Abstract
Although it is clear that T helper (Th)17 cells play a pathologic role in the pathogenesis of several inflammatory diseases, the contribution and regulation of pathogenic Th17 cells in the development of glomerulonephritis are still not fully understood. Herein, we show that IL-10–deficient mice exhibit exacerbation of glomerulonephritis after induction with anti–glomerular basement membrane globulin, with enhanced pathogenic Th17 immune responses. We further demonstrate that Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells develop more severe glomerulonephritis after induction of anti–glomerular basement membrane disease, with more infiltration of inflammatory cells into the kidneys. Finally, IL-17 and interferon γ double-positive cells were significantly increased in IL-10−/− CD4+ T-cell cultures under pathogenic Th17 conditions compared with wild-type cell cultures. These findings suggest that T-cell–derived IL-10 plays a critical suppressive role in the control of pathogenic Th17 cell differentiation and highlights the importance of IL-10 as protection against glomerulonephritis development.
T helper (Th)17 cells, which are clearly distinct from Th1 and Th2 cells, have been defined as an additional Th cell subset that mediates proinflammatory and autoimmune responses through the production of Th17 signature cytokines, including IL-17A, IL-17F, and IL-22.1–4 Synergy between the cytokines transforming growth factor (TGF)-β and IL-6 induces in vitro development of Th17 cells,5–8 whereas IL-23 promotes the survival and expansion of Th17 cell populations.2,5,9,10 IL-23 is also believed to play an important role in the development of pathogenic Th17 cells.11 Several transcription factors are involved in the regulation of Th17 cell differentiation. Among them, RORγt, a member of the orphan nuclear receptor family, has been identified as the master transcription factor for Th17 cell development.12 Other transcription factors, including RORα, STAT3, IRF4, and IRF8, are also involved in the control of Th17 cell differentiation.13 In addition, the differentiation of Th17 cells is also regulated by several positive and negative feedback loops involving IL-21, IL-23R, IL-10, and IL-27.6,7,14–18 There is increasing evidence that Th17 cells are involved in the pathogenesis of various autoimmune/inflammatory diseases, including multiple sclerosis, rheumatoid arthritis, inflammatory bowel diseases, and asthma.19 Thus, a more complete understanding of the molecular mechanisms involved in the regulation of Th17 immune responses and its roles in different inflammatory diseases should provide insights into the pathogenesis and treatment of inflammatory diseases.
Glomerulonephritis (GN) is a renal disease observed as inflammation in glomeruli and small blood vessels of the kidneys.20 The presentation of GN may include hematuria, proteinuria, and a variable degree of renal failure.20 The mechanisms underlying the pathogenesis of GN are incompletely understood. It has been believed that Th1-mediated immune responses are involved in the development of GN,21,22 but more recent studies suggest that Th17 cells instead of Th1 cells contribute to the pathogenesis of GN.23–26 However, the functions and regulation of Th17 cells in the development of GN still need to be further explored.
IL-10, identified by Mosmann and colleagues in 1989,27 is a pluripotent cytokine produced by many activated immune cell types, including Th cells, B cells, macrophages, monocytes, and keratinocytes.28 IL-10 activates through the IL-10 receptor (IL-10R), which is expressed on a variety of cell types.28 The IL-10R is composed of two chains, IL-10R1 and IL-10R2.28 Interaction of IL-10 with the IL-10R results in STAT3-mediated signal transduction.29 IL-10 inhibits Th1 cell differentiation and IL-12 production in macrophages. We recently demonstrated that IL-10 plays a negative role in the regulation of Th17 cell development.18 IL-10–deficient mice spontaneously develop colitis; a condition once attributed to an enhanced Th1 immune response. Recent studies suggest that Th17 cells may contribute to the development of colitis in IL-10–deficient mice.30 Kitching et al31 reported that endogenous IL-10 regulates Th1 immune responses that induce crescentic GN. However, the importance of IL-10 in the regulation of pathogenic Th17 cell differentiation during the development of GN is still not fully understood.
In the present study, we show that mice deficient in IL-10 exhibit more severe GN after induction with anti–glomerular basement membrane (aGBM) globulin, with enhanced Th17 immune responses. We further demonstrate that Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells develop more severe GN after induction of aGBM disease, with more infiltration of inflammatory cells into the kidneys. Finally, the IL-17 and interferon (IFN)-γ double-positive cell populations were significantly higher in IL-10−/− CD4+ T-cell cultures under pathogenic Th17 conditions compared with wild-type (WT) cell cultures, and the double-positive cells were significantly increased in IL-10−/− mice with GN. These findings suggest that IL-10 plays a critical suppressive role in the control of pathogenic Th17 cell differentiation and highlights the importance of IL-10 as protection against GN development.
Materials and Methods
Mice
C57BL/6J and IL-10–deficient mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and were maintained in the barrier facility at the Icahn School of Medicine at Mount Sinai (New York, NY). The animal study protocols were approved by the Institutional Animal Care and Use Committees of the Icahn School of Medicine at Mount Sinai.
Antibodies
The following antibodies were purchased from BD Biosciences (San Diego, CA), as conjugated to fluorescein isothiocyanate, phosphatidylethanolamine, phosphatidylethanolamine-Cy5, perCP-Cy5.5, or APC: CD4 (L3T4), CD8 (53-6.7), CD3e (145-2C11), CD25 (PC61.5), CD44 (IM7), CD62L (MEL-14), CD45RB (C363-16A), IL-17 (TC11-18H10), IFN-γ (XMG1.2), and isotype controls. Antibodies for IL-2 (JES6-1A12), RORγ (B2D), IL-10 (JES5-16E3), and Foxp3 (FJK-16S) were purchased from eBioscience Inc. (San Diego, CA).
Induction of Accelerated aGMB GN
WT and IL-10−/− mice (n = 8 per group) were sensitized by s.c. injection of 0.5 mg of sheep globulin (SG) in Freund complete adjuvant. Five days later, SG-sensitized mice were injected i.p. with sheep anti-mouse GMB antibody at 30 mg per mouse. Renal injury and systemic immune responses were assessed 14 days later. For assessment of antigen-specific immune responses, a separate group of mice was injected s.c. with SG in Freund complete adjuvant, and the mice were sacrificed 1 week later.
