Abstract
Heat stress, when combined with drought, is one of the major limitations to food production worldwide, especially in areas that use rainfed agriculture. As the world population continues to grow, and water resources for the crop production decline and temperature increases, so the development of heat- and drought-tolerant cultivars is an issue of global concern. In this context, four barley and two wheat genotypes were evaluated in south-eastern Russia to identify heat- and drought-tolerant genotypes for future breeding programmes by identifying suitable sowing times for specific genotypes. High temperature stress, when combined with drought during late sowing, decreased the days to visible awns, days to heading and days to ripe harvest, finally negatively affecting the growth and development of plants and resulting in a lower plant population m−2, tillers plant−1, plant height and dry matter production m−2. On the other hand, low temperature in combination with early sowing increased the number of days to germination, reduced seedling stand establishment and tillering capacity, finally affecting the growth and development of the crops. Compared to overall performance and optimum sowing date, barley genotypes ‘Zernograd.770’ and ‘Nutans’, and wheat genotype ‘Line4’ performed best in both late (high temperature with drought) and early (low temperature) stress conditions.
Keywords: High temperature, Drought, Spring wheat, Barley, Russia
1. Introduction
Agriculture in Russia has always had to contend with unfavourable climates. For centuries, farming was concentrated near the populated areas of European Russia where crop yields were limited by short growing seasons. At the end of the 18th century the need to feed a growing population finally led to the expansion of cropland into the southern steppe region where better soils (e.g. Chernozems, Greyzems, Phaeozems and Kastanozems) and a warmer climate provided higher crop yields than in the traditional agricultural regions in the north (Alcamo et al., 2007).
The agricultural production of Russia is six times smaller than that of the OECD (Organization for Economic Co-operation and Development), as reported by Izrael and Avdjushin (1997), who also reported that the yields of winter and spring wheat were rather low when compared to the world mean values (1.4 and 2.4 metric tones ha−1, respectively). Despite that report, Russia currently holds a leading position in the volume of wheat and barley production, ranked 3rd and 1st in terms of total production (MT) and price ($1000), respectively among the 20 wheat- and barley-producing countries of the world (FAOSTAT, 2011). On the other hand, the export share of the Russian wheat and barley market is 13–14% of total world export and main buyers of Russian wheat are Middle-Eastern and North African countries (15–20 million tons/year) such as Egypt, Turkey, Syria and Iran (Marubeni Corporation, 2010).
Under rainfed conditions, wheat and barley frequently suffer from drought resulting in a significant loss of yield (Trethowan and Pfeiffer, 1999; Hossain et al., 2012a) and decreased revenue: total losses due to drought and other natural disasters in 2000 alone amounted to 20 billion Rubles (US$ 800 million) (Albit, 2011). The USDA estimated a crop loss of 21% due to drought and heat stress over a period of 55 years from 1948 to 2002 in Russia (USDA–NASS, 2004).
Drought, the result of low precipitation or high temperature, is thus one of the main problems underlying the success of modern agriculture around the globe and is one of the most important environmental factors that affect the growth, development and production of plants (Hasanuzzaman et al., 2012; Hossain et al., 2012a). Drought is a non-uniform phenomenon that influences plants differently depending on the development stage at the time of its occurrence (Lopez et al., 2003; Martiniello and Teixeira da Silva, 2011; Hossain et al., 2012a).
For spring wheat and barley, an air temperature of about 20–25 °C is considered to be optimum for growth and development (Acevedo et al., 2002; Hakim et al., 2012; Hossain et al., 2012b–d). Optimal crop growth requires a non-limiting supply of water, nutrients, and radiation; as temperatures rise, the demand for growth resources increases due to higher rates of metabolism, development, and evapotranspiration (Rawson, 1988). When growth resources are limited by heat stress, the size of plant organs such as leaves, tillers, and spikes, is reduced (Martiniello and Teixeira da Silva, 2011; Hossain et al., 2012b–d). The apparent sensitivity of metabolic processes to heat stress in the field (Reynolds et al., 2000), coupled with the reduced length of the life cycle at high temperatures (Midmore et al., 1984; Hakim et al., 2012; Hossain et al., 2012b–d), results in low grain yield with lower total plant biomass in hot environments.
Several research findings also indicated that not only high temperature and drought have an effect on spring crops, but also that low temperature is one of the major constraints of late sowing in sub-tropical climates (Farooq et al., 2008; Hakim et al., 2012; Hossain et al., 2011, 2012b–d) and early sowing in temperate spring crops (Timmermans et al., 2007). Representative research findings related to high temperature, drought, low temperature stress and their effect on different wheat and barley cultivars in different countries around the world are presented in Table 1.
Table 1.
Relevant studies related to heat, drought and low temperature stress in different countries around the world (chronological order); all cultivar names in single inverted commas. Barley = italics entries.
| Country | Tested cultivars | Main research findings | Reference |
|---|---|---|---|
| Denmark | Tested 2,255 Mexican wheat landraces | Landraces were evaluated for traits associated with heat tolerance: canopy temperature depression (LCC), and 1000-kernel weight. Three landrace cultivars with superior and consistent LCC values were identified. These accessions are potentially useful sources for improving heat tolerance in cultivated wheat | Hede et al. (1999) |
| Australia | Wheat ‘Lyallpur’ | Despite favourable day/night temperature (18/13 °C), drought reduced kernel dry weight at anthesis | Wardlow (2002) |
| Sudan | Wheat ‘Debira’, ‘El Nelein’ and ‘Donki’ | A 2-year field study in two regions showed that ‘El Nelein’ performed best when sown late (air temp. 17–24 °C) | Ahmed et al. (2003) |
| China | Spring wheat ‘Ningchun18’ | Soil water deficit both during the middle vegetative stage (jointing) and the late reproductive stages (filling and maturity or filling) and no-soil–water-deficit both during the late vegetative stage (booting) and the early reproductive stage (heading) had the highest yield increase of 25.0% and 14.0% | Zhang et al. (2006) |
| Bahrain | Three barley cultivars ‘Rehani-3’, ‘SLB’ and ‘Rum’ | High temperature (27–33 °C) combined with water stress (−3 to −0.9 MPa) effect was the most pronounced than individual effect | Al-Karaki et al. (2007) |
| Egypt | Wheat ‘Sakha8’, ‘Sakha93’, ‘Sakha61’, ‘Chinese spring’ | Based on drought susceptibility index ‘Sakha8’ and ‘Sakha93’ were tolerant while ‘Sakha61’ and ‘Chinese spring’ were susceptible to drought | El-Fadly et al. (2007) |
| Argentina | Three seasons and at each season a wheat, barley and triticale were evaluated | Wheat, barley and one triticale cultivars were evaluated in three seasons under three thermal conditions: control and two timing of heating before anthesis; stem elongation stage was most sensitive to high temperature stress. Growth chamber temperatures were maintained at an average 5.5 °C higher than air temperature | Ugarte et al. (2007) |
| Slovak Republic | Barley ‘Kompakt’ | When stress was induced during shooting or earing, grain yields declined by >50% compared to optimal water regime | Krček et al. (2008) |
| Hungary | Wheat ‘GK-Elet’, ‘Mv-Emese’ | Pot culture experiment in growth chamber. ‘Mv-Emese’ had better drought stress tolerance than ‘GK-Elet’ | Lukacs et al. (2008) |
