens (mg kg−1) | Percent survival index |
|||
|---|---|---|---|---|
| VH | VA | VP | PD | |
| 850 | 68 | 65 | 68 | 69 |
| 1150 | 64 | 55 | 64 | 61 |
VH – V. harveyi; VA – V. alginolyticus; VP – V. parahaemolyticus; PD – Ph. damsela.
Table 3.
Second dose titration trial of control and medicated shrimps received 850 and 1150 mg kg−1 of dose levels for 21 days and subsequently challenged (ca.105 CFU shrimp−1) with respective Vibrio spp.
| Treatment regimens (mg kg−1) | Mean % infection (I) and mortality (M) |
|||||||
|---|---|---|---|---|---|---|---|---|
| VH |
VA |
VP |
PD |
|||||
| %I | %M | %I | %M | %I | %M | %I | %M | |
| 850 | 31 ± 2.3 | 32 ± 2.5 | 18 ± 2.6 | 21 ± 2.5 | 16 ± 3.9 | 20 ± 2.8 | 19 ± 3.5 | 21 ± 2.2 |
| 1150 | 51 ± 3.5 | 36 ± 2.2 | 40 ± 2.9 | 27 ± 2.8 | 45 ± 4.2 | 23 ± 3.5 | 39 ± 3.4 | 26 ± 3.1 |
| +Control | 100 | 100 | 100 | 61 ± 1.5 | 100 | 64 ± 1.8 | 100 | 68 ± 2.2 |
| – Control | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 |
Mean ± SD; VH – V. harveyi; VA – V. alginolyticus; VP – V. parahaemolyticus; PD – Ph. damsela.
3.2. Effect of treatment duration on the survival and infection
In time titration study, group IV type of treatment regimen significantly reduces pathological signs and mortality (Table 5). Indeed, there was a trend for lower mortality and lesser pathological signs. Although spontaneous recoveries do not occur, infection may continue for four to five days and lessened completely after the 14th day of treatment. The rate of mortality was also diminished dramatically to 13.25% (10–17%). The degree of infection significantly increased in groups (I and III) fed with normal feed after challenge experiment. This may be attributed to the colonization of Vibrios due to the discontinuation of medicated feed. It is assumed that, cessation of medicated feed after challenge experiment provided ample opportunity for surviving Vibrios to multiply. The study revealed that the treatment for shrimps should always be at an optimum protective dose of 850 mg kg−1 biomass day−1 for four weeks and has to be fed for a minimum of two weeks even if it appears to have recovered from the infection. Therefore, feeding lower dose of medicated feed or decreasing the number of days (28 days of prophylatic and 14 days of therapeutic intervals) can permit the bacterial pathogens to resurface.
Table 5.
Efficacy of medicated feed on protection and survival of experimentally infected P. monodon.
| Treatment regimens | Mean % infection (I) and mortality (M) |
|||||||
|---|---|---|---|---|---|---|---|---|
| VH |
VA |
VP |
PD |
|||||
| I% | M% | I% | M% | I% | M% | I% | M% | |
| Group I | 36 ± 2.5 | 38 ± 2.1 | 27 ± 5.3 | 31 ± 1.2 | 29 ± 5.7 | 26 ± 2.2 | 34 ± 1.8 | 28 ± 1.9 |
| Group II | 35 ± 3.4 | 34 ± 1.7 | 28 ± 2.7 | 25 ± 1.6 | 20 ± 3.1 | 18 ± 2.1 | 25 ± 1.5 | 22 ± 3.1 |
| Group III | 31 ± 4.7 | 25 ± 1.4 | 25 ± 4.2 | 22 ± 2.5 | 22 ± 4.3 | 21 ± 3.5 | 21 ± 3.5 | 19 ± 1.5 |
| Group IV | 26 ± 3.1 | 17 ± 2.2 | 23 ± 5.5 | 14 ± 1.9 | 22 ± 2.8 | 10 ± 1.5 | 20 ± 2.3 | 12 ± 4.2 |
| +Control | 100 | 100 | 100 | 64 ± 1.5 | 100 | 61 ± 1.5 | 100 | 62 ± 3.1 |
| –Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
VH – V. harveyi; VA – V. alginolyticus; VP – V. parahaemolyticus; PD – Ph. damesla.
