Abstract
Molecular analyses for the study of soil microbial communities often depend on the direct extraction of DNA from soils. The present work compares the effectiveness of three different methods of extracting microbial DNA from seven different paddy soils. Comparison among different DNA extraction methods against different paddy soil samples revealed a marked variation in DNA yields from 3.18–20.17 μg DNA/g of dry soil. However, irrespective of the soil samples and extraction methods the DNA fragment size was >10 kb. Among the methods evaluated, method-C (chemical–enzymatic–mechanical) had better cell lysis efficiency and DNA yield. After purification of crude DNA by Purification Kit, A260/A230 and A260/A280 ratios of the DNA obtained by method-C reached up to 2.27 and 1.89, respectively, sustaining the efficacy of this technique in removing humic acid, protein and other contaminants. Results of the comprehensive evaluation of DNA extraction methods suggest that method-C is superior to other two methods (chemical–enzymatic and chemical–mechanical), and was the best choice for extraction of total DNA from soil samples. Since soil type and microbial community characteristics influence DNA recovery, this study provides guidance for choosing appropriate extraction and purification methods according to experimental goals.
Keywords: Extraction method, Microbial DNA, Purification, Paddy soil
1. Introduction
Microbial communities play a critical role in maintaining soil productivity by regulating the cycling, retention and release of major nutrients in soil (Torsvik and Øvreås, 2002; Islam et al., 2011). But till to date, up to 99% of the microbes present in soil are neither cultivable nor accessible for basic biotechnological research (Knietsch et al., 2003; Lakay et al., 2007). Conventional approaches currently being used appear to be inaccurate, and the results obtained hardly indicate comprehensive profile of soil microbial diversity in situ (Luo et al., 2003). On the other hand, molecular techniques such as PCR amplification of 16S rRNA genes or other genes of ecological significance yield relatively less biased information about microbial communities than traditional culturing approaches. Therefore, molecular analyses of microbial communities in complex environmental samples such as soil warrant efficient unbiased DNA extraction procedures.
Numerous techniques have been developed for direct extraction and purification of total community DNA from different environmental samples (Bürgmann et al., 2001; Roose-Amsaleg et al., 2001; Luna et al., 2006). Among them, the most commonly applied approach involves the in situ lysis of cells (Roose-Amsaleg et al., 2001) through chemical and/or enzymatic and/or mechanical lysis (Robe et al., 2003; Luna et al., 2006). Though these methods generally provide the highest DNA yields within acceptable processing times by complete in situ lysis of all microorganisms, each method has its own disadvantages (Robe et al., 2003). The lysis efficiency in any nucleic acid extraction procedure is critical in determining its success, such that an accurate representation of the microbial community can be achieved (Robe et al., 2003; de Lipthay et al., 2004).
The purity of the DNA from soil is often found unsatisfactory, particularly in soils rich in humic compounds (Courtois et al., 2001) such as bulk soil from paddy fields. Because of its physico-chemical similarity with nucleic acids, humic substances are usually co-extracted during extraction of DNA from soils and this can interfere with DNA detection, measurement and purification (Zhou et al., 1996). This contamination can inhibit the activity of Taq DNA polymerase during PCR amplification of genes (Luo et al., 2003).
Paddy soils represent one of the principal agricultural systems in Korea. Fertile soil provides essential nutrients for crop growth, and then supports a diverse and active microbial community. Knowledge of the microbial community structure in different paddy soils can advance our understanding of soil processes and microbial functions in rice-based cropping system (Islam et al., 2009). Though many methods for community DNA extraction from soil samples have already been described, none of these have been shown to be robust enough to be accepted by the scientific community as a standard protocol. Moreover, most of the methods involve re-purifying process, which are not only time consuming and costly but also subject to DNA loss. In the present study, we compared and evaluated three different methods for extraction of microbial community DNA from seven different paddy soils through analyzing simplicity, purity, and yields of DNA.
2. Materials and methods
2.1. Sample collection
The soil samples were collected from seven different paddy fields located at the National Institute of Agricultural Science and Technology, Suwon city, Republic of Korea in October 2008. The sampling was done by collecting soils from nine randomly selected points within each field at 0–20 cm depth using a 1.45 cm diameter soil core. Samples from each field were then combined to form one composite sample and stored at 4 °C during experimental period. The properties of bulk soil texture are described in Table 1.