| Portugal | 4 Triticum genotypes: ‘Golia’, ‘Sever’, ‘Acalou’, ‘TE9306’ | After treated by heat stress (day/night 31/20 °C), analysis of plant samples showed that stress tolerant plant had more Fe and Mn than susceptible plants. Fe and Mn helped to overcome stress | Dias et al. (2009) |
| Iran | Wheat ‘Azar-2’, ‘Sardari’, ‘Frankia’, ‘Trakia’ | ‘Frankia’ performed better at various levels of terminal drought stress | Dalirie et al. (2010) |
| United Kingdom | Wheat ‘Damani’, ‘Gomal-8’, ‘Hashim-8’, ‘DN-73’, ‘Zam-04’, ‘Dera-98’ | Only ‘Hashim-8’ was drought tolerant | Khakwani et al. (2011) |
| Iran | 10 advanced spring barley cultivars ‘L1’ to ‘L10’ | Crop physiological status of plants was remarkably affected by terminal heat stress, which ultimately reduced grain yield. Overall, ‘L6’ and ‘L8’ were heat-tolerant | Bavei et al. (2011) |
| India | Wheat ‘HD2851, ‘HI8498’, ‘HDR77’, ‘PBW343’, ‘HD2936’ | Temperature (<18–20 °C air temperature) at reproductive stage caused sterility of pollen grains. ‘HD2851, ‘HI8498’and ‘HDR77’ were highly affected by low temperature (<15 °C). ‘PBW343’ and ‘HD2936’ were tolerant to air temperature at 11.6–15 °C | Chakrabarti et al., (2011) |
| Banglade-sh | 8 wheat cultivars ‘Sourav’, ‘Gourav’, ‘Shatabdi’, ‘Sufi’, ‘Bijoy’, ‘BARI-Gom-25’, ‘BARI Gom-26’ | Growth of early sown crop, and germination and grain-filling stages of late sown crops were highly affected by low air temperature and heat stress | Hossain et al. (2011) |
| Pakistan | 5 wheat cultivars: ‘TJ-83’, ‘Imdad-2005’, ‘Abadgar-93’ , ‘Moomal-2000’, ‘Mehran-89’ | ‘Moomal-2000’ and ‘Mehran- 89’ performed better at 20–30 °C (air temperature) heat stress. ‘TJ-83’, ‘Imdad-2005’ and ‘Abadgar-93’ were heat-sensitive | Buriro et al. (2011) |
| Saudi Arabia | 3 wheat cultivars: ‘KSU-105’, ‘KSU-106’, ‘Yecora Roja’ | ‘KSU-105’ performed better in late heat stress (25–30 °C). ‘KSU-106’ and ‘Yecora Roja’ were heat-sensitive | Refay (2011) |
| Russia | 8 wheat ‘Amir’, ‘Aestina’, ‘Zlata’, ‘Lada’, ‘Priokskaya’, ‘Ester’, ‘MIS’, ‘Yubileinaya’ | ‘Zlata’ was sensitive to low air temperature (−3 °C) and ‘Ester’ and ‘Yubileinaya’ were tolerant to low temperature | Karmanenko et al. (2011) |
| Jordan | 16 wheat ‘Hourani-27’, ‘Omguer-5’, ‘Genil-3’, ‘Stork’, ‘Korifla’, ‘Omrabi-5’, ‘Waha-1’, ‘Stojocri-3’, ‘Massara-1’, ‘Omsnima-1’, ‘Lagost-3’, ‘Heina’, ‘Ombar’, ‘Gersabil-2’, ‘Moulsabil-2, ‘Zeina-3 | Mediterranean adapted cultivars had long pre-heading periods, followed by short periods and high rates of grain filling to avoid terminal drought and high temperature (25–31 °C) stress. ‘Waha-1’, ‘Omrabi-5’, and ‘Massara-1’ performed best | Al-Karaki (2012) |
The Intergovernmental Panel on Climatic Change (IPCC) reported that global mean temperature is expected to rise 0.3 °C decade−1 (Jones et al., 1999) reaching approximately 1 and 3 °C above the present value by 2025 and 2100, respectively, and leading to further global warming (IPCC, 2007). Rising temperatures may lead to altered geographic distribution and growing season of agricultural crops by allowing the threshold temperature for the start of the season and crop maturity to be reached earlier (Porter, 2005). Based on 60 years of data, seasonal temperature around the world has been shown to be increasing every year (NASA, 2011). The FAO (2011), in February of 2011, issued an alert on drought in China stating that drought affected about 5.16 million ha of winter wheat. In 2010, drought damaged at least 10.3 million ha of crop land in Russia and wheat harvest was forecasted to fall to 50 million metric tons (FAO, 2010).
The development of stress-tolerant varieties and halophytic-like crops is a judicial way of mitigating the adverse effects of abiotic stresses (Ruan and Teixeira da Silva, 2011). However, the adverse effect of drought and high temperature on a crop can be minimized by avoiding stress at the most sensitive stages of crop development such as reproductive and grain-filling periods (Saini and Westgate, 2000; Hossain et al., 2012a). This is usually achieved by adjusting seeding date or by growing early-maturing varieties. However, as abiotic stresses are unpredictable, the best way to cope with them is to develop tolerant varieties that perform well under stress and under optimum environments (Prasad et al., 2008; Nouri et al., 2011; Hossain and Teixeira da Silva, 2012).
Considering that high temperature and drought strongly affect spring wheat and barley in southern European Russia and in other drought-prone regions of the world (Table 1), this study had, as its primary objective, to identify genotypes that would be most suitable for growth under early, optimal and late sowing dates under these harsh climates. Genotypes were assessed by observing variation at different phenological stages of plant development so as to better predict stand establishment of the crop populations, tiller production capacity and dry mater partitioning (i.e. yield). Such data would be valuable for future breeding programmes involving these select genotypes.
2. Materials and methods
The experiment was conducted in a research field of the Caspian Scientific Research Institute of Arid Agriculture (CSRIAA), Salt Zaymische, Chernoyarsky district, Astrakhan region, Russia, during the spring (April to July) of 2011.
2.1. Location of the experimental site
The Astrakhan region is located in the south-east of the East European Plain in the middle latitudes in the northern zone of a semi-desert and covers an area of 57,600 km2. The extreme northern point of the region lies on the border with the Volgograd region at 48° 52′ N (lat.). The western most point is located in Chernoyarsky district on the border with the Volgograd region at 44° 58′, where this experiment was conducted. The CSRIAA is situated in Salt Zaymische, Chernoyarsky district, Astrakhan region, Russia.
2.2. Treatments and experimental design
The experiment was laid out in a randomized complete block design (RCBD) with 3 replications. Treatments were three sowing dates viz., early sowing (ES) (sown on 8 April), optimum sowing (OS) (sown on 15 April) and late sowing (LS) (sown on 22 April) using four spring barley cultivars (‘Zernograd.770’, ‘Sokol’, ‘Nutans’, ‘Ratnik’) and two spring wheat cultivars (‘Saratov.70’ and ‘Line 4’). According to the CSRIAA (http://www.pniiaz.ru/), optimum sowing time for spring wheat and barley is a very narrow window in mid-April for this region, hence the sowing dates for ES, OS and LS. The unit plot size was 4 × 1 m wide with 6 rows and a 20-cm inter-row distance. The experiment was conducted under rainfed condition without irrigation or fertilizers.
2.3. Data collection
Development of wheat plants can be classified into three broad phases: the seed germination and seedling establishment phase, the vegetative phase, and the reproductive phase followed by maturity and ripening. Each developmental phase can be further classified into distinct growth stages. Even though there are several methods or scales to describe them such as Zadoke’s (0–94 stages), Acevedo et al. (2002) (G to GS3) and Feekes’ (1–11.4 stages) stage systems, Zadoke’s stages (0–94 stages) are very detailed while those of Acevedo et al. (2002) are usually categorized into E (germination to emergence), GS1 (emergence to double ridge), GS2 (double ridge to anthesis) and GS3 (anthesis to maturity). In this experiment, we used the Feeke’s stage (FS) system (Large, 1954). Phenological data on days to germination (FS 1.3), days to first visible awn (FS 10.1), days to heading (FS 10.5) and days to ripe harvest (FS 11.4), growth data on plant population m−2 (FS 1.3), number of tillers plant−1 (FS 3.0), and green biomass and dry biomass (both g m−2) (FS 10.5.2) were recorded. Soil moisture (%) and total moisture content (mm) were also noted (Table 2). Maximum, minimum and mean air temperature, the temperature at three soil depths (5, 10 and 15 cm), rainfall and, mean and minimum relative humidity (RH) were recorded daily during the experimental period from the information division of the CSRIAA website (http://rp5.ru/archive.php?wmo_id=34578&lang) (Table 3). Data were analysed using MSTAT-C (Russell, 1994). Treatment means were compared for significance by using the Least Significance Difference (LSD) test at P = 0.05.
Table 2.