CD4+ T-Cell Preparation and Differentiation in Vitro
Naive CD4+ T cells (CD62L+CD44lo) were prepared by fluorescence-activated cell sorting (FACS) from the spleens and lymph nodes of IL-10−/− mice and their WT littermates. The sorted cells were primed for 96 hours with 1 μg/mL of anti-CD3 (145-2C11; BD Biosciences) and 2 μg/mL of soluble anti-CD28 (37.51; BD Biosciences). The cells were rested for 48 hours and were then restimulated for 5 hours with phorbol 12-myristate 13-acetate (PMA) plus ionomycin in the presence of brefeldin A, and intracellular cytokines were measured by flow cytometry. Cells stimulated under neutral conditions were defined as Th0 cells. Cells were stimulated to differentiate into Th1 cells by supplementation with IL-12 plus anti–IL-4 or into Th2 cells by supplementation with IL-4 and anti–IFN-γ. For Th17 cell differentiation, cells were stimulated with 5 ng/mL of TGF-β1, 20 ng/mL of IL-6 added with or without 10 ng/mL of IL-23 (all from R&D Systems, Minneapolis, MN) in the presence of 10 μg/mL of anti–IL-4 antibody (11B11; BD Bioscences).
Assessment of Renal Injuries
Glomerular abnormalities were analyzed on PAS-stained, Bouin-fixed, paraffin-embedded sections (3 μm thick) using coded slides. Abnormalities included crescent formation and severe necrosis. A minimum of 50 glomeruli were analyzed per animal to determine the percentage of crescentic glomeruli. Semiquantitative analysis of tubulointerstitial damage was performed in each mouse as previously published32,33 using 10 randomly selected cortical areas (×200). Injury was defined as tubular dilation, tubular atrophy, sloughing of tubular epithelial cells, or thickening of the basement membrane.32,33 Each cortical field was scored (0 to 4) according to the amount of injury: 0 indicates no interstitial damage; 1, 25% of the tubulointerstitium damaged; 2, 25% to 50% damaged; 3, 50% to 75% damaged; and 4, >75% of the tubulointerstitium damaged. For urine collection, mice were housed for 24 hours in metabolic cages with free access to tap water, whereas serum was collected after the mice were sacrificed. Albuminuria was analyzed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience).
Renal Leukocyte Infiltration
Kidney sections were fixed in periodate-lysine-paraformaldehyde for 4 hours, washed with 20% sucrose solution, and then frozen. Tissue sections were cut, and immunofluorescence staining was used to stain for T cells, macrophages, and neutrophils. The antibodies of anti-mouse CD3 (145-2C11), anti–MAC-2, and anti–Gr-1(RB6-8C5) were used as primary antibodies to detect T cells, macrophages, and neutrophils, respectively (BD Biosciences). At least 25 consecutively viewed glomeruli were assessed per animal, and the results were expressed as cells per glomerular cross section.
Intracellular Staining and Flow Cytometry
Cells were stimulated with PMA and ionomycin for 5 hours in the presence of brefeldin A before intracellular staining. Cells were fixed with intracellular fixation buffer (BD Biosciences), incubated with permeabilization buffer, and stained with phosphatidylethanolamine–anti-mouse IL-17, APC–anti–IFN-γ, and phosphatidylethanolamine-Cy5.5 anti-mouse CD4 antibodies. Flow cytometry was performed using a FACSCalibur system (BD Biosciences).
RNA Isolation and Real-Time RT-qPCR
Total RNA was extracted using an RNeasy Plus kit (Qiagen Inc., Valencia, CA), and cDNA was generated using an oligo (dT) primer and the SuperScript II system (Invitrogen, Carlsbad, CA), followed by analysis using iCycler PCR with SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). Results were normalized based on the expression of ubiquitin. The following primer sets (sense and antisense) were used: IL-17A, 5′-CTCCAGAAGGCCCTCAGACTAC-3′ and 5′AGCTTTCCCTCCGCATTGACACAG-3′; IL-21, 5′-CGCCTCCTGATTAGACTTCG-3′ and 5′-GCCCCTTTACATCTTGTGGA-3′; RORγt, 5′-CCGCTGAGAGGGCTTCAC-3′ and 5′-TGCAGGAGTAGGCCACATTACA-3′; and ubiquitin, 5′-TGGCTATTAATTATTCGGTCTGCA-3′ and 5′-GCAAGTGGCTAGAGTGCAGAGTAA-3′.
Cell Transfer Studies
Splenocytes or CD4+ T cells from WT and IL-10−/− mice were injected i.p. into Rag1−/− recipients (1 × 107 splenocytes or 4 × 106 CD4+ T cells per mouse in 200 μL of sterile PBS per injection). Mice were then immunized by s.c. injection of 0.5 mg in Freund complete adjuvant, and 5 days later the SG-sensitized mice were i.p. injected with sheep anti-mouse GBM globulin at a dose of 30 mg per mouse. Fourteen days later, renal injury and systemic immune responses were assessed. For evaluation of antigen-specific immune responses, a separate group of mice was weighed every week throughout the course of the experiments. After 5 weeks, the mice were sacrificed and their kidney tissues were excised. The tissues were fixed in 10% buffered formalin and were paraffin embedded. Tissue sections (5 μm thick) were stained with H&E. All the slides were read and scored by an experienced pathologist without previous knowledge of the type of treatment. The degree of inflammation in the epithelium, submucosa, and submuscularis propria was scored separately as described previously.34
Cytokine ELISA
Supernatants from cell cultures were collected after activation under various conditions, and secreted cytokines in the supernatants were measured by ELISA kits with purified coating and biotinylated detection antibodies: anti–IL-17 (R&D Systems).
Statistical Analysis
Statistical analysis was performed using Student's t-test. P < 0.05 was considered statistically significant.