3.3. In vivo confirmatory experiments on shrimp disease control using effective dose of algal extract
The results of efficacy validation envisaged that, shrimp fed with 850 mg kg−1 medicated feed experienced a significant increase in the percent survival index relative to control groups (Table 6). The survival rate of treated and control shrimps against various Vibrio pathogens is depicted in Figs. 1–4. The mortality rate of medicated shrimp challenged with single culture of pathogens viz., V. harveyi, V. alginolyticus, V. parahaemolyticus and Ph. damsela were 19.8 ± 1.5%, 15.7 ± 1.3%, 10.6 ± 2.5% and 11.2 ± 2.2%, resulting in percent survival index of 79.6 ± 1.5, 74.6 ± 1.3, 83.5 ± 2.5 and 80.9 ± 2.2, respectively, whereas in the control group, the mortality rate was 97.3 ± 2.2, 62.3 ± 1.8% 64.6 ± 1.4% and 58.7 ± 2.5%. The clinical signs and mortalities of control group are consistent with the earlier pathogenicity studies. In treated shrimps, mortality began on the 48th hour after exposure to V. harveyi and continued for approximately 24 h. The onset of mortality was after 72 h in V. alginolyticus, V. parahaemolyticus and Ph. damsela injected groups. Half of the control shrimps died 48 h after inoculation with V. harveyi and mortality raised above 80% in 24 h and after that.
Table 6.
Efficacy of Asparagopsis extract incorporated feed (850 mg kg–1) and PSI of P. monodon towards shrimp pathogens.
| Pathogenic Vibrio spp. | Dose (CFU shrimp−1) | Cumulative mortality within 15 days (%) |
PSI | % Infection in the treated shrimp | |
|---|---|---|---|---|---|
| Control (n = 10) | Treated⁎ (n = 50) | ||||
| V. harveyi | 105 | 97.3 ± 2.2 | 19.8 ± 1.5 | 79.6 | 25 ± 4.5 |
| V. alginolyticus | 105 | 62.3 ± 1.8 | 15.7 ± 1.3 | 74.7 | 18 ± 3.2 |
| V. parahaemolyticus | 105 | 64.6 ± 1.4 | 10.6 ± 2.5 | 83.5 | 15 ± 2.8 |
| Ph. damsela | 105 | 58.7 ± 2.5 | 11.2 ± 2.2 | 80.9 | 17 ± 3.1 |
| V. harveyi + V. alginolyticus | 5 × 102 + 5 × 102 | 98.5 ± 1.4 | 35.8 ± 1.4 | 63.6 | 45 ± 4.8 |
| V. harveyi + V. parahaemolyticus | 5 × 102 + 5 × 102 | 95.6 ± 2.3 | 31.3 ± 2.5 | 67.2 | 38 ± 5.2 |
| V. harveyi + Ph. damsela | 5 × 102 + 5 × 102 | 94.2 ± 1.8 | 32.1 ± 1.7 | 65.9 | 41 ± 3.5 |
Mean ± SD; n= No of shrimps used for the experiment.
Figure 1.
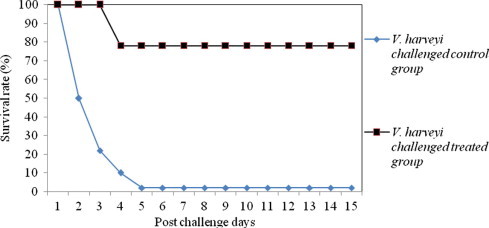
Survival rate of medicated and non-medicated P. monodon challenged with single culture of V. harveyi.
Figure 2.
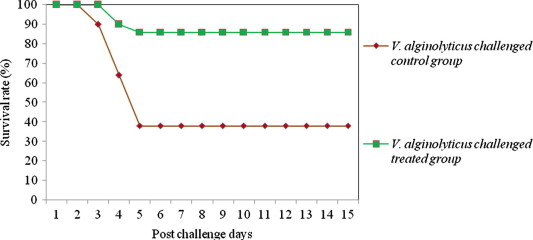
Survival rate of medicated and non-medicated P. monodon challenged with single culture of V. alginolyticus.
Figure 3.