Soil moisture (%) and total moisture content (mm) of experimental field soil in Chernoyarsky district, SE Russia.
| Soil horizon (cm) | First moisture data were taken immediately prior to all three sowing dates |
Second soil moisture data were measured at three Feeke’s stages (FS) [10.2 (heading completed), 10.1 (awn visible) and 10.0 (boot stage)] of early, optimum and late sowing |
||||||
|---|---|---|---|---|---|---|---|---|
| Early sowing (8 April) |
Optimum sowing (15 April) |
Late sowing (22 April) |
||||||
| % Soil moisture | Total moisture content (mm) | % Soil moisture | Total moisture content (mm) | % Soil moisture | Total moisture content (mm) | % Soil moisture | Total moisture content (mm) | |
| 0–10 | 12.8 | 16.4 | 6.0 | 7.4 | 5.8 | 7.2 | 5.6 | 6.9 |
| 10–20 | 14.0 | 18.2 | 5.2 | 6.2 | 6.7 | 8.1 | 9.4 | 11.3 |
| 20–30 | 15.0 | 20.2 | 6.8 | 8.5 | 6.8 | 8.5 | 10.9 | 13.7 |
| 30–40 | 15.7 | 22.0 | 7.2 | 9.7 | 12.0 | 16.1 | 13.1 | 17.5 |
| 40–50 | 15.8 | 22.3 | 10.3 | 14.5 | 13.6 | 19.0 | 12.4 | 17.3 |
| 50–60 | 14.6 | 20.7 | 13.6 | 19.1 | 12.5 | 17.4 | 12.3 | 17.2 |
| 60–70 | 10.8 | 15.7 | 13.4 | 18.2 | 13.2 | 18.5 | 13.3 | 18.6 |
| 70–80 | 8.8 | 12.3 | 13.0 | 18.2 | 13.0 | 18.2 | 13.3 | 18.6 |
| 80–90 | 6.4 | 9.1 | 11.2 | 15.7 | 10.5 | 14.7 | 10.7 | 15.0 |
| 90–100 | 5.8 | 8.4 | 9.8 | 13.7 | 8.9 | 12.5 | 9.4 | 13.2 |
| 0–30 | 54.8 | 22.1 | 23.8 | 31.9 | ||||
| 0–50 | 99.1 | 46.3 | 58.9 | 66.7 | ||||
| 0–100 | 165.3 | 131.2 | 140.2 | 149.3 | ||||
Previous research findings (Zhang et al., 2006) indicated that soil moisture status is most important at the reproductive stages of wheat and barley. On the basis of previous finding data were taken in 10.2, 10.2 and 10.0 (FS) in early, late and optimum sowing.
Table 3.
Meteorological and agro-climatic conditions during April–July 2011 at the experimental site.
| Meteorological parameters | April |
May |
June |
July |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (10 days) |
Mean | Mean (10 days) |
Mean | Mean (10 days) |
Mean | Mean (10 days) |
Mean | |||||||||
| I | II | III | I | II | III | I | II | III | I | II | III | |||||
| Air temperature (°С) | ||||||||||||||||
| Mean | 7.3 | 8.5 | 12.7 | 9.5 | 17.1 | 17.8 | 21.8 | 19.0 | 22.4 | 24.7 | 25.6 | 24.3 | 29.5 | 28.5 | 31.7 | 29.9 |
| Maх. | 16.3 | 19.5 | 24.0 | 24.0 | 27.5 | 30.2 | 33.0 | 33.0 | 32.1 | 33.3 | 38.3 | 38.3 | 42.7 | 37.6 | 42.1 | 42.7 |
| Min. | −4.4 | 1.0 | −1.2 | −4.4 | 8.4 | 6.4 | 9.9 | 6.4 | 12.0 | 13.4 | 13.2 | 12.0 | 19.6 | 17.4 | 16.8 | 16.8 |
| Total amount of active temperature above 5 °С | 27.0 | 34.5 | 76.6 | 138.1 | 120.9 | 128.4 | 184.5 | 433.8 | 173.3 | 197.7 | 205.9 | 576.5 | 244.8 | 235.2 | 244.6 | 724.6 |
| Total amount of active temperature (°С) | 2.0 | 3.0 | 33.9 | 38.9 | 70.9 | 78.4 | 86.2 | 235.5 | 129.5 | 123.3 | 147.4 | 400.2 | 194.8 | 185.2 | 238.4 | 618.4 |
| Rainfall (mm) | 4.0 | 2.4 | – | 6.4 | 5.1 | 1.0 | 4.7 | 10.8 | 0.3 | 4.0 | 3.2 | 7.5 | – | – | – | – |
| Average relative humidity (%) | 57 | 68 | 50 | 58 | 62 | 53 | 44 | 53 | 41 | 49 | 47 | 46 | 42 | 32 | 29 | 34 |
| Min. relative humidity (%) | 18 | 27 | 17 | 17 | 28 | 18 | 17 | 17 | 18 | 21 | 15 | 15 | 9 | 11 | 10 | 9 |
| Soil surface temperature (°С) | −7 | −2 | −5 | −7 | 7 | 5 | 8 | 5 | 9 | 11 | 19 | 13 | 21 | 22 | 25 | 22.7 |
| Soil temperature in different depth (°С) | ||||||||||||||||
| 5 cm | 6.5 | 8.5 | 14.7 | 9.9 | 19.5 | 21.1 | 25.2 | 22.1 | 27.3 | 28.6 | 29.8 | 28.6 | 30.7 | 31.3 | 31.9 | 31.3 |
| 10 cm | 5.3 | 7.6 | 13.4 | 8.8 | 18.3 | 19.9 | 23.8 | 20.8 | 25.6 | 26.8 | 28.3 | 26.9 | 28.2 | 29.4 | 30.5 | 29.4 |
| 15 cm | 4.5 | 7.2 | 12.6 | 8.1 | 17.7 | 19.3 | 23.2 | 20.1 | 25.0 | 26.6 | 27.0 | 26.2 | 27.2 | 27.3 | 28.4 | 27.6 |
I, II and III indicates 1st 10 days, 2nd 10 days and 3rd 10 days average data in every month.
3. Results
Temperature is an important environmental factor influencing the growth and development, and finally yield of crop plants. During the growth and development of a cereal crop several growth stages are distinguishable in which important physiological processes occur. In our present research, high temperature, when combined with drought, affected the phenology and growth, and finally yield and other parameters (data not presented) of rainfed spring wheat and barley, under field condition in southern European Russia. Here we explain the phenological variation in relation to the growth of development of spring wheat and barley under different sowing dates.
3.1. Soil moisture (%) and total moisture content (mm) in experimental soil
Soil moisture is the most important parameter for the growth and development of a plant, especially in dry land farming where temperature is high, relative humidity (RH) and rainfall are very low, irrigation is limited and evapotranspiration is very high. In this research, soil moisture (%) and total moisture content (mm) in the soil were recorded before sowing and also recorded at different stages of different sowing times (Table 2). It was found that pre-sowing soil moisture was higher than at other sowing dates. There was a relation between increasing temperature and RH: as temperature increased, soil moisture and RH decreased, ultimately affecting the plant’s response (Tables 2 and 3). In particular, the LS crop was strongly affected from germination to reproductive stages due to a deficit in soil moisture and high air and soil temperature (Tables 2 and 3).
3.2. Climatic conditions during growing season
Climatic factors are most important for the growth and development of plant, by which morpho-anatomical, physiological and biochemical changes are occurred in plants. In this study, the ES crop was exposed to very low temperature (soil surface temperature reaching −0 °C) at germination and seedling stages which delayed germination, affected stand establishment and tillering, ultimately affecting biomass and grain yield (Table 3).
RH is one of the most important constraints in dry land farming due to low rainfall and high temperatures. In our research, RH decreased from ES to LS (Table 3), which may have affected evapotranspiration and metabolic processes of plants; finally decreasing biomass of the evaluated crops (Fig. 8).
Figure 8.
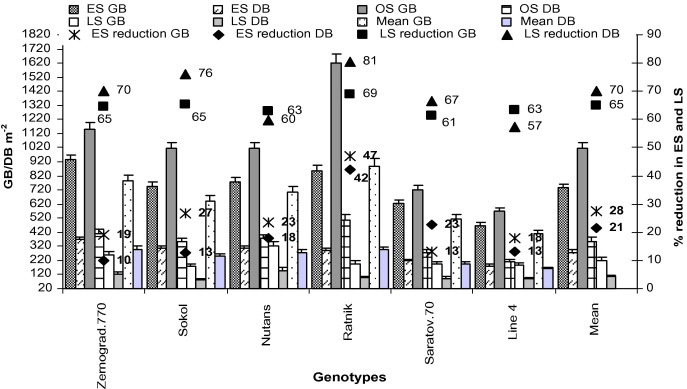
Green and dry biomass weight (m−2) of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. Means (±SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at s ⩽ 0.05 (LSD test). GB – green biomass and DB – dry biomass.
3.3. Days to germination (FS 1.3)
In our present study, due to low temperature, all genotypes took long time to geminate in ES than OS (Table 3; Fig. 1). As compared with OS, the days required for germination increased in all genotypes by 17% and 33% in ES and LS. Germination required the most time at LS (Tables 2 and 3; Fig. 1) due to a deficit in soil moisture.