Results
IL-10 Deficiency Exacerbates GN
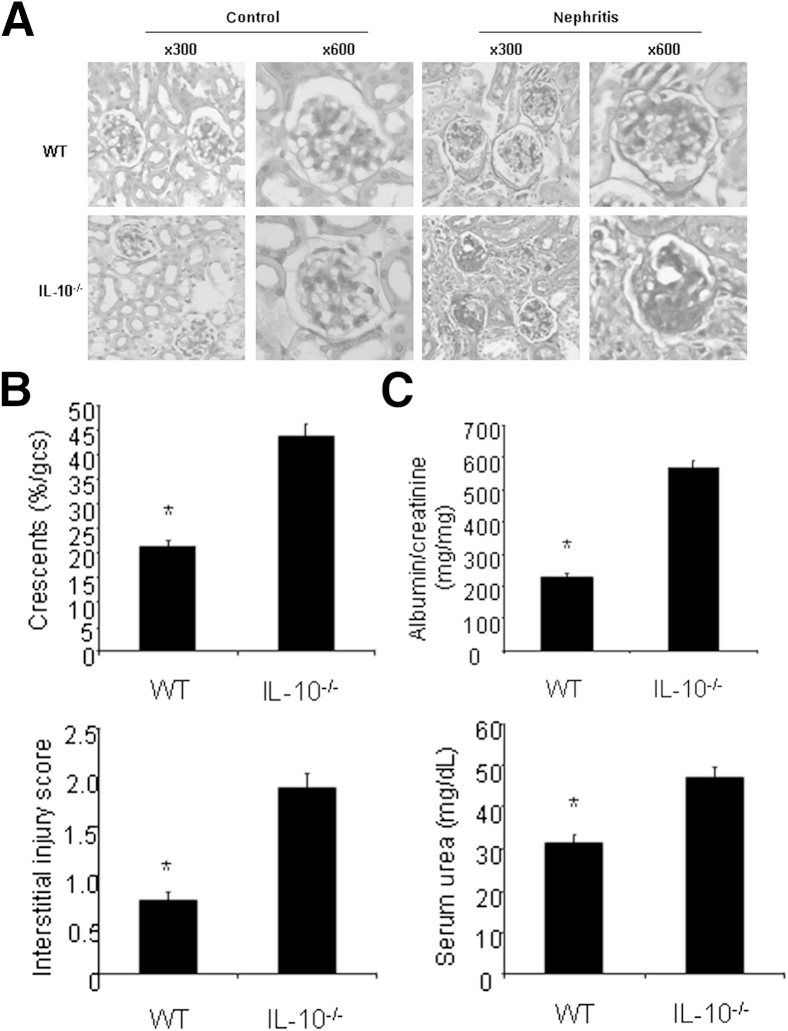
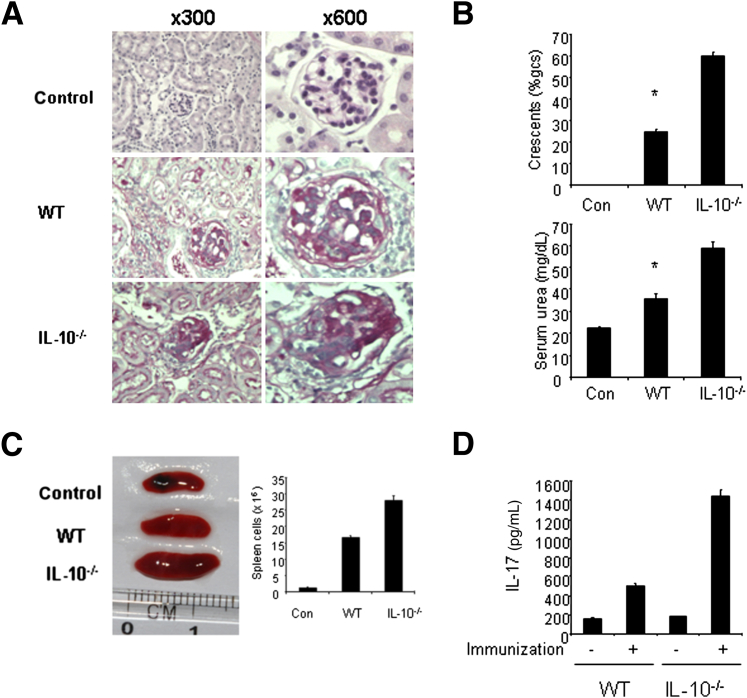
To investigate the function of IL-10 in the development of GN, we induced GN by using the aGBM disease model. IL-10–deficient mice developed more severe nephritis after injection of aGBM globulin into sensitized mice compared with WT mice (Figure 1A). IL-10−/− mice displayed more severe histologic and functional renal injury and exhibited more glomerular crescents and severe interstitial injury (Figure 1B). In addition, IL-10−/− mice also had more albuminuria and higher serum urea nitrogen levels compared with WT mice (Figure 1C). Taken together, the results suggest that anti-inflammatory cytokine IL-10 plays a protective role in the development of GN.
Figure 1.
IL-10–deficient mice develop more severe GN. WT and IL-10−/− mice were sensitized with SG for 5 days, and the mice were then were injected i.p. with sheep anti-mouse GBM globulin at a dose of 30 mg per mouse. Renal injuries were assessed 14 days later. A: PAS staining of glomeruli in WT and IL-10−/− mice. B: Quantification of histologic injury showed aggravation of GN in IL-10−/− mice (n = 6 per group). gcs, glomerular cross section. C: Functional injury indicated more albuminuria and higher serum urea levels in IL-10−/− mice (n = 6 per group). Data are given as means ± SD. ∗P < 0.05 versus IL-10−/− mice.
Enhanced Immune Responses in IL-10−/− Mice with aGBM Disease
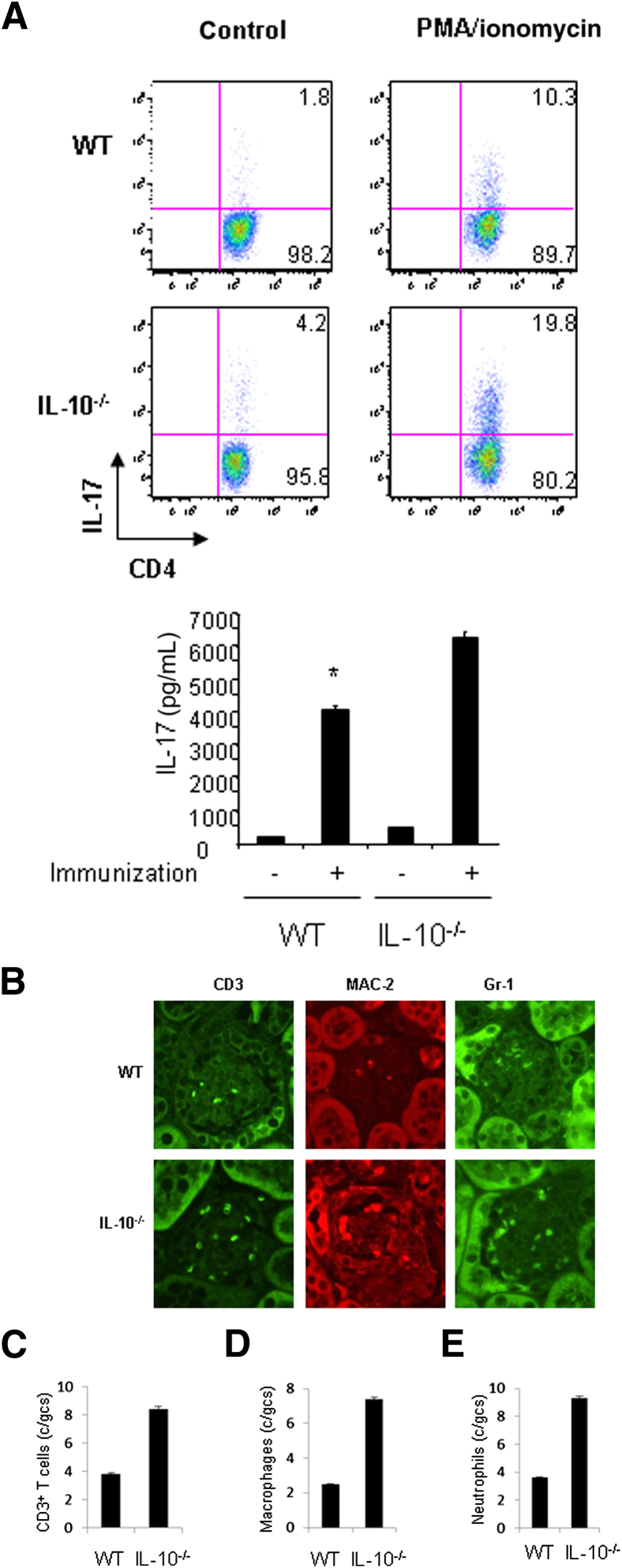
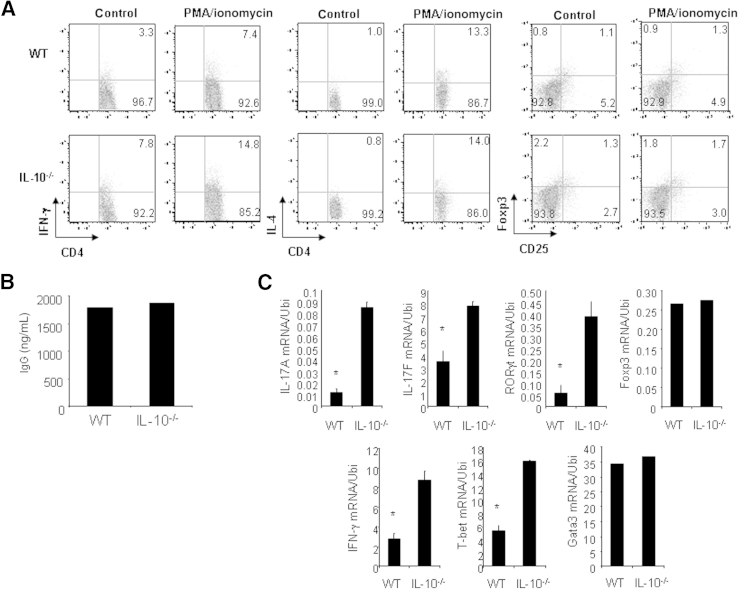
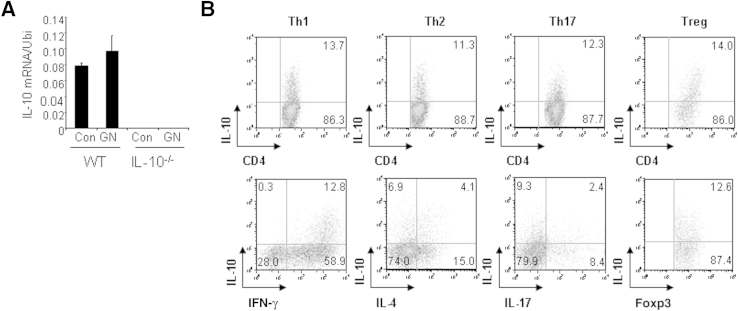
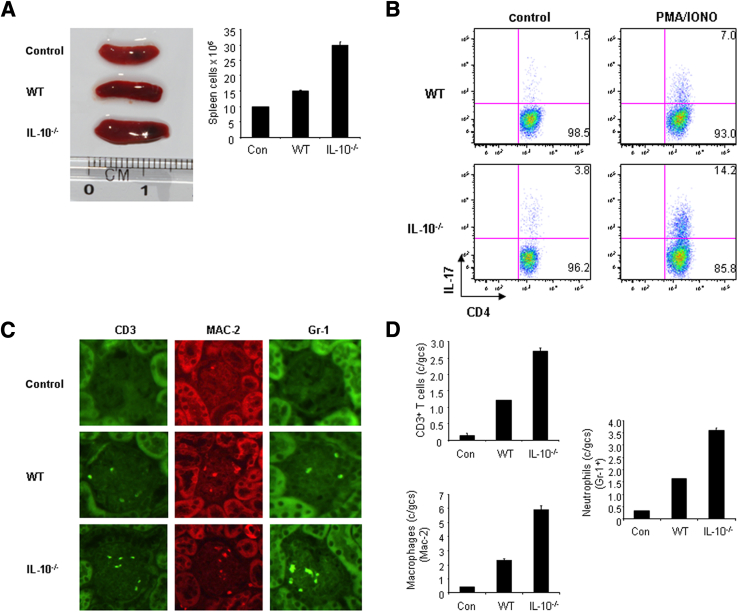
Th17 cells are thought to be pathogenic in several inflammatory diseases. As such, a study of Th17 cell development seems critical to understanding disease progression. We began to investigate the development of Th17 cells in WT and IL-10−/− mice with GN. We prepared splenocytes from WT and IL-10−/− mice with GN induced by aGBM and restimulated the cells with SG for 24 hours. After stimulation, IL-17–producing CD4+ T cells were analyzed by FACS, and IL-17 protein production was determined by