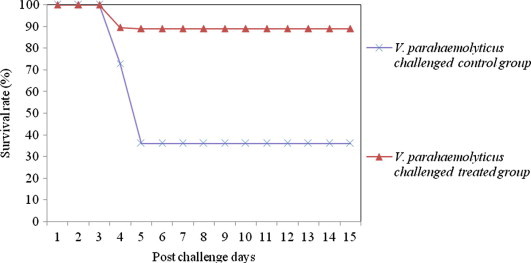
Survival rate of medicated and non-medicated P. monodon challenged with single culture of V. parahaemolyticus.
Figure 4.
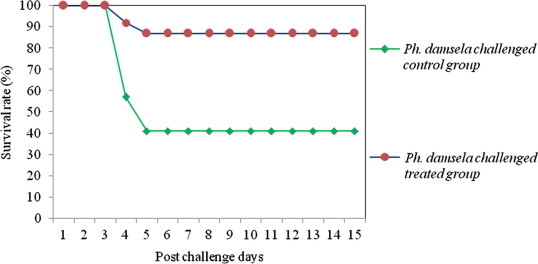
Survival rate of medicated and non-medicated P. monodon challenged with single culture of Ph. damsela.
Challenged animals began to exhibit pathological signs 24 h after injection. The degree of infection was below 25% in V. harveyi inoculated groups whereas, V. alginolyticus, V. parahaemolyticus and Ph. damsela caused less than 18% of infection. Duration of infection ranged from three to six days or more. Following ten days of medicated feed treatment, the pathological signs were averaged for each animal for everyday. The higher degree of percent survival index and lower degree of morbidities could be an indication of improved health status of shrimp fed with this medicated feed.
The shrimps challenged with mixed culture of pathogen produced more infection or mortality in the treated group than those caused by the single culture of pathogen (Figs. 5–7). The percent survival indices of shrimps challenged with mixed culture of pathogens (V. harveyi + V. alginolyticus; V. harveyi + V. parahaemolyticus; V. harveyi + Ph. damsela) were 63.6 ± 1.4, 67.2 ± 2.5, 65.9 ± 1.7, respectively. The onset of the mortality was after 24 h in treated groups and reached ca. 35% in 48 h. While in control groups the mortality began after 24 h and scaled up to 90% on 72 h of post challenge. The shrimps injected with the mixture of Vibrio culture exhibited an average 41.3% of infection. The severity of infection was higher during the 72 h of post challenge in survivor shrimp. The closed observation of infected shrimps revealed several clinical symptoms of Vibriosis such as discoloration (dark spot), atypical swimming, empty digestive tract and aversion to feed. These symptoms were completely recovered after twelve to fourteen days of medicated treatment. At the end of the experiment, majority of the shrimps exhibited the same external signs of health like quick movements, bright color and lack of disease as it was in the beginning. The shrimps that survived the Vibrio challenge or that recovered after medication molted at a rate analogous to that of the unchallenged shrimps. Accordingly, the same dosage regimen is often pragmatically recommended for the management of single and mixed culture of Vibrio challenge.
Figure 5.
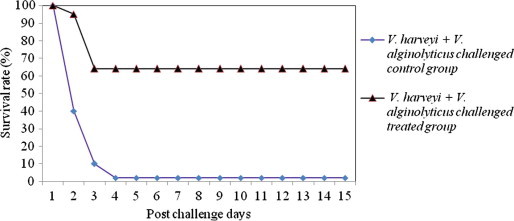
Survival rate of medicated and non-medicated P. monodon challenged with mixed culture of V. harveyi + V. alginolyticus.
Figure 6.
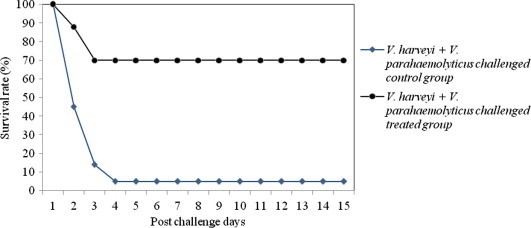
Survival rate of medicated and non-medicated P. monodon challenged with mixed culture of V. harveyi + V. parahaemolyticus.
Figure 7.
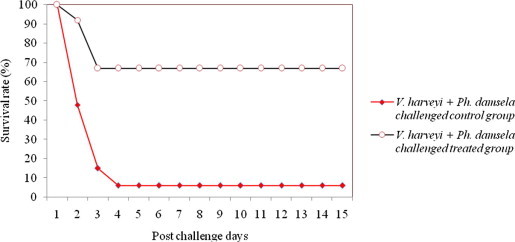
Survival rate of medicated and non-medicated P. monodon challenged with mixed culture of V. harveyi + Ph. damsela.