Figure 1.
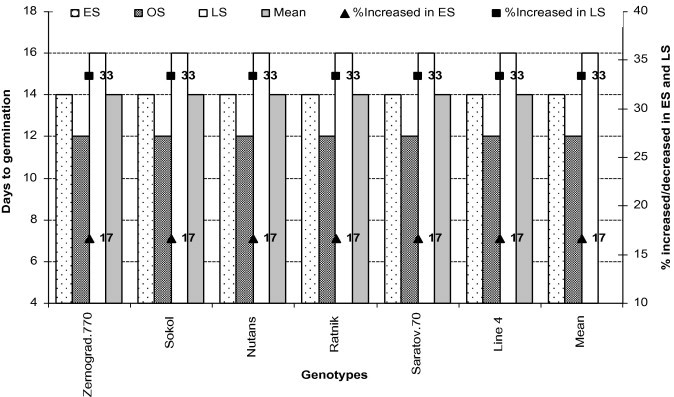
Days to germination of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. ES – early sowing, OS – optimum sowing, LS – late sowing; % increase and reduction were calculated in ES and LS compared to OS.
3.4. Days to first visible awn (FS-10.1)
From Fig. 2, it can be observed that all genotypes in ES – compared to OS and LS – required a long time until the first visible awn, possibly caused by delayed germination and a long period of vegetative growth due to low temperature (Table 3). Compared to all genotypes, ‘Line4’ took the longest time to complete FS-10.1 in all three sowing conditions, followed by ‘Ratnik’ in OS and ‘Saratov.70’ in both OS and LS (Fig. 2). More days were required to complete FS-10.1 in wheat than in barley. Because of low temperature in ES, days required for FS-10.1 increased by 15% in ‘Sokol’ than in ‘Line4’ (2%). On the other hand, in LS, days to visible awn was reduced the most (by 25%) in ‘Line4’ and ‘Saratov.70’ than in ‘Sokol’ (19%).
Figure 2.
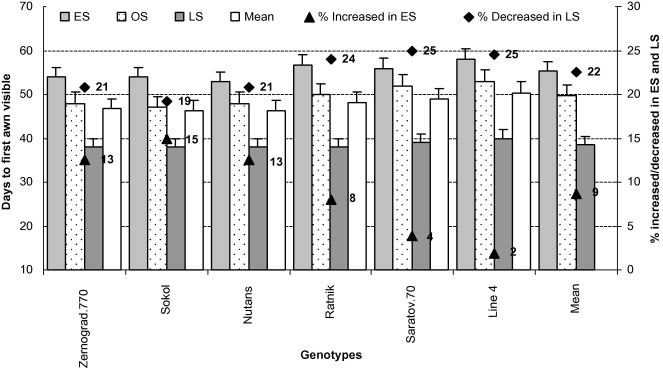
Days to first awn visible of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. Means (±SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P ⩽ 0.05 (LSD test).
3.5. Days to heading (FS-10.5)
Both wheat and barley under ES took more time than under LS to reach heading. Among these, ‘Line4’ needed the most time (Fig. 3). The two crops under LS took less time to reach heading due to high temperature stress (air and soil), lack of rainfall and low RH (drought), which influenced the inherent characteristics of growing plants and reduced their life span (Tables 2 and 3). ‘Sokol’ and ‘Ratnik’ took more time (13% increase) while ‘Nutans’ (4% increase) took less time to complete FS-10.5 in ES due to low temperature which delayed germination at the vegetative stage. On the other hand, due to high temperature (air and soil) and low soil moisture (drought) in LS, ‘Line4’ took less time (25% reduction) while ‘Sokol’ took more time (21% reduction) to heading.
Figure 3.
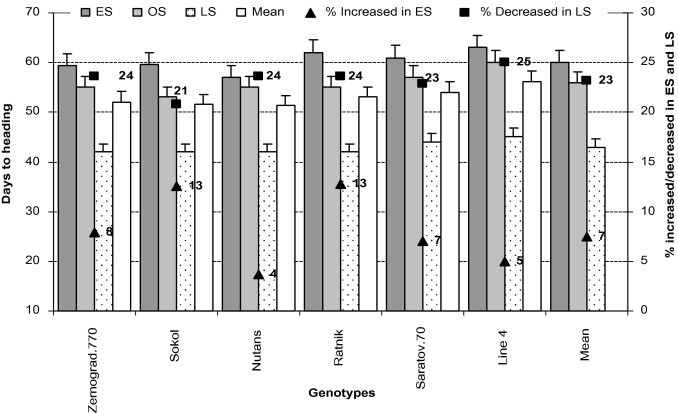
Days to heading of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. Means (±SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P ⩽ 0.05 (LSD test).
3.6. Days to ripe harvest (FS-11.4)
It is well-known that the duration of maturity of any crop is reduced by stress and varies with genotype, due to their inherent nature. From our experimental result, we found that LS crop was highly affected by high temperature combined with drought, resulting in fewer days (reduced 18–20%) to reach ripe harvest than OS. ES crop at seedling and vegetative stages affected by low temperature, finally took more time (increased 4–7%) to complete the life span (Tables 2 and 3; Fig. 4). Among the genotypes, ‘Line4’ took the highest duration (increase 7%) than ‘Zernograd.770’ and ‘Nutans’ (4%) in ES, due to low temperature stress. On the other hand, in LS all barley genotypes took more time (18% reduction) than wheat (‘Saratov.70’ (20%) and ‘Line4’ (19%)), due to high temperature (air and soil) and drought (deficit soil moisture) (Tables 2 and 3; Fig. 4).
Figure 4.
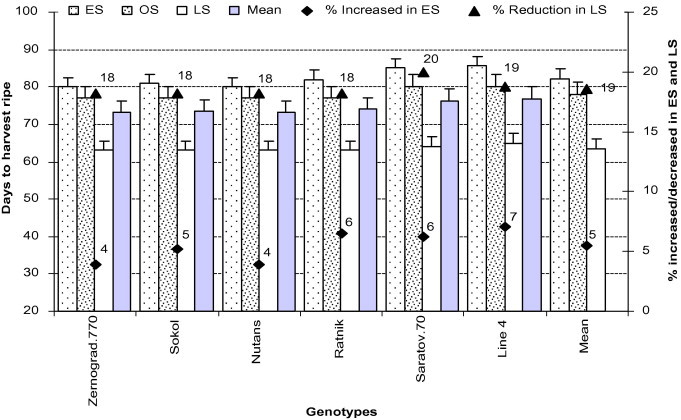
Days to harvest ripe of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. Means (±SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P ⩽ 0.05 (LSD test).
3.7. Plant population m−2 (FS-1.3)
Plant population m−2 is an important growth parameter to get maximum yield m−2. It is fully depending on seed rate, genotypes, percent germination and environmental condition. In our research, the plant population m−2 of all genotypes was higher in ES followed by OS and LS due to the available soil moisture at the time, which helped to increase germination, resulting in an increased plant population (Fig. 5; Table 2). Among the genotypes, ‘Line4’ and ‘Saratov.70’ showed most plants m−2 in ES but plant population was lower in LS due to a decrease in soil moisture and an increase in temperature from ES to LS, which postponed germination and stand establishment, ultimately reducing plant population m−2 (decreased by 12–70%) (Tables 2 and 3; Fig. 5).
Figure 5.
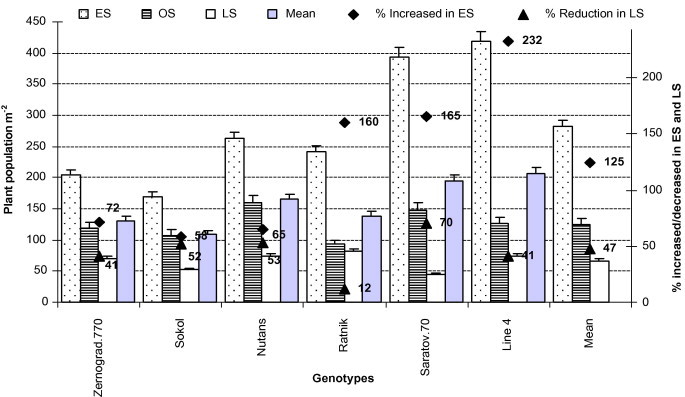
Plant population (m−2) of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. Means (±SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P ⩽ 0.05 (LSD test).