ELISA. The percentage of IL-17–producing CD4+ T cells was significantly higher in IL-10−/− cell cultures (19.8%) than in WT cell cultures (10.3%) (Figure 2A). Similarly, IL-17 protein production was significantly enhanced in IL-10−/− cell cultures compared with WT cell cultures (Figure 2A). Furthermore, we assessed infiltration of inflammatory cells in the kidneys of WT and IL-10−/− mice with GN. Histologic analysis showed that IL-10−/− mice have increased numbers of glomerular CD3+ T cells, MAC-2+ macrophages, and Gr-1+ neutrophils compared with WT mice (Figure 2, B–E), suggesting that more inflammatory cells were present in the kidneys of IL-10−/− mice with GN. In addition, we also analyzed Th1, regulatory T (Treg), and Th2 cells in WT and IL-10−/− mice with GN. Splenocytes from WT and IL-10−/− mice with GN were restimulated with SG for 24 hours. IFN-γ–producing, IL-4–producing, and Treg cells were analyzed by FACS. The percentage of IFN-γ–producing CD4+ T cells was significantly increased in IL-10−/− cell cultures (14.8%) compared with WT cell cultures (7.4%) (Figure 3A). The percentages of IL-4–producing and Treg cells were comparable in WT and IL-10−/− cell cultures (Figure 3A). Furthermore, IgG levels in WT and IL-10−/− mice with GN were similar, ruling out B-cell involvement as a cause of IL-10−/− mice developing worse GN (Figure 3B). Real-time PCR analysis indicated that the mRNA expression of signature molecules for Th17 and Th1 cells was significantly enhanced in the kidneys of IL-10−/− compared with WT mice with GN (Figure 3C). Transcript levels of transcription factors (Gata3 and FOXP3) for Th2 and Treg cells, however, were not altered (Figure 3C). In addition, IL-10 mRNA expression was slightly increased in WT mice with GN (Figure 4A). To investigate IL-10 expression from different effector T cells, we activated naive CD4+ T cells from C57Bl/6 mice under Th1, Th2, Th17, or Treg cell conditions and found that all these effector T cells express IL-10 (Figure 4B). Taken together, these results suggest that IL-10 negatively regulates Th17 and Th1 immune responses in the development of GN.