3.4. Bacteriological enumeration
Bacterial vegetation densities were determined in medicated and untreated controls at the beginning and the end of the experiment. Regarding the Vibrio count in shrimps challenged with single species of Vibrio culture, the rapid proliferation of Vibrio was successfully arrested in the treated group relative to the control. The Vibrio count was initially high in treated groups and further decreased as the experiment progressed. Mean bacterial densities for medicated shrimp at initiation of post challenge treatment was 8.2 × 104. Following 14 days of treatment with medicated feed, proliferation of Vibrio spp. was suppressed in treated shrimps. The density of Vibrios declined steadily from 105 to 101 CFU mL−1 in the majority of medicated shrimps that underwent ecdysis. In contrast, the Vibrio count in control groups remained steady toward the end of the experiment. On the other hand, shrimps injected with NS (negative control) showed few numbers of Vibrio spp. in hepatopancreas. The presence of bacteria in the negative control group may be explained by the entrance of microorganisms from the water through the wound caused by the injection. There was no significant difference in Vibrio count among the four different challenged control groups. The mean bacterial density for survivor shrimp ranged from 1.38 × 101 to 4.23 × 101 CFU mL–1 on the 16th day of post challenge period.
Results of microbiological examination of specimens challenged with mixed culture of Vibrio revealed that there was a gradual reduction in the Vibrio count of treated groups relative to the control group. The mean bacterial density in treated group was ca. 101 and 105 CFU mL−1 in the untreated control group on the 16th day of microbiological examination. In the medicated shrimp challenged with V. harveyi + V. alginolyticus, the bacterial density was 8.3 ± 0.5 × 104 in the 1st day of the experiment, which reduced to 4.2 ± 0.2 × 101 CFU mL–1 on the 16th day of experiment, whereas in the control this value remained steady during the post challenge days. The Vibrio count was significantly reduced in the specimens that underwent ecdysis. The medicated groups challenged with V. harveyi + V. parahaemolyticus and V. harveyi + Ph. damsela also exhibited similar pattern of reduction in Vibrio count during the end of the experiment.
4. Discussion
4.1. In vivo dose titration trials
The results of this preliminary dose titration trial suggest that the treatment with moderate dose level of medicated feed increases or confers considerable disease resistance in P. monodon. This study also revealed that the higher doses of algal extract may not be beneficial to animal performance and the lower doses did not influence the Vibrio count after infection. Based on these results, moderate dose levels (850 and 1150 mg kg−1) exhibiting highest efficacy was preferred for subsequent experiments.
The efficacy of algal metabolites on the survival of shrimp depends on the source of algae, concentration of algal metabolites, administration route and exposure time. In the second dose titration study, 850 mg kg−1 treatment was shown to be more potent than the 1150 mg kg−1. This may be due to the difference in the content of extract in two preparations which in turn influence the feed intake and efficacy. It probably concluded that the beneficial effect of Asparagopsis extract incorporated feed on disease resistance of P. monodon is dose-dependent and 850 mg kg−1 is an optimal dose after three weeks of feeding against Vibrio infection. The optimal dosage rate and administration schedule are integral agents governing the effectiveness of a drug, therefore further experiments are obviously warranted to determine the duration of feeding regime.