3.8. Number of tillers plant−1 (FS-3.0)
Wheat tillers grow from the axils of the main shoot leaves. The potential number of tillers varies from genotype to genotype and depends on environmental conditions. In our research, we found that the tillering ability of all genotypes was higher in OS and lower in ES (Fig. 6). In ES, soil moisture was favourable, but air temperature was low (occasionally minus °C) (Tables 2 and 3) in seedling and vegetative stages, which may have hampered stand establishment and tillering ability. Because of low temperature in ES and high temperature (air, soil) and deficit soil moisture in LS, ‘Ratnik’ was more highly affected than other genotypes (76% reduction in ES and 68% in LS). On the other hand, as compared with OS, the tillering stage of ‘Nutans’ was less affected in ES (reduced by 50%) and LS (increased by 17%) than other genotypes. On the other hand, the tillering ability of the LS crop was lower due to low soil moisture (drought), high soil and air temperature, and low RH, which ultimately reduced the number of tillers plant−1 (Tables 2 and 3; Fig. 6).
Figure 6.
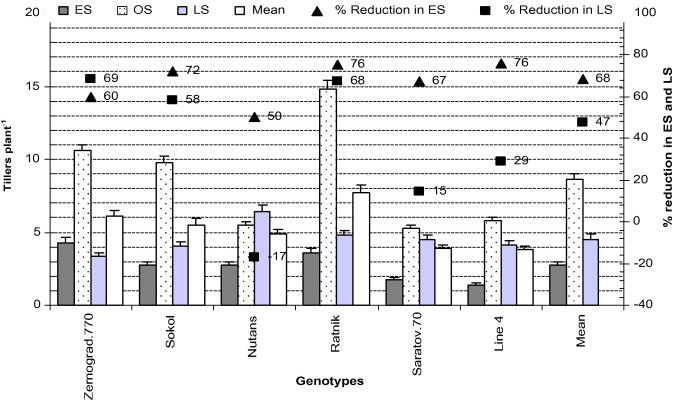
Tillers plant−1 of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. Means (±SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P ⩽ 0.05 (LSD test).
3.9. Plant height (cm) (FS-11.0)
Plant height is an inherent character and varies with genotype and environmental conditions. From Fig. 7, it can be observed that plant height of all genotypes in ES and LS was lower than at OS. Low temperature may have reduced plant height at the seedling and vegetative stages in ES while high temperature combined with drought may have reduced plant height in LS (Tables 2 and 3). The effect of low temperature stress was greater in ‘Sokol’ (25% decrease) than ‘Zernograd.770’ (6% reduction) (Fig. 7). On the other hand, due to high temperature (air, soil) and deficit available soil moisture (drought), ‘Sokol’ was more affected (41% reduction) than ‘Nutans’ (31% reduction) in LS (Fig. 7).
Figure 7.
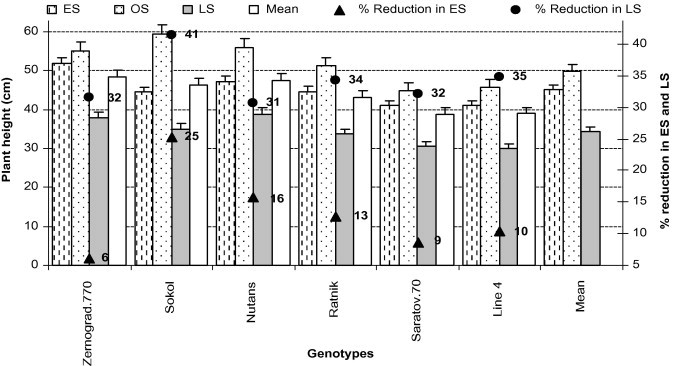
Plant height (cm) of all genotypes in early (low temperature) and late stress (high temperature with drought) conditions. Means (±SD) were calculated from three replicates for each treatment. Bars with different letters are significantly different at P ⩽ 0.05 (LSD test).
3.10. Green and dry biomass (g m−2) (FS-10.5.2)
In our research, biomass (green and dry) of all genotypes in OS was higher than in LS and ES (Fig. 8). Despite a higher plant population m−2 in ES, tillers plant−1 was lower, ultimately reducing biomass (green and dry) which might have been due to unfavourable conditions at the tillering stage (low temperature). However, in LS, plant population m−2 and tillers plant−1 was lower than OS due to high temperature, low soil moisture and RH in air, which ultimately affected germination, tillering capacity and dry matter partitioning (Tables 2 and 3; Fig. 8). In our study, we found that all genotypes were highly sensitive (57–81% reduction in dry matter) to the combined effect of high temperature and drought than low temperature stress (10–42% reduction in dry matter) (Fig. 8).
4. Discussion
Changes in evapotranspiration during a drought depend largely on the availability of moisture at the onset of a drought and the severity and duration of a drought (Hanson, 2003; Hossain et al., 2012a). Siahpoosh et al. (2011) stated that evapotranspiration efficiency (ETE), a reliable physiological indicator for evaluating cultivars with regard to water deficit tolerance in different growing stages, was calculated by dividing the total biomass by ET. Based on this indicator, they discovered that wheat cultivar ‘Mahdavie’ had the highest ETE from planting to stem elongation, ‘Niknejad’ from planting to flowering, ‘Pishtaz’ from planting to dough development and ripening, while ‘Kavir’ was tolerant to water deficit throughout the entire growing season. In this situation, two major processes are involved: (i) water absorption by the crop, which is controlled by root characteristics and soil physical properties; and (ii) crop evapotranspiration, which depends on atmospheric properties, notably net radiation and vapour pressure deficit, and crop characteristics, such as crop ground cover and stomatal conductance (Acevedo et al., 2002). In a study by Zhang et al. (2006), the yield of wheat decreased under soil–water deficit both during the middle vegetative stage (jointing) and the late reproductive stages (filling and maturity or filling) while subjected to no-soil–water-deficit both during the late vegetative stage (booting) and the early reproductive stage (heading), resulting in the highest increase in yield of 25.0% and 14.0%, respectively. These results are in agreement with our data set (Tables 2 and 3).
In our research, fluctuations in weather conditions were reflected in the crop growth and development (Tables 2 and 3; Fig. 8) and ultimately by yield (data not presented), which is common among several crops (Martiniello and Teixeira da Silva, 2011; Hakim et al., 2012; Hossain and Teixeira da Silva, 2012; Hossain et al. 2012b–d). Weather parameters such as maximum and minimum air and soil temperature (soil surface and root zone), rainfall and RH are the most important climatic factors affecting the growth and development of plants, especially in dry land cultivation area (Hossain et al., 2012a; Table 3). Ju et al. (2010) also found a relation between air temperature, water distribution and water storage in the soil profile: rising atmospheric temperature, increased evaporation, evapotranspiration and decreased RH in the air, by which water distribution and storage in the soil profile changed, decreased biomass, number of spikelets/spike and number of grains/spike of wheat. Chakrabarti et al. (2011) and Hossain and Teixeira da Silva (2012) also found that air temperature <15 °C was not suitable for the growth and development (germination, seedling stand establishment and tillering) of spring wheat.
RH, when decreased (Table 3; Fig. 8), may have affected ET and metabolic processes, finally affecting the biomass of the tested plant. This assumption is supported by Reynolds et al. (2001), who stated that the patterns of heat stress may vary widely between wheat-growing regions and that, in low RH areas, crops are affected by high temperature combined with drought, while in high RH environments, disease pressure may be an additional and possibly a more serious limitation.
Evans et al. (1975) found that heat seeds are usually stored at around 12% moisture content with the minimum water content required in grain for germination being 35–45% by weight; when moisture is in shortage, germination fails or is delayed, as observed in our results (Fig. 1). Al-Qasem et al. (1999) and Hossain et al. (2012c) found maximum germination of spring wheat cultivars at 20–30 °C (air temperature). They also reported that when soil moisture and air temperature were low (<12 °C), germination was delayed, adversely affecting crop establishment, similar to our findings in which germination of all genotypes at ES took longer than at OS, possibly because of low temperature at ES (Table 3; Fig. 1). Hossain and Teixeira da Silva (2012) also reported that low air temperature during germination in LS spring wheat had a detrimental effect on germination, crop establishment, tillering and finally productivity.
The variation in required days to FS-10.1 (Fig. 2) observed in ES and LS between genotypes is due to environmental factors (Hossain and Teixeira da Silva, 2012; Hossain et al., 2012a–d). Araus et al. (2007) also stated that the environment affects the number of days required to reach or achieve different growth stages in wheat, but that this varies with genotype due to their different genetic makeup. Reynolds et al. (2000) stated that the sensitivity of metabolic processes of wheat in response to heat stress in the field reduced the length of the life cycle due to a shortage of favourable resources, resulting in low grain yield and low total plant biomass.