Figure 2.
Enhanced immune responses in IL-10−/− mice with GN. A: Splenocytes were prepared from WT or IL-10−/− mice with GN induced by aGBM, and the cells were activated with SG for 24 hours. IL-17–producing CD4+ T cells were analyzed by FACS, and IL-17 protein levels in the supernatants were determined by ELISA. ∗P < 0.05, versus immunized IL-10−/− mice. B: WT or IL-10−/− mice were sensitized with SG for 5 days, and the mice were then injected i.p. with sheep anti-mouse GBM antibody at a dose of 30 mg per mouse. Renal injuries were assessed 14 days later. The infiltration of immune cells was analyzed by immunostaining in the kidneys. Quantification of T cells (C), macrophages (D), and neutrophils (E) in the kidneys of WT and IL-10−/− mice. gcs, glomerular cross section. Data are given as means ± SD.
Figure 3.
Th17 and Th1 immune responses are enhanced in IL-10−/− mice with GN. A: Cells from spleens and mesenteric lymph nodes were prepared from WT and IL-10−/− mice with GN induced by aGBM, and the cells were activated with SG for 24 hours. IFN-γ–producing, IL-4–producing, and Treg CD4+ T cells were analyzed by FACS. B: Serum was collected from WT and IL-10−/− mice with GN induced by aGBM, and the serum IgG level was determined by ELISA. C: Total RNA was extracted from the kidneys of WT and IL-10−/− mice with GN induced by aGBM, and real-time PCR was performed for the analysis of mRNA expression of the indicated genes. ∗P < 0.05 versus immunized IL-10−/− mice. Data are given as means ± SD. Ubi, ubiquitin.
Figure 4.
IL-10 is expressed in different effector T cells. A: WT and IL-10−/− mice were sensitized with SG for 5 , and the mice were then injected i.p. with sheep anti-mouse GBM globulin at a dose of 30 mg per mouse. Total RNA was extracted from the kidneys of WT and IL-10−/− mice with GN induced by aGBM or control mice. Real-time PCR was performed for the analysis of IL-10 mRNA expression. Data are given as means ± SD. B: Naive CD4+ T cells from WT and IL-10−/− mice were differentiated under nonpathogenic Th17, pathogenic Th17, or Th1 conditions for 3 days. Cells were then restimulated with PMA/ionomycin for 5 hours; stained for intracellular IL-10, IL-17, IFN-γ, IL-4, and Foxp3; and analyzed by flow cytometry. Representative FACS dot plots gated on CD4+ cells and the percentages of IL-17–producing or IFN-γ–producing or double-positive CD4+ cells are shown.
Transfer of CD4+ T Cells Induces More Severe GN in RAG1−/− Mice
These data suggest that Th17 cells may be involved in the development of GN in IL-10−/− mice. Next we wanted to confirm that the disease development is really due to CD4+ T cells. We first prepared splenocytes from WT and IL-10−/− mice and transferred the cells into Rag1−/− mice. Rag1−/− mice reconstituted with IL-10−/− splenocytes developed more severe GN, with more severe histologic injury and functional impairment of the kidney compared with mice reconstituted with WT splenocytes after induction of aGBM disease (Figure 5, A and B). In addition, mice reconstituted with IL-10−/− exhibited significantly larger spleens (Figure 5C), with more cells in the spleens after induction of GN (Figure 5C), suggesting that alterations of the immune responses and immune microenvironment had occurred. Meanwhile, significantly higher Th17 immune responses were observed in Rag1−/− mice reconstituted with IL-10−/− splenocytes (Figure 5D).
Figure 5.
Transfer of IL-10−/− spleen cells induces more severe GN in Rag1−/− mice. A: Spleen cells from WT and IL-10−/− mice were prepared and were injected i.p. into Rag1−/− mice. Beginning the next day, mice were sensitized with SG for 5 days, and the mice were then injected i.p. with sheep anti-mouse GBM globulin at a dose of 30 mg per mouse. Renal injuries were assessed 20 days later. A: PAS staining of glomeruli in mice reconstituted with either WT or IL-10−/− spleen cells. B: Quantification of histologic injury showed aggravation of GN in mice reconstituted with either WT or IL-10−/− spleen cells. gcs, glomerular cross section. ∗P < 0.05 versus mice transferred with IL-10−/− spleen cells. C: Spleens were enlarged and spleen cells were significantly increased in Rag1−/− mice reconstituted with IL-10−/− splenocytes. D: Splenocytes were prepared from Rag1−/− mice reconstituted with either WT or IL-10−/− spleen cells with GN induced by aGBM globulin, and the cells were activated with SG for 24 hours. The supernatants were harvested, and IL-17 protein levels were determined by ELISA. Data are given as means ± SD.
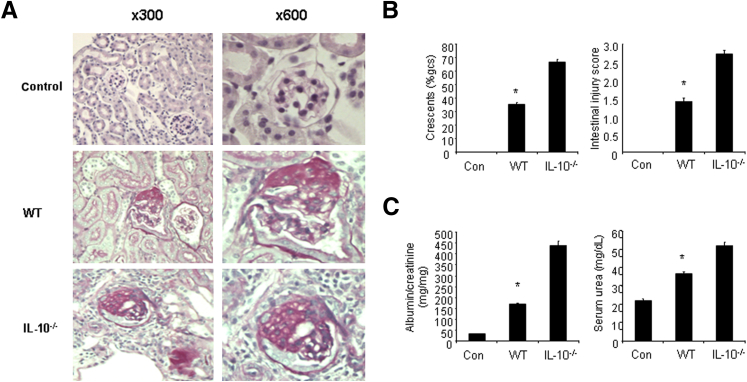
To further clarify the results, CD4+ T cells were extracted from spleens of WT and IL-10−/− mice and then were transferred into Rag1−/− mice. After adoptive transfer of CD4+ T cells into Rag1−/− mice, aGBM disease was induced in these mice. Mice reconstituted with IL-10−/− CD4+ T cells developed more severe renal disease compared with mice reconstituted with WT CD4+ T cells (Figure 6A). IL-10−/−CD4+ T cells transferred to Rag1−/− mice developed severe glomerular and interstitial injury (Figure 6B) and higher albuminuria and serum urea (Figure 6C). Taken together, these results demonstrate that the anti-inflammatory cytokine IL-10 plays an important role in the control of GN development, suggesting that IL-10 could be a therapeutic choice for the treatment of immune-related GN.