Vibrios are continued to be a significant cause of morbidity and mortality in shrimp aquaculture, and they are frequent isolates causing Vibriosis. The multi-resistance of these isolates plays an important role in the colonization or infection of P. monodon. The application of algal metabolites for the management of infectious pathogens of P. monodon was reported to be most effective by different workers (Selvin et al., 2009; Jose et al., 2008). The results obtained in the present study confirm preliminary evaluation by Selvin et al. (2011) that Ulva diet is effective against Vibrio infestation in P. monodon. The results of the present study extend previous observations on the in vivo efficacy of microalgae Tetraselmis suecica for the control of Vibrio spp. in a commercial shrimp hatchery of the white prawn F. indicus (Regunathan and Wesley, 2004). Immanuel et al. (2004) demonstrated that herbal and algal extracts may be effectively used as a dietary source to enhance the disease resistance as well as to have better survival and production of P. indicus in aquaculture systems. Banana shrimp F. merguiensis fed with S. platensis showed resistance against V. harveyi infection (Lee et al., 2003). L. vannamei treated with hot-water extract of Gracilaria tenuistipitata via injection displayed resistance against V. alginolyticus (Hou and Chen, 2005). Leano et al. (2007) reported the efficacy of stevia extract in enhancing disease resistance of juvenile P. monodon against V. harveyi. According to Yeh et al. (2009) treatment with twig hot-water extract from Cinnamomum kanehirae can enhance disease resistance of white shrimp to V. alginolyticus. Tayag et al. (2010) reported that L. vannamei that received extract of Spirulina platensis showed increased resistance against V. alginolyticus. Recently, Manilal et al. (2012) envisaged the effect of Acrosiphonia orientalis incorporated medicated feed on the survival rate of P. monodon against V. harveyi and V. alginolyticus infection.
4.2. Effect of treatment duration on the survival and infection
One of the important parameters to be determined for the development of rational dose regimen is the optimal duration of administration. A period of 28 days of prophylactic medication followed by 14 days of post challenge therapy was sufficient to manage the severity and resurgence of infection to a great extent. This treatment regimen was significantly superior to other treatment in reducing the degree of morbidity. The reason for this efficacy may be related to prompt treatment with effective dosage of medicated feed which prevents bacterial re-growth even after the challenge experiment. Manilal et al. (2012) reported that prophylactic treatment with 1200 mg kg–1 of A. orientalis extract for a period of 28 days and subsequent two weeks of post challenge therapy was an effective means of controlling vibriosis in P. monodon. Similarly, P. monodon following 15 days feeding on Ulva diet at a dose level of 1000 mg kg−1 enhanced its survival rate against vibrio infection (Selvin et al., 2011). Pholdaeng and Pongsamart (2010) reported that P. monodon pre-fed with Durio zibethinus supplemented diet showed higher disease resistance with both viral and bacterial pathogens than in the control group.
4.3. In vivo confirmatory experiments on shrimp disease control using effective dose of algal extract
The confirmatory experiment was aimed to optimize the dose level of Asparagopsis extract required by experimentally infected P. monodon for survival. Therefore, 850 mg of Asparagopsis extract incorporated feed with optimal feeding schedule which exhibited higher efficacy in dose/time titration experiment was preferred. In general, the percent survival index of shrimps challenged with single and mixed culture of pathogens was significantly higher than that of control groups. It has been reported that hot-water extract of red alga Gelidium amansii increased the resistance of L. vannamei against V. alginolyticus (Fu et al., 2007). Similarly, Yeh and Chen (2009) showed that survival rates of shrimp immersed in hot-water extract of Gracilaria tenuistipitata were significantly higher than those of control shrimp. In addition, G. tenuistipitata also had a beneficial effect on the prevention and treatment for Vibrio disease in L. vannamei (Hou and Chen, 2005).
The confirmatory study revealed that prompt and effective administration of effective dose level of medicated feed eradicated the Vibrios from experimentally challenged animals. In the present study, survival rate and degree of infection in effective dose treated shrimp varied in the single and mixed pathogenic exposure. The reason for the difference in the efficacy of the effective dose related to different factors such as the route of pathogenic challenge, virulence of the pathogen, environmental and physiological factors affecting both pathogens and hosts. The medicated feed rationalized with 850 mg kg–1of Asparagopsis extract was manifold. This effective dose level was found to be optimal for the control of four pathogenic Vibrio species. In addition, the experiment proves that the same feeding regimen is enough for the management of single and mixed culture of Vibrio infection in P. monodon. In the present study, bacterial concentration in the experimentally infected medicated shrimp subsequently reduced upon treatment.
The oral administration with medicated feed provided complete recovery of clinical signs in the experimentally infected shrimp in treatment. The ability of medicated feed to reduce the Vibrio load in the treated shrimp could be due to the antibacterial effect of Asparagopsis sp. (Manilal et al., 2009a). The increased disease resistance in P. monodon fed algal-based medicated feed after pathogen challenge is in line with other studies (Selvin et al., 2009, 2011; Manilal et al., 2012). The protective and therapeutic effect of Asparagopsis extract in defending shrimps from infections could be attributed due to the presence of halogenated compounds (Paul et al., 2006). It has been claimed that the antibacterial effect of Asparagopsis sp. has a broad spectrum (Manilal et al., 2009a).