In Fig. 3, a reduction in life span of the LS crop can be observed due to the negative effect of high temperature (air and soil) and drought (deficit soil moisture). Ubaidullah et al. (2006) also noticed that in general, LS had negative effects {due to high temperature (25–33 °C) stress at reproductive stage} on all traits with as much as 23 days difference between ES and LS for heading. Wollenweber et al. (2003) and Howarth (2005) stated that the different phenological stages of different genotypes differ in their sensitivity to climatic factors, especially high temperature (air and soil) and low RH (drought), and that this depends on species and genotype as there are great inter- and intra-specific variations, which is similar to our present result (Fig. 3). Prasad et al. (2008) and Hossain and Teixeira da Silva (2012) reported a decrease in time to flowering, grain set, and physiological maturity in spring wheat when grown at high night temperature (>14 °C).
Crops that are highly affected by stress attempt to survive and complete all the developmental stages within a shortened period of time (Young et al., 2004; Hakim et al., 2012; Hossain et al., 2012b–d). On the other hand, Ubaidullah et al. (2006) noticed from their two-year study that heading, grain filling and grain maturity were 23, 3 and 29 days earlier when wheat was sown late than when sown normally due to heat stress during LS; these two assumptions are also true for our study (Fig. 4).
Seed germination is one of the most important phases affecting yield and quality in crop production (Almansouri et al., 2001). Further, the interaction between the seedbed environment and seed quality plays an important role in the establishment of a crop (Khajeh-Hosseini et al., 2003). Al-Karaki et al. (2007) and Hossain et al. (2012a) stated that the combined effect of high air temperature (27–33 °C) and water stress (−3 to −0.9 MPa) was most critical to reducing germination rate and percentage than individual stressor effects. Water shortage in soil postpones and reduces seed germination, causes unequal seedling emergence, and results in variation in the number of plants/unit area, ultimately decreasing seed yield and quality (Hampson and Simpson, 1990), as also found in our study (Fig. 5; Table 2).
The results of our study, with respect to number of tillers plant−1 (Fig. 6), show a parallel resemblance to the following two findings in wheat: Differentiation into tillers and the appearance of tillers generally ended just before stem elongation started (Baker and Gallagher 1983); low air temperatures (<12 °C) negatively affected seedling establishment, tillering and finally reduced grain yield (Hossain et al., 2011). On the other hand, Herbek and Lee (2009) observed that under weather stress conditions such as high temperature, drought, high plant populations, and low soil fertility, or pests, plants responded by producing fewer tillers or even aborting initiated tillers.
In our research, plant height varied under LS and ES (Fig. 7) due to genotype effects and environmental stress. Ahamed et al. (2010) also found similar results in ES and LS. On the other hand, Khan et al. (2009) found, in rice, a positive and significant association between plant height and all morphological traits at the genotypic level. They also found that grain yield/plant was positively and significantly correlated with plant height, panicle length, flag leaf area and number of grains/panicle at the genotype level.
In Mediterranean climates high temperatures combined with drought cause considerable damage, including scorching of leaves and twigs, sunburn on leaves, branches and stems, leaf senescence and abscission, shoot and root growth inhibition, and fruit discoloration and damage, consequently reducing the yield (Martiniello and Teixeira da Silva, 2011; Vollenweider and Gunthardt-Goerg, 2005). Similarly, in temperate regions, heat stress has been reported to be one of the most important causes of reduction in yield and dry matter production in many crops, including maize (Giaveno and Ferrero, 2003). In wheat, under field conditions, high temperature stress is associated with drought (low soil moisture and low RH), affecting the growth and development, and finally reducing yield (Fig. 8; Ahamed et al., 2010; Machado and Paulsen, 2001; Mazorra et al., 2002; Simões-Araújo et al., 2003; Hossain et al., 2012a).
5. Conclusions
From the above results and discussion, it may be concluded that the combined effect of high temperature (soil, air) and drought (deficit soil moisture) is more destructive than low temperature stress. There is a relation between phenological variation and growth and development of wheat and barley: high temperature stress, when combined with drought during LS, decreased the days to visible awns, days to heading and days to ripe harvest, finally negatively affecting the growth and development of plants and resulting in a lower plant population m−2, number of tillers plant−1, plant height and dry matter production m−2. On the other hand, low temperature in combination with ES increased the number of days to germination, reduced seedling stand establishment and tillering capacity, finally affecting the growth and development of both cereal crops. Compared to overall performance and optimum sowing date, barley genotypes ‘Zernograd.770’ and ‘Nutans’, and wheat genotype ‘Line4’ performed best in both late (high temperature with drought stress) and early (low temperature stress) sowing conditions, making them potential target genotypes for use in southern European Russia.
Acknowledgement
We are very grateful to all staff of “The Caspian Scientific Research Institute of Arid Agriculture”, Astrakhan, Russia, especially the staff of the “Arid Agriculture and Crop Production Division” for all help during experiments, data collection, data analysis and writing up of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Akbar Hossain, Email: tanjimar2003@yahoo.com.
Jaime A. Teixeira da Silva, Email: jaimetex@yahoo.com.
References
- Acevedo, E., Silva, P., Silva, H., 2002. Wheat growth and physiology. In: Curtis, B.C. (Ed.), Bread Wheat: Improvement and Production, FAO Plant Production and Protection Series No. 30. Rome, Italy, p. 567. Available from: <http://www.fao.org/docrep/006/y4011e/y4011e00.htm> (last access 17.07.12).
- Ahamed K.U., Nahar K., Fujita M. Sowing date mediated h e a t stress affects the leaf growth and dry matter partitioning in some spring wheat (Triticum aestivum L.) cultivars. IIOAB J. 2010;1(3):1–9. [Google Scholar]
- Ahmed, M.F., Ahmed, A.S.H., Burhan, H.O., Ahmed, F.E., 2003. Effects of Sowing Date on Growth and Yield of Wheat at Different Elevations in Jebel Marra Highlands under Rain-fed Conditions. Faculty of Agriculture, University of Khartoum, Shambat and Agricultural Research Corporation, Nyala Research Station, Sudan. Available from: <http://www.arcsudan.sd/proceedings/39thmeeting/fulltext%20pdf%2039/Wheat.pdf> (last access 17.07.12).
- Albit (Albit Scientific and Industrial LLC), 2011. An innovative biological product effectively protecting plants against drought, diseases, and other stresses: increase of resistance to drought and other adverse environmental factors. In: Zlotnikov, K. M., Pustovoitova T. N., Zlotnikov A. K. (Eds.), Metabolites of Pseudomonas aureofaciens H16 and Bacillus megaterium PC2 increase drought resistance of spring wheat. In: Kulaev I. S. (Ed.), Modern Problems of Microbial Biochemistry and Biotechnology. Abstr. Int. Symp., Puschino, June 25–30, 2000. IBPM. Puschino. Albit Scientific and Industrial LLC, 142290 Russia, pp. 138–139. <http://www.albit.com/2/2_04.php> (last access 17.07.12).
- Alcamo, J., Dronin, N., Endejan, M., Golubev, G., Kirilenko, A., 2007. A new assessment of climate change impacts on food production shortfalls and water availability in Russia. Global Environmental Change 1–16, Available from: <www.elsevier.com/locate/gloenvcha> (last, access 17.07.12).
- Al-Karaki, G.N., 2012. Phenological development–yield relationships in durum wheat cultivars under terminal high temperature stress in semiarid conditions. ISRN Agronomy. 2012, Article ID 456856, p. 7.