Table 1.
Selected properties of the different paddy soil samples. The values are averages ± standard errors based on three replications.
| Soil sample No. | Soil texture | pH | EC (dS/m) | Organic matter (g/kg) | Available N (mg/kg) | Available P (mg/kg) | Available K (mg/kg) |
|---|---|---|---|---|---|---|---|
| 1 | Sandy loam | 6.0 ± 0.35 | 0.65 ± 0.05 | 38 ± 2.21 | 112.36 ± 4.56 | 76.14 ± 2.27 | 227.30 ± 6.32 |
| 2 | Silty loam | 6.4 ± 0.11 | 0.79 ± 0.03 | 27 ± 1.64 | 105.17 ± 1.98 | 25.72 ± 0.85 | 124.15 ± 2.55 |
| 3 | Sandy loam | 6.5 ± 0.23 | 0.83 ± 0.17 | 31 ± 0.92 | 103.84 ± 6.12 | 68.55 ± 4.38 | 87.60 ± 3.24 |
| 4 | Clay loam | 5.8 ± 0.38 | 1.21 ± 0.09 | 43 ± 1.13 | 120.57 ± 3.50 | 45.70 ± 3.14 | 175.16 ± 8.05 |
| 5 | Loamy | 6.9 ± 0.19 | 0.57 ± 0.03 | 25 ± 2.27 | 115.20 ± 2.03 | 105.61 ± 7.42 | 256.42 ± 2.86 |
| 6 | Silty clay | 5.9 ± 0.42 | 1.06 ± 0.06 | 29 ± 0.64 | 131.91 ± 4.35 | 83.46 ± 2.95 | 96.50 ± 4.50 |
| 7 | Clay loam | 6.7 ± 0.24 | 0.72 ± 0.11 | 36 ± 1.08 | 137.82 ± 7.40 | 117.23 ± 5.08 | 306.15 ± 7.12 |
2.2. Extraction of soil DNA
To extract total microbial community DNA from paddy soils, we applied three different methods; method-A (chemical–enzymatic lysis), method-B (chemical–mechanical lysis), and method-C (chemical–enzymatic–mechanical lysis). The basic–differences among the three extraction methods are shown in Table 2.
Table 2.
Treatment differences for soil lyses in three different methods.
| Treatment | Method-A | Method-B | Method-C |
|---|---|---|---|
| Chemical | 20% SDS | 1% SDS | 10% SDS |
| 1% CTAB | 0.1 M NaCl | 0.15 M NaCl for solution I | |
| 1.5 M NaCl | 0.1 M NaCl for solution II | ||
| Enzymatic | Proteinase-K | – | Lysozyme |
| Mechanical | – | Freezing and thawing | Freezing and thawing |
In method-A, DNA was extracted by the protocol of Zhou et al. (1996) with a little modification. Briefly, 5 g of soil samples were mixed with 13.5 mL of DNA extraction buffer (100 mM Tris–HCl, pH 8.0; 100 mM sodium EDTA, pH 8.0; 100 mM sodium phosphate, pH 8.0; 1.5 M NaCl; 1% CTAB [Hexadecylmethylammonium bromide]) and 100 μL Proteinase-K (10 mg/mL) in a Oakridge tube by horizontal shaking at 225 rpm under 37 °C for 30 min. 1.5 mL of 20% sodium dodecyl sulfate (SDS) was added to the sample mixture, which was then incubated for 2 h at 65 °C in a water bath with gentle end-over-end inversions every 15–20 min. After centrifugation at 6000 rpm for 10 min under room temperature the supernatants were collected, and the pellets were transferred into a 50 mL centrifuge tube. The pellets remaining were then extracted two more times by adding 4.5 mL of the extraction buffer and 0.5 mL of 20% SDS, vortexed for 10 s, followed by incubation at 65 °C for 10 min, and centrifugation as described earlier.