5. Conclusion
The crude extract of the marine red algae species of Asparagopsis was rationalized with commercial shrimp feed and orally administered for different duration of time followed by the artificial bacterial challenge experiment. In dose titration experiments, the oral administration of Asparagopsis sp. at a dosage of 850 mg kg–1 of biomass was highly efficacious in the treatment of natural infestations of Vibriosis in P. monodon. The results of the confirmatory dose experiment revealed that the prophylactic treatment with moderate dose of 850 mg kg–1 of biomass day–1 for four weeks followed by fourteen days of post infection therapy was highly effective in controlling Vibrio infection in shrimps. Based on the present findings, it could be inferred that the secondary metabolites of Asparagopsis sp. may be an excellent source for developing potent and cost effective formulations for sustainable shrimp farming. In addition to this, it is necessary to identify the exact lineage of Asparagopsis sp. from Indian littoral by molecular characterization. Therefore, further investigations both with the aim of identifying the taxonomic lineage and to evaluate the mechanism of action of algal extract on shrimp immunity are warranted.
Acknowledgment
The author Aseer Manilal is gratefully acknowledged to Council of Scientific and Industrial Research for providing Senior Research Fellowship. (File No. 09/475 (0149)/2010-EMR-I).
References
- Abutbul S., Golan-Goldhirsh A., Barazani O., Zilberg D. Use of Rosmarinus officinalis as a treatment against Streptococcus iniae in tilapia (Oreochromis sp.) Aquaculture. 2004;238:97–105. [Google Scholar]
- Auro de Ocampo A., Jimenez E.M. Herbal medicines in the treatment of fish diseases in Mexico. Vet. Mex. 1993;24:291–295. [Google Scholar]
- Bansemir A., Blume M., Schröder S., Lindequist U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture. 2006;252:79–84. [Google Scholar]
- Cano-Gomez A., Høj L., Owens L., Andreakis N. Multilocus sequence analysis provides basis for fast and reliable identification of Vibrio harveyi-related species and reveals previous misidentification of important marine pathogens. Syst. Appl. Microbiol. 2011;34(8):561–565. doi: 10.1016/j.syapm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Castro S.B.R., Leal C.A.G., Freire F.R., Carvalho D.A., Oliveira D.F., Figueiredo H.C.P. Antibacterial activity of plant extracts from Brazil against fish pathogenic bacteria. Braz. J. Microbiol. 2008;39:756–760. doi: 10.1590/S1517-838220080004000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotigeat W., Tongsupa S., Supamataya K., Phongdara A. Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture. 2004;233:23–30. [Google Scholar]
- Chythanya R., Karunasagar I., Karunasagar I. Inhibition of shrimp pathogenic vibrios by a marine Pseudomonas I-2 strain. Aquaculture. 2002;208:1–10. [Google Scholar]
- Das B.K., Mukherjee S.C., Sahu B.B., Murjani G. Neem (Azadirachta indica) extract as an antibacterial agent against fish pathogenic bacteria. Indian J. Exp. Biol. 1999;37(11):1097–1100. [PubMed] [Google Scholar]
- Fu Y.W., Hou W.Y., Yeh S.T., Li C.H., Chen J.C. The immunostimulatory effects of hot-water extract of Gelidium amansii via immersion, injection and dietary administrations on white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2007;22(6):673–685. doi: 10.1016/j.fsi.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Gatesoupe F.J.E., Arakawa T., Watanabe T. The effect of bacterial additives on the production rate and dietary value of rotifers as food for Japanese flounder, Paralichthys olivaceus. Aquaculture. 1989;83(2):39–44. [Google Scholar]
- Hameed A.S.S., Rahaman K.H., Alagan A., Yoganandhan K. Antibiotic resistance in bacteria isolated from hatchery-reared larvae and post-larvae of Macrobrachium rosenbergii. Aquaculture. 2003;217(3):39–48. [Google Scholar]