- Al–Karaki G.N., Al–Ajmi A., Othman Y. Seed germination and early root growth of three barley cultivars as affected by temperature and water stress. American–Eurasian J. Agric. Environ. Sci. 2007;2(2):112–117. [Google Scholar]
- Almansouri M., Kinet J.M., Lutts S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.) Plant Soil. 2001;231:243–254. [Google Scholar]
- Al–Qasem H., Kafawin O., Duwayri M. Effects of seed size and temperature on germination of two wheat cultivars. Dirasat Agril. Sci. 1999;26(1):1–7. [Google Scholar]
- Araus J., Ferrio J., Buxo R., Voltas J. The historical perspective of dry land agriculture: lessons learned from 10000 years of wheat cultivation. J. Exp. Bot. 2007;58(2):131–145. doi: 10.1093/jxb/erl133. [DOI] [PubMed] [Google Scholar]
- Baker C.K., Gallagher J.N. The development of winter wheat in the field. The control of primordium initiation rate by temperature and photoperiod. J. Agric. Sci. 1983;101:337–344. [Google Scholar]
- Bavei V., Vaezi B., Abdipour M., Kamali M.R.J., Roustaii M. Screening of tolerant spring barleys for terminal heat stress: different importance of yield components in barleys with different row type. Intl. J. Plant Breed. Genet. 2011;5(3):175–193. [Google Scholar]
- Buriro M., Oad F.C., Keerio M.I., Tunio S., Gandahi A.W., Hassan S.W.U., Oad S.M. Wheat seed germination under the influence of temperature regimes. Sarhad J. Agric. 2011;27(4):539–543. [Google Scholar]
- Chakrabarti B., Singh S.D., Nagarajan S., Aggarwal P.K. Impact of temperature on phenology and pollen sterility of wheat varieties. Aust. J. Crop Sci. 2011;5(8):1039–1043. [Google Scholar]
- Dalirie M.S., Sharif R.S., Farzaneh S. Evaluation of yield, dry matter accumulation and leaf area index in wheat genotypes as affected by terminal drought stress. Not. Bot. Horti Agrobotanici Cluj–Napoca. 2010;38(1):182–186. [Google Scholar]
- Dias A.S., Lidon F.C., Ramalho J.C. Heat stress in Triticum: Kinetics of Fe and Mn accumulation. Braz. J. Plant Physiol. 2009;21(2):153–164. [Google Scholar]
- El-Fadly, G.A.B., Menshawy, A.M., Farhat, W.Z.E., 2007. Molecular and biochemical studies on some bread wheat genotypes in relation to water stress tolerance. In: African Crop Science Conference Proceedings. Printed by African Crop Science Society, El-Minia, Egypt, 8, pp. 605–612.
- Evans L.T., Wardlaw I.F., Fischer R.A. Wheat. In: Evans L.T., editor. Crop Physiology. Cambridge University Press; Cambridge, UK: 1975. pp. 101–149. [Google Scholar]
- FAO, 2010. Monthly news report on grains, No. 64. Food and Agriculture Organization of the United Nations, Rome, Italy. Available from: <http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Grains/Documents/MNR0710.pdf> (last access 17.07.12).
- FAO, 2011. Special alert No. 330: A severe winter drought in the north china plain may put wheat production at risk. FAO Global Information and Early Warning System on Food and Agriculture (GIEWS), Rome, Italy. Available from: <http://www.fao.org/docrep/013/al975e/al975e00.pdf> (last access 17.07.12).
- FAOSTAT. 2011. FAOSTAT, 2009, Food and Agricultural commodities production, Rome, Italy. Available from: <http://faostat.fao.org/site/339/default.aspx> (last access 17.07.12).
- Farooq M., Basra S.M.A., Rehman H., Saleem B.A. Seed priming enhancement. The performance of late sown wheat by improving chilling tolerance. J. Agron. Crop Sci. 2008;194:55–60. [Google Scholar]
- Giaveno C., Ferrero J. Introduction of tropical maize genotypes to increase silage production in the central area of Santa Fe. Argentina. Crop Breed. Appl. Biotech. 2003;3:89–94. [Google Scholar]
- Hakim M.A., Hossain A., Teixeira da Silva J.A., Zvolinsky V.P., Khan M.M. Yield, protein and starch content of 20 wheat (Triticum aestivum L.) genotypes exposed to high temperature under late sowing conditions. J. Sci. Res. 2012;4(2):477–489. [Google Scholar]
- Hampson C.R., Simpson G.M. Effect of temperature, salt and osmotic potential on early growth of wheat (Triticum aestivum L.) I. Germination. Can. J. Bot. 1990;68:524–528. [Google Scholar]
- Hanson, R.L., 2003. Climate Variability: Evapotranspiration and Droughts. U.S. Department of the Interior, U.S. Geological Survey. Available from: <http://geochange.er.usgs.gov/sw/changes/natural/et/> (last access 17.07.12).
- Hasanuzzaman M., Hossain M.A., Teixeira da Silva J.A., Fujita M. Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Bandi V., Shanker A.K., Shanker C., Mandapaka M., editors. Crop Stress and its Management: Perspectives and Strategies. Springer; The Netherlands: 2012. pp. 261–315. [Google Scholar]
- Hede R., Skovmand B., Reynolds M.P., Crossa J., Vilhelmsen A.L., Stolen O. Evaluating genetic diversity for heat tolerance traits in Mexican wheat landraces. Genet. Res. Crop Evol. 1999;46:37–45. [Google Scholar]
- Herbek, J., Lee, C., 2009. A Comprehensive Guide to Wheat Management in Kentucky. U.S. Department of Agriculture, M. Scott Smith, Director, Cooperative Extension Service, University of Kentucky College of Agriculture, Lexington, and Kentucky State University, Frankfort. <http://www.uky.edu/Ag/GrainCrops/ID125Section2.htm> (last access 17.07.12).
- Hossain A., Teixeira da Silva J.A. Phenology, growth and yield of three wheat (Triticum aestivum L.) varieties as affected by high temperature stress. Not. Sci. Biol. 2012;4(3):97–109. [Google Scholar]
- Hossain A., Sarker M.A.Z., Hakim M.A., Lozovskaya M.V., Zvolinsky V.P. Effect of temperature on yield and some agronomic characters of spring wheat (Triticum aestivum L.) genotypes. Intl. J. Agril. Res. Innov. Tech. 2011;1(1&2):44–54. [Google Scholar]
- Hossain, A., Lozovskaya, M.V., Zvolinsky, V.P., Tutuma, N.V., 2012a. Effect of soil resources and climatic factors (temperature) on spring wheat and barley in the northern Bangladesh and southern Russia. Paper presented in “International scientific and practical conference on problems of environmental management and conservation of ecological balance in the arid zones”, held in “Caspian Scientific Research Institute of Arid Agriculture”, Salt Zaymische, Chorniarsky district, Astrakhan State, Russia, from 16–18 May, 2012.
- Hossain A., Teixeira da Silva J.A., Lozovskaya M.V., Zvolinsky V.P. The effect of high temperature stress on the phenology, growth and yield of five wheat (Triticum aestivum l.) genotypes. Asian Australasian J. Plant Sci. Biotech. 2012;6(1):14–23. [Google Scholar]
- Hossain A., Lozovskaya M.V., Zvolinsky V.P., Teixeira da Silva J.A. Effect of soil and climatic conditions on yield-related components performance of spring wheat (Triticum aestivum L.) varieties in the northern Bangladesh. Nat. Sci.: J. Fund. Appl. Sci. 2012;2(39):69–78. [Google Scholar]
- Hossain A., Lozovskaya M.V., Zvolinsky V.P., Teixeira da Silva J.A. Effect of soil and climatic conditions on phenology of spring wheat varieties in the northern Bangladesh. Nat. Sci.: J. Fund. Appl. Sci. 2012;2(39):78–86. [Google Scholar]
- Howarth C.J. Genetic improvements of tolerance to high temperature. In: Ashraf M., Harris P.J.C., editors. Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches. Haworth Press Inc; New York: 2005. pp. 277–300. [Google Scholar]
- IPCC, 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. p. 996. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Izrael Y.A., Avdjushin, S.I., 1997. Russian Federation Climatic Changes Country Study (Co-Operative Agreement DE–FCO2–93PO10118). In: Sirotenko, O.D. (Ed.), All-Russian Institute of Agricultural Meteorology, Final report, volume 3: Vulnerability and adaptation assessments. Moscow. pp. 4–111. Available from: <https://www.usgcrp.gov/CSP/pdf/russianfed_vuladap.pdf> (last access 17.07.12).
- Jones P.D., New M., Parker D.E., Mortin S., Rigor I.G. Surface air temperature and its change over the past 150 years. Rev. Geophysics. 1999;37:173–199. [Google Scholar]
- Ju, Z., Hu, C., Zhang, Y., Chen, S., 2010. Effects of temperature rising on soil hydrothermal properties, winter wheat growth and yield. Climate change: Agriculture, food, security and human health, 9th European IFSA Symposium, WS3, 4–7 July 2010, Vienna (Austria). pp. 307–1316. Available from: <http://ifsa.boku.ac.at/cms/fileadmin/Proceeding2010/2010_WS3.1_JuZhaoqiang.pdf> (last access 17.07.12).