For Method-B, Kuske’s (1997) extraction protocol was followed with slight modifications. Ten milliliters of TENS buffer (50 mM Tris, pH 8.0; 20 mM disodium EDTA; 0.1 M NaCl; 1% [w/v] SDS) was added to 5 g of soil samples and vortexed. The samples were incubated in a water bath at 70 °C for 1 h, and centrifuged at 6000 rpm for 10 min to collect the supernatant. The soil pellet was then washed with 5 mL of TEN buffer (TENS buffer without SDS), and the supernatant was collected upon centrifugation. Thereafter, the soil pellet was re-suspended in 7.5 mL of TEN buffer and exposed to three sets of thermal shocks by immersion of the tubes at −20 °C for 10 min followed by rapid thawing in a 65 °C water bath, centrifugation at 6000 rpm, and the supernatant was collected.
For method-C, DNA extraction procedure by Tsai and Olson (1991) was used with modification. Shortly, 5 g of soil samples were mixed with 10 mL of 120 mM sodium phosphate buffer (pH 8.0) by shaking at 150 rpm for 15 min. The slurry was pelleted by centrifugation at 6000 rpm for 10 min. The pellet was washed again with phosphate buffer, re-suspended in 10 mL lysis solution I (0.15 M NaCl; 0.1 M disodium EDTA, pH 8.0) containing 15 mg/mL of lysozyme, and incubated in a 37 °C water bath for 2 h with agitation at 20–30 min intervals, and then 10 mL of lysis solution II (0.1 M NaCl; 0.5 M Tris–HCl, pH 8.0; 10% SDS) was added. Three cycles of freezing in −20 °C and thawing at 65 °C in water bath was conducted to release DNA from the microbial cells, and the suspension centrifuged at 6000 rpm for 15 min to get the supernatant.
Supernatants obtained from all three cycles of extractions were combined for each of the three different methods, and then mixed with an equal volume of chloroformisoamyl alcohol (24:1, v/v). The aqueous phase was recovered by centrifugation and precipitated with 0.6% v/v of isopropanol at room temperature for 1 h. Crude nucleic acid pellet was obtained by centrifugation at 12,000 rpm for 20 min at room temperature, washed with cold 70% ethanol, and re-suspended in sterile deionized water to give a final volume of 500 μL and stored at −20 °C for future use.
2.3. Evaluation of DNA quality and quantity
The quality and quantity of DNA were evaluated and estimated using a Spectrophotometer (UV-1601, Shimadzu) by calculating the A260/A230 and A260/A280 ratios as described by Sambrook et al. (1989). This method was based on the principle that co-extracted humic acids, phenol, and other aromatic compounds are absorbed at 230 nm whereas DNA at 260 and protein at 280 nm; high A260/A230 and A260/A280 ratios are indicative of purity of DNA (Yeates et al., 1998; De Maeseneire et al., 2007). A pure sample of DNA has the A260/A280 ratio as 1.80, and the A260/A230 ratio as 2.00, whereas DNA preparation that is contaminated with protein will have an A260/A280 ratio lower than 1.80 (Sambrook et al., 1989). Samples of extracted DNA were also assessed by agarose gel electrophoresis. The band size of each extracted crude DNA was determined by comparing with known concentration of molecular weight marker (DirectLoad™ Wide Range DNA Marker) on 0.8% (w/v) agarose gel containing 0.5 μg/mL of ethidium bromide. Gel images were visualized under ultraviolet light (Bio-Rad Laboratories, CA, USA).
2.4. Purification of DNA
All extracted crude DNA were purified by GeneAll® DNA Purification Kit (Biofrontier Technology). The purification protocols (gel-plus-column method) were performed according to the manufacturer’s instructions.
2.5. Statistical analyses
The data were analyzed using the SAS package version 9.1 (SAS Institute Inc., Cary, NC, USA). The values given represent mean ± standard errors based on three replications.
3. Results and discussion
In the present investigation, we used three different extraction methods to separate microbial community DNA from seven different paddy soil samples. The efficiency of soil microbial DNA extraction depends on soil qualities, including the cell content, pH value, and humic acid content (Roose-Amsaleg et al., 2001; Gu et al., 2005). Different paddy soil samples revealed a noticeable variation in DNA yields, and this variation observed in our work may be due to the differences in soil properties. Johnson et al. (2003) reported that bacterial DNA fingerprints are significantly correlated with soil electrical conductivity, soil texture, inorganic carbon, and nitrogen content but not with pH and organic carbon content.