- Hou W.Y., Chen J.C. The immunostimulatory effect of hot-water extract of Gracilaria tenuistipitata on the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2005;19(2):127–138. doi: 10.1016/j.fsi.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhou H., Zhang H. The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 2006;20:750–757. doi: 10.1016/j.fsi.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Immanuel G., Vincybai V.C., Sivaram V., Palavesam A., Marian M.P. Effect of butanolic extracts from terrestrial herbs and seaweeds on the survival, growth and pathogens (Vibrio parahaemolyticus) load on shrimp Penaeus indicus juveniles. Aquaculture. 2004;236(1–4):53–65. [Google Scholar]
- Jose J.J., Lipton A.P., Subhash S.K. Impact of marine secondary metabolites (MSM) from Hypnea musciformis as an immunostimulant on hemogram count and Vibrio alginolyticus infection in the prawn, Penaeus monodon, at different salinities. Isr. J. Aquacult-Bamid. 2008;60(1):65–69. [Google Scholar]
- Kanjana K., Radtanatip T., Asuvapongpatana S., Withyachumnarnkul B., Wongprasert K. Solvent extracts of the red seaweed Gracilaria fisheri prevent Vibrio harveyi infections in the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 2011;30(1):389–396. doi: 10.1016/j.fsi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Karunasagar I., Pai R., Malathi G.R., Karunasagar I. Mass mortality of Penaeus monodon larvae due to antibiotic resistant Vibrio harveyi infection. Aquaculture. 1994;128:203–209. [Google Scholar]
- Kromna V., Wachirapakorn C., Luangthongkum P., Juntarasanit W., Soicome C. The effect of Psidium guajava Linn. leaves treatment on diarrhea in diary calves. Khonkhan Univ. Res. J. 2006;6:38–47. [Google Scholar]
- Leano E.M., Xi Y.C., Liao I.C. Effects of Stevia extract on growth, non-specific immune response and disease resistance of grass prawn, Penaeus monodon (Fabricious), juveniles. J. Fish. Soc. Taiwan. 2007;34:165–175. [Google Scholar]
- Lee Y.K., Chew P.F., Soh B.S., Tham L.Y. Enhancing phagocytic activity of hemocytes and disease resistance in the prawn Penaeus merguieneis by feeding Spirulina platensis. J. Appl. Phycol. 2003;15:279–287. [Google Scholar]
- Lipton A.P., Pramitha V.S., Jose J.J. Marine secondary metabolites (MSM) from macro algae enhance bacterial clearance in hemolymph of Penaeus monodon. Isr. J. Aquacult-Bamid. 2009;61(1):42–47. [Google Scholar]
- Loomis G.A. third ed. Lea and Febiger; Philadelphia: 1978. Essentials of Toxicology. p. 241. [Google Scholar]
- Manilal A., Sujith S., Selvin J., Seghal Kiran G., Shakir C. In vivo antiviral activity of polysaccharide from the Indian green alga, Acrosiphonia orientalis (J. Agardh): potential implication in shrimp disease management. World J. Fish Mar. Sci. 2009;1(4):278–282. [Google Scholar]
- Manilal A., Sujith S., Kiran G.S., Selvin J., Shakir C., Gandhimathi R., Lipton A.P. Antimicrobial potential and seasonality of red algae collected from southwest coast of India tested against shrimp, human and phytopathogens. Ann. Microbiol. 2009;59(2):207–219. [Google Scholar]
- Manilal A., Sujith S., Sabarathnam B., Shakir C., Kiran G.S., Lipton A.P. Bioactivity of the red algae Asparagopsis taxiformis collected from the Southwestern coast of India. Braz. J. Oceangr. 2010;58(2):93–100. [Google Scholar]
- Manilal A., Sujith S., Selvin J., Seghal Kiran G., Shakir C., Gandhimathi R. Virulence of vibrios isolated from diseased black tiger shrimp Penaeus monodon Fabricious. J. World Aquacult. Soc. 2010;41(3):332–343. [Google Scholar]
- Manilal, A., 2011. Bioactive halogenated derivatives from the marine red algae, Asparagopsis taxiformis: formulation and development of shrimp therapeutics. Ph.D. Thesis, Bharathidasan University, Tiruchirapalli, India, p. 129.