Figure 6.
Transfer of IL-10−/− CD4+ T cells induces more severe GN in Rag1−/− mice. A: CD4+ cells from spleens of WT and IL-10−/− mice were prepared and injected i.p. into Rag1−/− mice. From next day, mice were sensitized with SG for 5 days, and the mice were then injected i.p. with sheep anti-mouse GBM globulin at a dose of 30 mg per mouse. Renal injuries were assessed 20 days later. A: PAS staining of glomeruli in mice reconstituted with either WT or IL-10−/− CD4+ T cells. B: Quantification of histologic injury showed aggravation of GN in mice reconstituted with either WT or IL-10−/− CD4+ T cells (n = 6 per group). gcs, glomerular cross section. C: Functional injury indicated more albuminuria and higher serum urea levels in Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells (n = 6 per group). Data are given as means ± SD. ∗P < 0.05 versus IL-10−/− mice.
IL-10 Regulates Pathogenic Th17 Cell Differentiation in GN
To explore how IL-10 regulates pathogenic Th17 cell development during GN, we focused on Rag1−/− mice reconstituted with WT and IL-10−/− CD4+ T cells followed by immunization with SG for 6 days. We found that recipient mice reconstituted with IL-10−/− CD4+ T cells displayed enlarged spleens and increased splenocyte counts (Figure 7A) compared with mice transferred with WT CD4+ T cells. The spleen cells were restimulated with SG for 24 hours, and IL-17–producing CD4+ T cells were analyzed by flow cytometry. As expected, the percentage of Th17 cells was significantly higher in mice reconstituted with IL-10−/− CD4+ T cells (Figure 7B). In addition, significantly more inflammatory cells, including CD3+ T cells, MAC-2 macrophages, and Gr-1+ neutrophils were present in the kidneys of mice reconstituted with IL-10−/− CD4+ T cells (Figure 7, C and D). These results demonstrate that Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells develop more severe GN, with enhanced Th17 immune responses.
Figure 7.
Th17 immune responses are enhanced in Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells. CD4+ cells from spleens of WT and IL-10−/− mice were prepared and injected i.p. into Rag1−/− mice. Beginning the next day, mice were sensitized with SG for 5 days, and the mice were then injected i.p. with sheep anti-mouse GBM globulin at a dose of 30 mg per mouse. The mice were sacrificed 20 days later. A: Spleens were enlarged and spleen cells were significantly increased in Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells. B: Splenocytes were prepared from Rag1−/− mice reconstituted with either WT or IL-10−/− spleen cells with GN induced by aGBM globulin, and the cells were activated with SG for 24 hours. The cells were harvested, and IL-17–producing CD4+ T cells were analyzed by FACS. C: The infiltration of immune cells was analyzed by immunostaining in the kidneys. D: Quantification of T cells, macrophages, and neutrophils in the kidneys of mice reconstituted with either WT or IL-10−/− CD4+ T cells. gcs, glomerular cross section. Data are given as means ± SD.
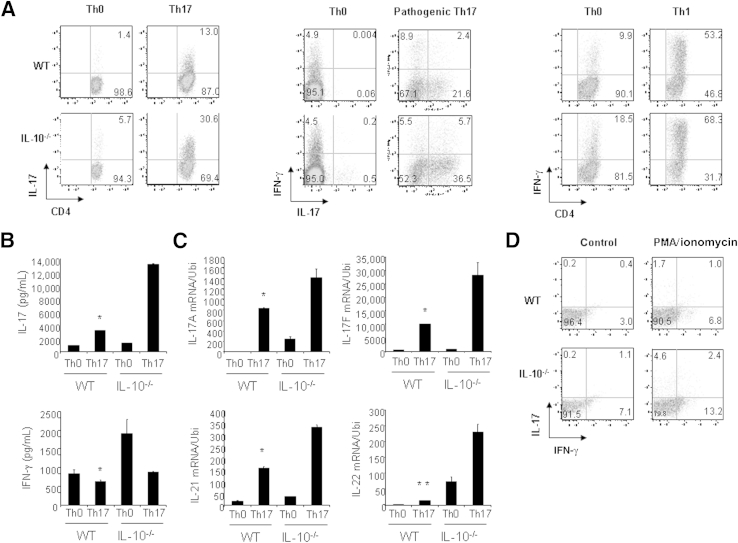
To further investigate the regulation of pathogenic Th17 cell differentiation by IL-10, we purified naive CD4+ T cells from the spleens and lymph nodes of WT and IL-10−/− mice and stimulated the cells under nonpathogenic Th17 conditions (TGF-β1 and IL-6), pathogenic Th17 conditions (TGF-β1, IL-6, and IL-23), or Th1 conditions (IL-12) for 3 days. The IL-17–producing CD4+ T-cell population was significantly higher in IL-10−/− cell cultures compared with WT cell cultures (Figure 8A).18 Under pathogenic Th17 conditions, the IL-17 and IFN-γ double-positive cell populations also significantly increased, which is similar to the response in IL-17 single-positive cells (Figure 8A). In addition, IFN-γ–producing cells were moderately increased (Figure 8A). IL-17 production and mRNA expression of Th17 signature molecules were also greatly enhanced in IL-10−/− mice under pathogenic Th17 conditions (Figure 8, B and C). To confirm that IL-10 regulates the production of Th17 cells in vivo, we prepared cells from the spleens and mesenteric lymph nodes of WT and IL-10−/− mice with GN and then stimulated those cells with PMA/ionomycin for 6 hours. Stimulated cells were harvested, and the IL-17 and IFN-γ double-positive CD4+ T cells were analyzed by FACS. As expected, the percentage of IL-17 and IFN-γ double-positive CD4+ T cells was significantly higher in IL-10−/− mice than in WT mice (Figure 8D). Taken together, these results suggest that IL-10 derived from T cells regulates pathogenic Th17 cell development in GN.
Figure 8.
IL-10 suppresses pathogenic Th17 cell differentiation. A: Naive CD4+ T cells from WT and IL-10−/− mice were differentiated under nonpathogenic Th17, pathogenic Th17, or Th1 conditions for 3 days. Cells were then restimulated with PMA/ionomycin for 5 hours, stained for intracellular IL-17 and IFN-γ, and analyzed by flow cytometry. Representative FACS dot plots gated on CD4+ cells and the percentages of IL-17–producing or IFN-γ–producing or double-positive CD4+ cells are shown. B: IL-17 and IFN-γ protein levels in the supernatants of cell culture under the pathogenic Th17 conditions prepared in A were determined by ELISA. C: Total RNA was extracted from the pathogenic Th17 cells prepared in A, and real-time RT-qPCR was performed for the detection of mRNA expression of cytokines. D: Cells from spleens and mesenteric lymph nodes were prepared from WT or IL-10−/− mice with GN induced by aGBM, and the cells were activated with PMA/ionomycin for 24 hours. IL-17 and IFN-γ double-positive cells were analyzed by FACS. Data are given as means ± SD. ∗P < 0.05, ∗∗P < 0.01 versus IL-10−/− Th17 cells.