The purity of DNA in crude extracts for three methods was determined by the UV-Spectrophotometer, and the results are presented in Table 3. UV absorption ratio of A260/A230 was 0.91 to 1.22 for method-A, 0.88 to 1.26 for method-B, and 1.16 to 1.49 for method-C. Similarly, UV absorption ratio of A260/A280 of extracted crude DNA was ranged between 1.12–1.26, 1.12–1.30 and 1.08–1.40 by methods-A, -B, and -C, respectively. These results indicate DNA extracted via method-C yielded DNA that was relatively free from humic acids and protein contamination. On the other hand, the ratios of A260/A230 and A260/A280 for method-B were comparatively lower than those of method-A and method-C, suggesting that method-B was less effective in removing the contamination although it had a higher OD value at 260 nm. Nevertheless, it can be seen from our result that the respective averages of A260/A230 and A260/A280 ratios of DNA from all soil samples were above 1.00, suggesting that DNA extracted by the three methods contained low humic acid and protein impurities (Yeates et al., 1998; Roh et al., 2006).
Table 3.
Purity ratios of extracted microbial DNA, and DNA yield from paddy soils by three different methods. The values are averages ± standard errors based on three replications.
| Method | Soil sample | Crude DNA |
Purified DNA |
DNA yields (μg/g of dry soil)a | ||
|---|---|---|---|---|---|---|
| A260/A230 | A260/A280 | A260/A230 | A260/A280 | |||
| A | 1 | 0.92 ± 0.03 | 1.14 ± 0.04 | 1.89 ± 0.04 | 1.68 ± 0.03 | 10.93 ± 1.17 |
| 2 | 0.93 ± 0.01 | 1.12 ± 0.02 | 1.91 ± 0.05 | 1.72 ± 0.04 | 12.20 ± 1.26 | |
| 3 | 0.91 ± 0.05 | 1.14 ± 0.02 | 1.73 ± 0.01 | 1.48 ± 0.06 | 8.78 ± 0.04 | |
| 4 | 0.92 ± 0.07 | 1.15 ± 0.07 | 1.86 ± 0.03 | 1.64 ± 0.02 | 6.94 ± 0.08 | |
| 5 | 1.02 ± 0.04 | 1.26 ± 0.05 | 2.01 ± 0.04 | 1.62 ± 0.07 | 13.02 ± 1.27 | |
| 6 | 1.14 ± 0.02 | 1.22 ± 0.03 | 2.05 ± 0.07 | 1.65 ± 0.05 | 18.65 ± 1.24 | |
| 7 | 1.22 ± 0.04 | 1.12 ± 0.06 | 2.01 ± 0.05 | 1.72 ± 0.02 | 13.17 ± 1.02 | |
| B | 1 | 0.89 ± 0.02 | 1.16 ± 0.01 | 1.78 ± 0.06 | 1.57 ± 0.05 | 7.16 ± 0.65 |
| 2 | 0.88 ± 0.06 | 1.17 ± 0.03 | 1.75 ± 0.02 | 1.60 ± 0.04 | 9.13 ± 1.34 | |
| 3 | 0.90 ± 0.08 | 1.15 ± 0.08 | 1.69 ± 0.05 | 1.50 ± 0.03 | 5.95 ± 0.05 | |
| 4 | 0.94 ± 0.01 | 1.15 ± 0.02 | 1.72 ± 0.06 | 1.47 ± 0.05 | 3.18 ± 0.14 | |
| 5 | 0.88 ± 0.04 | 1.16 ± 0.04 | 1.74 ± 0.01 | 1.59 ± 0.06 | 4.87 ± 0.06 | |
| 6 | 1.22 ± 0.08 | 1.12 ± 0.05 | 1.78 ± 0.03 | 1.49 ± 0.07 | 9.54 ± 1.09 | |
| 7 | 1.26 ± 0.05 | 1.30 ± 0.03 | 1.80 ± 0.07 | 1.75 ± 0.09 | 11.36 ± 1.21 | |
| C | 1 | 1.35 ± 0.09 | 1.08 ± 0.02 | 2.11 ± 0.08 | 1.76 ± 0.07 | 13.75 ± 1.07 |
| 2 | 1.16 ± 0.01 | 1.16 ± 0.06 | 2.13 ± 0.05 | 1.81 ± 0.02 | 15.87 ± 0.05 | |
| 3 | 1.27 ± 0.03 | 1.22 ± 0.04 | 2.13 ± 0.02 | 1.69 ± 0.05 | 14.05 ± 1.42 | |
| 4 | 1.17 ± 0.01 | 1.40 ± 0.03 | 2.10 ± 0.09 | 1.84 ± 0.06 | 13.74 ± 0.04 | |
| 5 | 1.49 ± 0.07 | 1.37 ± 0.05 | 2.27 ± 0.03 | 1.89 ± 0.08 | 20.17 ± 1.31 | |
| 6 | 1.21 ± 0.02 | 1.40 ± 0.03 | 2.11 ± 0.01 | 1.86 ± 0.04 | 18.50 ± 1.23 | |
| 7 | 1.27 ± 0.06 | 1.33 ± 0.01 | 2.16 ± 0.04 | 1.83 ± 0.03 | 16.04 ± 1.08 | |
DNA yields were calculated by the OD value of DNA determined by UV-Spectrophotometer.