- Manilal A., Selvin J., Sujith S., Panikkar M.V.N. Evaluation of therapeutic efficacy of Indian green alga, Acrosiphonia orientalis (J. Agardh) in the treatment of vibriosis in Penaeus monodon. Thalassas Int. J. Mar. Sci. 2012;28(1):33–46. [Google Scholar]
- Mtambo M.M.A., Mushi E.J., Kinabo L.D.B., MaedaMachang A., Mwamengele G.L.M., Yongolo M.G.S., Temu R.P.C. Evaluation of the efficacy of the crude extracts of Capsicum frutescens, Citrus limon and Opuntia vulgalis against Newcastle disease in domestic fowl in Tanzania. J. Ethnopharmacol. 1999;68:51–61. doi: 10.1016/s0378-8741(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Pachanawan A., Phumkhachorn P., Rattanachaikunsopon P. Potential of Psidium guajava supplemented fish diets in controlling Aeromonas hydrophila infection in tilapia (Oreochromis niloticus) J. Biosci. Bioeng. 2008;106(5):419–424. doi: 10.1263/jbb.106.419. [DOI] [PubMed] [Google Scholar]
- Paul N.A., de Nys R., Steinberg P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function. Mar. Ecol. Prog. Ser. 2006;306:87–101. [Google Scholar]
- Pholdaeng K., Pongsamart S. Studies on the immuno-modulatory effect of polysaccharide gel extracted from Durio zibethinus in Penaeus monodon shrimp against Vibrio harveyi and WSSV. Fish Shellfish Immunol. 2010;28(4):555–561. doi: 10.1016/j.fsi.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Regunathan C., Wesley S.G. Control of Vibrio spp. in shrimp hatcheries using the green algae, Tetraselmis suecica. Asian Fish. Sci. 2004;17:147–158. [Google Scholar]
- Roch P. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture. 1999;172:125–145. [Google Scholar]
- Selvin J., Ninawe A.S., Lipton A.P. ANE Publishers; New Delhi, India: 2009. Shrimp Disease Management: Prospective Approaches. [Google Scholar]
- Selvin J., Manilal A., Sujith S., Kiran G.S., Lipton A.P. Efficacy of marine green alga Ulva fasciata extract on the management of shrimp bacterial diseases. Lat. Am. J. Aquat. Res. 2011;39(2):197–204. [Google Scholar]
- Tayag C.M., Lin Y.C., Li C.C., Liou C.H., Chen J.C. Administration of the hot-water extract of Spirulina platensis enhanced the immune response of white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2010;28(5–6):764–773. doi: 10.1016/j.fsi.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Tukmechil A., Ownagh A., Mohebbat A. In vitro antibacterial activities of ethanol extract of Iranian propolis (eeip) against fish pathogenic bacteria (Aeromonas hydrophila, Yersinia ruckeri and Streptococcus iniae) Braz. J. Microbiol. 2010;41:1086–1092. doi: 10.1590/S1517-838220100004000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvrouw M., De Clercq E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Phamacol. 1997;29:497–511. doi: 10.1016/s0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- Xu D., Rogers W.A. Leaching loss from oxytetracycline medicated feeds. J. Appl. Aquacul. 1994;4(1):29–39. [Google Scholar]
- Yeh R.Y., Shiu Y.L., Shei S.C., Cheng S.C., Huang S.Y., Lin J.C., Liu C.H. Evaluation of the antibacterial activity of leaf and twig extracts of stout camphor tree, Cinnamomum kanehirae, and the effects on immunity and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2009;27:26–32. doi: 10.1016/j.fsi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Yeh S.T., Chen J.C. White shrimp Litopenaeus vannamei that received the hot-water extract of Gracilaria tenuistipitata showed earlier recovery in immunity after a Vibrio alginolyticus injection. Fish Shellfish Immunol. 2009;26(5):724–730. doi: 10.1016/j.fsi.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Yeh S.T., Lee C.S., Chen J.C. Administration of hot-water extract of brown seaweed Sargassum duplicatum via immersion and injection enhances the immune resistance of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2006;20(3):332–345. doi: 10.1016/j.fsi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Yeh S.T., Lin Y.C., Huang C.L., Chen J.C. White shrimp Litopenaeus vannamei that received the hot-water extract of Gracilaria tenuistipitata showed protective innate immunity and up-regulation of gene expressions after low-salinity stress. Fish Shellfish Immunol. 2010;28(5–6):887–894. doi: 10.1016/j.fsi.2010.02.005. [DOI] [PubMed] [Google Scholar]