- Karmanenko N.M., Osipova L.V., Nilovskaya N.T. Acid, cold and drought tolerance in cereal. J. Russian Acad. Sci. 2011;37(5):354–357. [Google Scholar]
- Khajeh–Hosseini M., Powell A.A., Bingham I.J. The interaction between salinity stress and seed vigour during germination of soybean seeds. Seed Sci. Tech. 2003;31:715–725. [Google Scholar]
- Khakwani A.A., Dennett M.D., Munir M. Drought tolerance screening of wheat varieties by inducing water stress conditions. Songklanakarin J. Sci. Tech. 2011;33(2):135–142. [Google Scholar]
- Khan A.S., Imran M., Ashfaq M. Estimation of genetic variability and correlation for grain yield components of rice. American-Eurasian J. Agric. Environ. Sci. 2009;6(5):585–590. [Google Scholar]
- Krček P., Slamka K., Olšovská M., Brestič, Benčíková M. Reduction of drought stress effect in spring barley (Hordeum vulgare L.) by nitrogen fertilization. Plant Soil Environ. 2008;54(1):7–13. [Google Scholar]
- Large E.C. Growth stages in cereals illustration of the Feekes scale. Plant Path. 1954;3:128–129. [Google Scholar]
- Lopez C.G., Banowetz G.M., Peterson C.J., Kronstad W.E. Dehydrin expression and drought tolerance in seven wheat cultivars. Crop Sci. 2003;43:577–582. [Google Scholar]
- Lukacs A., Partay G., Nemeth T., Csorba S., Farkas C. Drought stress tolerance of two wheat genotypes. Soil Water Res. 2008;3(Special Issue 1):95–104. [Google Scholar]
- Machado S., Paulsen G.M. Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil. 2001;233:179–187. [Google Scholar]
- Martiniello P., Teixeira da Silva J.A. Physiological and bio–agronomical aspects involved in growth and yield components of cultivated forage species in Mediterranean environments: A review. European J. Plant Sci. Biotech. 2011;5(Special Issue 2):64–98. [Google Scholar]
- Marubeni Corporation, 2010. Russian Grain Export/Future and Bottleneck. pp. 1–11. Available from: <http://www.jp-ru.org/4forum/presen/3higuchi.pdf> (last access 17.07.12).
- Mazorra L.M., Nunez M., Echerarria E., Coll F., Sánchez–Blanco M.J. Influence of brassinosteriods and antioxidant enzymes activity in tomato under different temperatures. Plant Biol. 2002;45:593–596. [Google Scholar]
- Midmore D.J., Cartwright P.M., Fischer R.A. Wheat in tropical environments. II. Crop growth and grain yield. Field Crops Res. 1984;8:207–227. [Google Scholar]
- NASA (National Aeronautics and Space Administration), 2011. GISS Surface Temperature Analysis. Goddard Space Flight Center, Sciences and Exploration Directorate, Earth Sciences Division. Columbia University, New York City. Available from: <http://data.giss.nasa.gov/gistemp/graphs/> (last access 17.07.12).
- Nouri A., Etminan A., Teixeira da Silva J.A., Mohammadi R. Assessment of yield, yield related traits and drought tolerance of durum wheat genotypes (Triticum turjidum var durum Desf.) Austr. J. Crop Sci. 2011;5(1):8–16. [Google Scholar]
- Porter J.R. Rising temperatures are likely to reduce crop yields. Nature. 2005;436:174. doi: 10.1038/436174b. [DOI] [PubMed] [Google Scholar]
- Prasad P.V.V., Pisipati S.R., Ristic Z., Bukovnik U., Fritz A.K. Impact of nighttime temperature on physiology and growth of spring wheat. Crop Sci. 2008;48:2372–2380. [Google Scholar]
- Rawson H.M. Effect of high temperatures on the development and yield of wheat and practices to reduce deleterious effects. In: Klatt A.R., editor. Wheat Production Constraints in Tropical Environments. Mexico, D.F; CIMMYT: 1988. pp. 44–62. [Google Scholar]
- Refay Y.A. Yield and yield components parameters of bread wheat genotypes as affected by sowing dates. Middle–East. J. Sci. Res. 2011;7(4):484–489. [Google Scholar]
- Reynolds M.P., Delgado M.I., Gutiérrez–Rodríguez M., Larqué–Saavedra A. Photosynthesis of wheat in a warm, irrigated environment. I: Genetic diversity and crop productivity. Field Crops Res. 2000;66:37–50. [Google Scholar]
- Reynolds M.P., Nagarajan S., Razzaque M.A., Ageeb O.A.A. Heat tolerance. In: Reynolds M.P., Ortiz–Monasterio J.I., McNab A., editors. Application of Physiology in Wheat Breeding. CIMMYT; Mexico, D.F: 2001. pp. 124–135. [Google Scholar]
- Ruan C.-J., Teixeira da Silva J.A. Metabolomics: Creating new potentials for unraveling mechanisms in response to salt and drought stress and for biotechnological improvement of xero–halophytes. Crit. Rev. Biotech. 2011;31(2):152–168. doi: 10.3109/07388551.2010.505908. [DOI] [PubMed] [Google Scholar]
- Russell, O. F., 1994. MSTAT–C v. 2.1 (A computer based data analysis software). Crop and Soil Science Department, Michigan State University, USA.
- Saini H.S., Westgate M.E. Reproductive development in grain crops during drought. Adv. Agron. 2000;68:59–96. [Google Scholar]
- Siahpoosh M.Z., Dehghanian E., Kamgar A. Drought tolerance evaluation of bread wheat genotypes, using water use efficiency, evapotranspiration efficiency, and drought susceptibility Index. Crop Sci. 2011;51(3):1198–1204. [Google Scholar]
- Simões–Araújo J.L., Rumjanek N.G., Margis–Pinheiro M. Small heat shock proteins genes are differentially expressed in distinct varieties of common bean. Braz. J. Plant Physiol. 2003;15:33–41. [Google Scholar]
- Timmermans B.G.H., Vos J., Nieuwburg J.V., Stomph T.J., Putten P.E.L., Molendijk L.P.G. Field performance of Solanum sisymbriifolium, a trap crop for potato cyst nematodes. I. Dry matter accumulation in relation to sowing time, location, season and plant density. Ann. Appl. Biol. 2007;150:89–97. [Google Scholar]
- Trethowan, R., Pfeiffer, W.H., 1999. Challenges and future strategies in breeding wheat for adaptation to drought stressed environments: A CIMMYT wheat program perspective. In: Ribaut, J.M., Poland, D. (Eds.), Molecular approaches for the genetic improvement of cereals for stable production in water-limited environments. A strategic planning workshop held at CIMMYT, El Batan, Mexico. Jun 21–25, 1999. CIMMYT, Mexico, DF, Mexico. pp. 45–48.
- Ubaidullah Raziuddin., Mohammad T., Hafeezullah, Ali S., Nassimi A.W. Screening of wheat (Triticum aestivum L.) genotypes for some important traits against natural terminal heat stress. Pak. J. Biol. Sci. 2006;9:2069–2075. [Google Scholar]
- Ugarte C., Calderini D.F., Slafer G.A. Grain weight and grain number responsiveness to pre–anthesis temperature in wheat, barley and triticale. Field Crops Res. 2007;100(2–3):240–248. [Google Scholar]
- USDA–NASS Agriculture Statistics, 2004. Taxes, Insurance, Credit, and Cooperatives. Chapter X. Available from: <http://www.usda.gov/nass/pubs/agr04/04_ch10.pdf> (last access 17.07.12).
- Vollenweider P., Gunthardt–Goerg M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2005;137:455–465. doi: 10.1016/j.envpol.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Wardlow L.F. Interaction between drought and chronic high temperature during kernel filling in wheat in a controlled environment. Ann. Bot. 2002;90:469–476. doi: 10.1093/aob/mcf219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber B., Porter J.R., Schellberg J. Lack of interaction between extreme high temperature events at vegetative and reproductive growth stages in wheat. J. Agron. Crop Sci. 2003;189:142–150. [Google Scholar]
- Young L.W., Wilen R.W., Bonham–Smith P.C. High temperature stress of Brassica napus during flowering reduces micro- and mega gametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004;55:485–495. doi: 10.1093/jxb/erh038. [DOI] [PubMed] [Google Scholar]
- Zhang B., Li F.M., Huang G., Cheng Z.Y., Zhang Y. Yield performance of spring wheat improved by regulated deficit irrigation in an arid area. Agric. Water Manag. 2006;79:28–42. [Google Scholar]