Total yields of the extracted DNA from soil samples using three different methods were determined spectrophotometrically and the yields were found to be varied with different extraction methods (Table 3). Depending upon soils, DNA extraction by the method-A, method-B, and method-C yielded to 6.94–18.65, 3.18–11.36, and 13.74–20.17 μg DNA/g dry soil sample, respectively. As it shows, quantity of DNA recovered using enzymatic cell lysis method (method-A and method-C) was higher compared to chemical–mechanical disruption (method-B). Maximum 20.17 μg DNA/g soil was obtained with method-C followed by 18.65 μg DNA/g soil with method-A. These results were a little higher than the previous finding (18.20 μg/g) of Howeler et al. (2003). The possible reason for method-A and method-C yielding the promising extraction result is that enzymatic digestion (proteinase-K and lysozyme) could effectively break up the cell wall of microorganism to release DNA easily (Zhang et al., 2003). Though we found method-B to yield the lowest (3.18 μg DNA/g of soil) in this study, it is still higher compared to earlier observation by Martin-Laurent et al. (2001) who have obtained 0.19–2.52 μg/g DNA from different soil samples using a similar technique.
The differences in DNA yields by three methods from each of the seven soil samples were visualized by band analysis after electrophoresis of extracted crude DNA (Fig. 1). The amount and quality of the DNA extracted from all soils were similar and the fragment size was larger than 10 kb. However, method-B and method-C yielded comparatively low molecular size DNA in case of Soil Sample 1 and Soil Sample 2.
Figure 1.
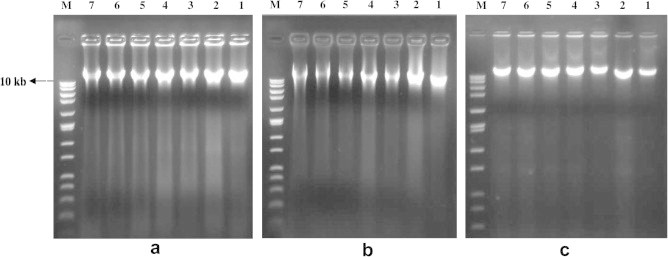
Comparison of total microbial DNA extracted from different paddy soils: (a) Method-A, (b) Method-B and (c) Method-C. Lanes: 1, Soil Sample 1; 2, Soil Sample 2; 3, Soil Sample 3; 4, Soil Sample 4; 5, Soil Sample 5; 6, Soil Sample 6; 7, Soil Sample 7; M, molecular weight marker (DirectLoad™ Wide Range DNA Marker).