Discussion
Although Th17 cells have been associated with the pathogenesis of inflammatory diseases, including GN, the regulation of pathogenic Th17 cell development during the process of inflammatory diseases is incompletely understood. Therefore, it is of great importance to identify the different factors that are responsible for the suppression of pathogenic Th17 cell differentiation in the target organs of inflammatory diseases. In the present study, we demonstrated that IL-10–deficient mice exhibit exacerbation of GN after induction with aGBM globulin, with enhanced Th17 immune responses. We further demonstrated that Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells develop more severe GN with aGBM globulin induction, which is associated with increased infiltration of inflammatory cells into the kidneys. Finally, the IL-17 and IFN-γ double-positive cell populations were significantly greater in IL-10−/− CD4+ T-cell cultures under pathogenic Th17 conditions compared with WT cell cultures, and double-positive cells were also significantly increased in IL-10−/− mice with disease. These results suggest that IL-10 plays a critical role in the control of pathogenic Th17 cell differentiation during GN development.
IL-10 is critical in setting immune response magnitude.28 Its genetic ablation or inhibition leads to spontaneous colitis and some other autoimmune and inflammatory diseases. Previously, Kitching et al31 reported that endogenous IL-10 protects against crescentic GN by suppressing Th1 immune responses. In addition, El-Shemi et al35 showed that IL-10 gene transfer suppressed experimental GN.35 However, the exact actions performed by IL-10 in preventing the development of GN are not well known. Recently, the new diagram indicates that Th17 cells instead of Th1 cells play the critical role in the development of inflammatory diseases. Therefore, the role of IL-10 in the development of immune-related GN needs to be revisited. In the present study, we demonstrated that IL-10–deficient mice exhibit worse GN in a mouse model induced with aGBM globulin, with enhanced Th17 immune responses. These results suggest that Th17 immune responses may be involved in the exacerbation of GN in IL-10–deficient mice.
IL-10 has been detected in human GN, in which IL-10 mRNA and protein are found in the more severe glomerular lesions.36 IL-10 mRNA has been detected in the kidneys of rats with accelerated anti-GBM GN.37 The expression of IL-10 in a disease situation may reflect initiation of protective mechanisms in the context of severe glomerular injury; however, the molecular regulation of IL-10 expression in a disease condition is still not fully understood. IL-10 is produced by different cell types, including macrophages, B cells, mesangial cells, keratinocytes, and T cells.28 In the present study, we focused on T-cell–expressed IL-10 in the pathogenesis of GN. Rag1−/− mice were reconstituted with either WT CD4+ T cells or IL-10−/− CD4+ T cells followed by induction with anti-GBM GN. The results show that Rag1−/− mice reconstituted with IL-10−/− developed more severe GN and renal injuries compared with mice transferred with WT cells. In addition, more Th17 cells were induced in mice reconstituted with IL-10−/− CD4+ T cells as well. These results indicate that IL-10 produced by T cells negatively regulates Th17 cell differentiation, resulting in the development of GN. Although we found that different Th cells produce IL-10 in vitro, it is still not clear which effector Th cells are an important cellular source for IL-10 expression in the development of GN.
Because Th1 and Th17 immune responses are significantly strengthened in IL-10−/− mice, it has been difficult to resolve the exact importance of which one (Th1 or Th17) is essential for the immune-related diseases in IL-10−/− mice. Yen et al30 investigated IL-10 and IL-23p19 or IL-10 and IL-12p35 double knockout mice and found that IL-10 and IL-12p35 double-knockout mice develop colitis in a similar way as IL-10 single-knockout mice. Although IL-10 and IL-23p19 double-knockout mice do not develop colitis,30 their results strongly support the idea that Th17 but not Th1 immune responses contribute to the pathogenesis of inflammatory diseases in IL-10−/− mice. In the present study, we observed that IL-10−/− or Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells developed worse GN in terms of glomerular crescent formation, serum urea nitrogen level, and inflammatory cell infiltration in the kidneys. Worsening of GN is correlated with enhanced Th17 and Th1 cell development in IL-10−/− mice. Taken together, we believe that Th17 and Th1cells regulated by IL-10 may contribute to the worsening of GN in IL-10−/− mice. However, the relative importance of Th17 and Th1 cells to the development of GN needs to be further explored in future studies.
Although Th17 cells have been associated with the induction of autoimmune/inflammatory diseases, emerging data suggest that not all Th17 cells are pathogenic and that exposure to IL-23 is crucial for their ability to induce autoimmunity.11,14,38,39 To investigate the regulation of nonpathogenic and pathogenic Th17 cell development by IL-10, we purified naive CD4+ T cells from WT and IL-10−/− mice and activated the cells under either nonpathogenic conditions or pathogenic conditions. The IL-17-single-positive cells were significantly increased in IL-10−/− cell culture under nonpathogenic or pathogenic conditions compared with WT cell cultures. However, IL-17/IFN-γ double-positive cells were significantly increased in IL-10−/− cell cultures under only pathogenic Th17 conditions. In addition, the double-positive cells were also significantly increased in IL-10−/− mice with GN. These results imply that IL-10 preferentially regulates pathogenic Th17 cell development. It is known that TGF-β1 is essential for nonpathogenic Th17 cell differentiation, whereas autonomously produced TGF-β3 from Th17 cells is critical in the induction of pathogenic Th17 cells.11 Although the present study demonstrated that IL-10 is clearly involved in the regulation of pathogenic Th17 cell induction, the molecular mechanisms underlying this phenomenon are incompletely understood. A future study will focus on the regulation of key transcription factors, including RORγt, IRF4, Ahr, and TGF-β3, by IL-10 under pathogenic Th17 conditions.
In summary, these studies clearly demonstrate that IL-10−/− mice or Rag1−/− mice reconstituted with IL-10−/− CD4+ T cells develop aggravated GN with enhanced Th17 and Th1 cell phenotypes. In addition, IL-10−/− mice display enhanced nonpathogenic and pathogenic Th17 cells. We suggest a novel regulation of pathogenic Th17 cell development by IL-10. These results support the concept that IL-10 expressed by T cells may play an important role in the control of pathogenesis of inflammatory diseases by controlling nonpathogenic and pathogenic Th17 immune responses.
Footnotes
Supported by NIH grants P01 DK072201 and R56AI091871 and by the Broad Medical Research Program of The Broad Foundation (H.X.).
R.Z. and Q.L. contributed equally to this work.
Contributor Information
John Cijiang He, Email: cijiang.he@mssm.edu.
Huabao Xiong, Email: huabao.xiong@mssm.edu.
References
- 1.Bettelli E., Korn T., Kuchroo V.K. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver C.T., Harrington L.E., Mangan P.R., Gavrieli M., Murphy K.M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 4.Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Korn T., Bettelli E., Gao W., Awasthi A., Jager A., Strom T.B., Oukka M., Kuchroo V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurieva R., Yang X.O., Martinez G., Zhang Y., Panopoulos A.D., Ma L., Schluns K., Tian Q., Watowich S.S., Jetten A.M., Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L., Ivanov, Spolski R., Min R., Shenderov K., Egawa T., Levy D.E., Leonard W.J., Littman D.R. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 9.Mangan P.R., Harrington L.E., O'Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y., Awasthi A., Yosef N., Quintana F.J., Xiao S., Peters A., Wu C., Kleinewietfeld M., Kunder S., Hafler D.A., Sobel R.A., Regev A., Kuchroo V.K. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov, McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang X., Zhang R., Yang J., Li Q., Qin L., Zhu C., Liu J., Ning H., Shin M.S., Gupta M., Qi C.F., He J.C., Lira S.A., Morse H.C., III, Ozato K., Mayer L., Xiong H. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat Commun. 2011;2:314. doi: 10.1038/ncomms1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeachy M.J., Chen Y., Tato C.M., Laurence A., Joyce-Shaikh B., Blumenschein W.M., McClanahan T.K., O'Shea J.J., Cua D.J. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L., Anderson D.E., Baecher-Allan C., Hastings W.D., Bettelli E., Oukka M., Kuchroo V.K., Hafler D.A. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 17.Stumhofer J.S., Laurence A., Wilson E.H., Huang E., Tato C.M., Johnson L.M., Villarino A.V., Huang Q., Yoshimura A., Sehy D., Saris C.J., O'Shea J.J., Hennighausen L., Ernst M., Hunter C.A. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y., Yang J., Ouyang X., Liu W., Li H., Bromberg J., Chen S.H., Mayer L., Unkeless J.C., Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 20.Hricik D.E., Chung-Park M., Sedor J.R. Glomerulonephritis. N Engl J Med. 1998;339:888–899. doi: 10.1056/NEJM199809243391306. [DOI] [PubMed] [Google Scholar]
- 21.Tipping P.G., Holdsworth S.R. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–1263. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- 22.Kitching A.R., Holdsworth S.R., Tipping P.G. Crescentic glomerulonephritis: a manifestation of a nephritogenic Th1 response? Histol Histopathol. 2000;15:993–1003. doi: 10.14670/HH-15.993. [DOI] [PubMed] [Google Scholar]
- 23.Ooi J.D., Phoon R.K., Holdsworth S.R., Kitching A.R. IL-23, not IL-12, directs autoimmunity to the Goodpasture antigen. J Am Soc Nephrol. 2009;20:980–989. doi: 10.1681/ASN.2008080891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinmetz O.M., Summers S.A., Gan P.Y., Semple T., Holdsworth S.R., Kitching A.R. The Th17-defining transcription factor RORgammat promotes glomerulonephritis. J Am Soc Nephrol. 2011;22:472–483. doi: 10.1681/ASN.2010040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner J.E., Paust H.J., Steinmetz O.M., Panzer U. The Th17 immune response in renal inflammation. Kidney Int. 2010;77:1070–1075. doi: 10.1038/ki.2010.102. [DOI] [PubMed] [Google Scholar]
- 26.Turner J.E., Paust H.J., Steinmetz O.M., Peters A., Riedel J.H., Erhardt A., Wegscheid C., Velden J., Fehr S., Mittrucker H.W., Tiegs G., Stahl R.A., Panzer U. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21:974–985. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorentino D.F., Bond M.W., Mosmann T.R. Two types of mouse T helper cell, IV: Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang W., Rutz S., Crellin N.K., Valdez P.A., Hymowitz S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 29.Moore K.W., de Waal Malefyt R., Coffman R.L. O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 30.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., Murphy E., Sathe M., Cua D.J., Kastelein R.A., Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitching A.R., Tipping P.G., Timoshanko J.R., Holdsworth S.R. Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int. 2000;57:518–525. doi: 10.1046/j.1523-1755.2000.00872.x. [DOI] [PubMed] [Google Scholar]
- 32.Dean E.G., Wilson G.R., Li M., Edgtton K.L., O'Sullivan K.M., Hudson B.G., Holdsworth S.R., Kitching A.R. Experimental autoimmune Goodpasture's disease: a pathogenetic role for both effector cells and antibody in injury. Kidney Int. 2005;67:566–575. doi: 10.1111/j.1523-1755.2005.67113.x. [DOI] [PubMed] [Google Scholar]
- 33.Phoon R.K., Kitching A.R., Odobasic D., Jones L.K., Semple T.J., Holdsworth S.R. T-bet deficiency attenuates renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2008;19:477–485. doi: 10.1681/ASN.2007030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Totsuka T., Kanai T., Nemoto Y., Makita S., Okamoto R., Tsuchiya K., Watanabe M. IL-7 Is essential for the development and the persistence of chronic colitis. J Immunol. 2007;178:4737–4748. doi: 10.4049/jimmunol.178.8.4737. [DOI] [PubMed] [Google Scholar]
- 35.El-Shemi A.G., Fujinaka H., Matsuki A., Kamiie J., Kovalenko P., Qu Z., Bilim V., Nishimoto G., Yaoita E., Yoshida Y., Anegon I., Yamamoto T. Suppression of experimental crescentic glomerulonephritis by interleukin-10 gene transfer. Kidney Int. 2004;65:1280–1289. doi: 10.1111/j.1523-1755.2004.00536.x. [DOI] [PubMed] [Google Scholar]
- 36.Niemir Z.I., Ondracek M., Dworacki G., Stein H., Waldherr R., Ritz E., Otto H.F. In situ upregulation of IL-10 reflects the activity of human glomerulonephritides. Am J Kidney Dis. 1998;32:80–92. doi: 10.1053/ajkd.1998.v32.pm9669428. [DOI] [PubMed] [Google Scholar]
- 37.Chadban S.J., Tesch G.H., Foti R., Lan H.Y., Atkins R.C., Nikolic-Paterson D.J. Interleukin-10 differentially modulates MHC class II expression by mesangial cells and macrophages in vitro and in vivo. Immunology. 1998;94:72–78. doi: 10.1046/j.1365-2567.1998.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., Zurawski S., Wiekowski M., Lira S.A., Gorman D., Kastelein R.A., Sedgwick J.D. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 39.Awasthi A., Riol-Blanco L., Jager A., Korn T., Pot C., Galileos G., Bettelli E., Kuchroo V.K., Oukka M. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]