It was observed in the present study that the contamination of humic materials and protein was very high in crude microbial DNA. After crude DNA was purified using Purification Kit, the A260/A230 and A260/A280 ratios of DNA from different soil samples were ranged from 1.73 to 2.05 and 1.48 to 1.72 by method-A, 1.69 to 1.80 and 1.47 to 1.75 by method-B, and 2.10 to 2.27 and 1.69 to 1.89 by method-C (Table 3). These results indicate that DNA extracted by all three methods was of good quality. By comparison, the DNA recovery efficiency of method-B was obviously lower than method-A and method-C. Sambrook et al. (1989) reported that A260/A230 ratio greater than 2.00 and A260/A280 ratio greater than 1.80 indicate pure DNA, while low ratios indicate humic acid or protein contamination. Similar results were obtained by method-C with all soils except Soil Sample 1 and Soil Sample 3, where A260/A280 ratios of purified DNA were 1.76 and 1.69, respectively. This shows the efficiency of this method compared to others in removing contaminants.
In our experiment, enzymatic techniques showed the best results with respect to cell lyses and DNA purity. Better quality of DNA with high molecular weight and purity was obtained with method-C. This may be due to the combination of chemical/enzymatic/mechanical lysis technique, which could give much higher DNA yields without severe shearing as previously reported by Zhou et al. (1996). On the other hand, method-B produced comparatively low quality and quantity of DNA. The reason behind this can be attributed to the fact that repeated freeze–thaw operation can cause a certain degree of damage to nucleic acid, especially large linear DNA molecules such as eukaryotic chromosomal DNA. Previously, Liesack et al. (1991) reported that bead mill homogenization and other mechanical approaches such as sonication generally cause severe DNA shearing.
4. Conclusion
The results of this comprehensive evaluation of DNA extraction methods suggest that all of these methods were suitable for use in a large-scale study involving the direct comparative analysis of different paddy soils. However, method-C (chemical–enzymatic–mechanical lysis followed by DNA purification) proved to be superior to other two methods (chemical–enzymatic and chemical–mechanical), and was the best choice to extract total DNA from soil samples. UV absorption profiles showed that extracted DNA was relatively free of adhering soil components such as humic acids and protein contaminants. This method was found to be reliable, simple, rapid, and affordable for microbial community DNA extraction from different soils. It is, however, important to recognize that no single method of DNA extraction or purification will be appropriate for all soil types and experimental goals, as there are multiple factors that may affect the performance of an extraction method. Combinations and modifications of different protocols might be needed for some conditions.
Acknowledgments
This work was supported by the research grant of Korea Research Foundation (KRF), Republic of Korea. We thank the anonymous reviewers for critical reading of this manuscript. The partial support from the research grant of Inha University, Republic of Korea, is also acknowledged.
References
- Bürgmann H., Pesaro M., Widmer F., Zeyer J. A strategy for optimizing quality and quantity of DNA extracted from soil. J. Microbiol. Methods. 2001;45:7–20. doi: 10.1016/s0167-7012(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Courtois S., Frostegard A.S., Goransson P. Quantification of bacterial subgroups in soil: comparison of DNA extracted directly from soil or from cells previously released by density gradient centrifugation. Environ. Microbiol. 2001;7:431–439. doi: 10.1046/j.1462-2920.2001.00208.x. [DOI] [PubMed] [Google Scholar]
- de Lipthay J.R., Enzinger C., Johnsen K., Aamand J., Sørensen S.J. Impact of DNA extraction method on bacterial community composition measured by denaturing gradient gel electrophoresis. Soil Biol. Biochem. 2004;36:1607–1614. [Google Scholar]
- De Maeseneire S.L., Van Bogaert I.N., Dauvrin T., Soetaert W.K., Vandamme E.J. Rapid isolation of fungal genomic DNA suitable for long distance PCR. Biotechnol. Lett. 2007;29:1845–1855. doi: 10.1007/s10529-007-9483-6. [DOI] [PubMed] [Google Scholar]
- Gu H.J., Li Y.X., Zhao M.W. Comparison of methods of DNA extraction from paddy soil. J. Jiangsu Univ. 2005;15:300–305. (in Chinese) [Google Scholar]
- Howeler M., Ghiorse W.C., Walker L.P. A quantitative analysis of DNA extraction and purification from compost. J. Microbiol. Methods. 2003;54:37–45. doi: 10.1016/s0167-7012(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Islam M.R., Trivedi P., Palaniappan P., Reddy M.S., Sa T. Evaluating the effect of fertilizer application on soil microbial community structure in rice based cropping system using Fatty acid methyl esters (FAME) analysis. World J. Microbiol. Biotechnol. 2009;25:1115–1117. [Google Scholar]
- Islam M.R., Chauhan P.S., Kim Y., Kim M., Sa T.M. Community level functional diversity and enzyme activities in paddy soils under different long-term fertilizer management practices. Biol. Fert. Soils. 2011;47:599–604. [Google Scholar]
- Johnson M.J., Lee K.Y., Scow K.M. DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma. 2003;114:279–303. [Google Scholar]
- Knietsch A., Waschkowitz T., Bowien S., Henne A., Daniel R. Metagenomics of complex microbial consortia derived from different soils as sources for novel genes conferring formation of carbonyls from short-chain polyols on Escherichia coli. J. Mol. Microbiol. Biotechnol. 2003;5:46–56. doi: 10.1159/000068724. [DOI] [PubMed] [Google Scholar]
- Kuske C.R., Barns S.M., Busch J.D. Diverse uncultivated bacterial groups from soils of the arid southwestern united states that are present in many geographic regions. Appl. Environ. Microbiol. 1997;63:3614–3621. doi: 10.1128/aem.63.9.3614-3621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakay F.M., Botha A., Prior B.A. Comparative analysis of environmental DNA extraction and purification methods from different humic acid-rich soils. Appl. Microbiol. 2007;102:265–273. doi: 10.1111/j.1365-2672.2006.03052.x. [DOI] [PubMed] [Google Scholar]
- Liesack W., Weyland H., Stackebrandt E. Potential risks of gene amplification by PCR as determined by 16S rDNA analysis of a mixed-culture of strict barophilic bacteria. Microb. Ecol. 1991;21:191–198. doi: 10.1007/BF02539153. [DOI] [PubMed] [Google Scholar]
- Luna G.M., Dell’Anno A., Danovaro R. DNA extraction procedure: a critical issue for bacterial assessment in marine sediments. Environ. Microbiol. 2006;8:308–320. doi: 10.1111/j.1462-2920.2005.00896.x. [DOI] [PubMed] [Google Scholar]
- Luo H., Qi H., Xue K., Zhang H. A preliminary application of PCR-DGGE to study microbial diversity in soil. Acta Ecologica Sini. 2003;23:1570–1575. (in Chinese) [Google Scholar]
- Martin-Laurent F., Philippot L., Hallet S., Chaussod R., Germon J.C., Soula G., Catroux G. DNA extraction from soils: old bias for new microbial diversity analysis methods. Appl. Environ. Microbiol. 2001;67:2354–2359. doi: 10.1128/AEM.67.5.2354-2359.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robe P., Nalin R., Capellano C., Vogel T.M. Extraction of DNA from soil. Eur. J. Soil Biol. 2003;39:183–190. [Google Scholar]
- Roh C., Villatte F., Kim B.G., Schmid R.D. Comparative study of methods for extraction and purification of environmental DNA from soil and sludge samples. Appl. Biochem. Biotechnol. 2006;134:97–112. doi: 10.1385/abab:134:2:97. [DOI] [PubMed] [Google Scholar]
- Roose-Amsaleg C.L., Garnier-Sillam E., Harry M. Extraction and purification of microbial DNA from soil and sediment samples. Appl. Soil Ecol. 2001;18:47–60. [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1989. Molecular cloning: a laboratory manual. ISBN 0879695773. [Google Scholar]
- Torsvik V., Øvreås L. Microbial diversity and function in soil: from genes to ecosystems. Curr. Opin. Microbiol. 2002;5:240–245. doi: 10.1016/s1369-5274(02)00324-7. [DOI] [PubMed] [Google Scholar]
- Tsai Y.L., Olson B.H. Rapid method for direct extraction of DNA from soil and sediments. Appl. Environ. Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates C., Gillings M.R., Davison A.D., Altavilla N., Veal D.A. Methods for microbial DNA extraction from soil for PCR amplification. Biol. Proc. Online. 1998;1:40–47. doi: 10.1251/bpo6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Cao H., Cui Z., Li S., Fan B. Extraction and purification of soil microbial total DNA. Acta Microbiol. Sini. 2003;43:276–282. (in Chinese) [Google Scholar]
- Zhou J.Z., Mary A.B., James M.T. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996;62:316–322. doi: 10.1128/aem.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


