Abstract
Glomerular injury leads to podocyte loss, a process directly underlying progressive glomerular scarring and decline of kidney function. The inherent repair process is limited by the inability of podocytes to regenerate. Cells of renin lineage residing alongside glomerular capillaries are reported to have progenitor capacity. We investigated whether cells of renin lineage can repopulate the glomerulus after podocyte injury and serve as glomerular epithelial cell progenitors. Kidney cells expressing renin were genetically fate-mapped in adult Ren1cCreER×Rs-tdTomato-R, Ren1cCre×Rs-ZsGreen-R, and Ren1dCre×Z/EG reporter mice. Podocyte depletion was induced in all three cell-specific reporter mice by cytotoxic anti-podocyte antibodies. After a decrease in podocyte number, a significant increase in the number of labeled cells of renin lineage was observed in glomeruli in a focal distribution along Bowman's capsule, within the glomerular tuft, or in both locations. A subset of cells lining Bowman's capsule activated expression of the glomerular parietal epithelial cell markers paired box protein PAX2 and claudin-1. A subset of labeled cells within the glomerular tuft expressed the podocyte markers Wilms tumor protein 1, nephrin, podocin, and synaptopodin. Neither renin mRNA nor renin protein was detected de novo in diseased glomeruli. These findings provide initial evidence that cells of renin lineage may enhance glomerular regeneration by serving as progenitors for glomerular epithelial cells in glomerular disease characterized by podocyte depletion.
Glomerular diseases are the leading cause of progressive chronic and end-stage kidney disease.1 Recent studies have demonstrated important roles for both podocytes and parietal epithelial cells (PECs) in these diseases. Diabetic nephropathy,2–5 membranous nephropathy,6 and classical focal segmental glomerulosclerosis (FSGS)7–10 are characterized by loss of podocytes. The conventional paradigm has been that, when podocyte number decreases after disease-induced injury, podocytes cannot replace themselves because they are terminally differentiated cells and cannot proliferate.11–17 This inadequate regeneration of podocytes directly underlies the development of progressive glomerulosclerosis and reduced kidney function.2,4,6,7,10,18–20 Recent studies have challenged this conventional paradigm, showing that podocyte number can be restored under certain circumstances.21–23 Importantly, this occurs in the absence of podocyte proliferation,22 suggesting that there may be one or more podocyte progenitors.
Several seminal studies have shown that the neighboring glomerular PECs might serve this role.24–28 After podocyte loss, PECs activate expression of proteins considered to be restricted to podocytes.24,29,30 Such cells may be in transition, because they express both PEC and podocyte proteins, and have therefore been called glomerular epithelial transition cells.24,29,30 The number of glomerular epithelial transition cells detected lining Bowman's capsule and within the glomerular tuft increases in membranous nephropathy,30 classical FSGS,30 and aging nephropathy.29 Based on these various studies, a new paradigm has emerged, that in proteinuric glomerular diseases characterized by reduced podocyte number subpopulations of PECs express podocyte markers and migrate to the glomerular basement membrane.31–33
Progenitor cells are oligopotent cells that frequently lie dormant in the tissue in which they reside; however, after local injury or death of mature, functioning cells, they replace the lost cell or cells by transdifferentiating into a new type of cell, acquiring its ultrastructure, activating transcriptional programs unique to those cells, and performing the biological functions of those cells. Although recent studies indicating that PECs may become podocytes are convincing, it remains to be shown that PECs become fully functional podocytes.
Previous studies have identified the juxtaglomerular compartment (JGC) as a reservoir of kidney progenitors.34,35 In adults, juxtaglomerular granular cells are modified smooth muscle cells (also called myoepithelioid-like cells) present in the vascular component of the juxtaglomerular apparatus, at the distal end of afferent arterioles and, to a lesser extent, of the efferent arterioles.36 These cells are the major source of total renin production and circulating active renin37,38 and therefore play critical roles in the regulation of vascular tone and the renin–angiotensin–aldosterone system.39 An elegant study showed that cells of renin lineage can also serve a progenitor function for smooth muscle, epithelial, mesangial, and extrarenal cells and can be detected in low numbers in normal glomeruli.34 Moreover, we have previously shown that non–renin-expressing cells of the extraglomerular mesangium,36 residing in the JGC, repopulate the glomerular tuft and restore mesangial cell number after mesangiolysis in a model of mesangioproliferative glomerulonephritis.35
The purpose of these studies was to apply genetic cell fate-mapping strategies in four transgenic gene–targeted mice that report for cells of renin lineage to test the hypothesis that these cells serve as progenitor cells for podocytes and PECs during experimental glomerular disease characterized by a decrease in podocyte number. Three newly generated renin-reporter mouse strains and one existing reporter mouse strain were used.
Materials and Methods
Reporter Mice
Four different reporter mouse strains were used to genetically fate-map cells of renin lineage, three of which were newly generated.
Ren1cCreER×Rs-tdTomato-R
The newly generated Ren1cCreER×Rs-tdTomato-R mouse labels the Ren1c gene with tomato red protein only after the administration of tamoxifen (Sigma-Aldrich, St. Louis, MO). Because renin expression might be switched on later in life, thus confounding the data from the constitutive reporter mice, we introduced a Cre recombinase fused to the human estrogen receptor (ER) ligand-binding domain40 into exon 1 of the Ren1c gene residing within a 240-kb bacterial artificial chromosome (BAC), here represented as RenCreER, using previously described methods41,42 (Supplemental Figure S1). The Ren1cCreER transgenic line, when crossed to the reporter Gt(ROSA)Sortm9(CAG-tdTomato)Hze/J,43 allows for the permanent tagging of cells of renin lineage with tomato red protein, which is detected by red fluorescent protein (RFP), within temporal windows defined by treatment with tamoxifen (Supplemental Figure S2A). Accordingly, 5-week-old bigenic mice, weighing 16 to 20 g, were given either 100 mg/kg tamoxifen or vehicle by intraperitoneal injection on alternate days for 6 days.
Ren1cCre×Rs-ZsGreen-R
The newly generated Ren1cCre×Rs-ZsGreen-R reporter mouse labels the Ren1c gene with ZsGreen. To tag cells of renin lineage indefinitely, even after renin is no longer being transcribed, a Cre-recombinase cassette40 was cloned into the Ren1c BAC using homologous recombination, as described previously42,43 (Supplemental Figure S1). The renin regulatory region can then control Cre recombinase, which recognizes and excises loxP sites in DNA. When the Ren1cCre transgenic line is crossed with the commercially available B6.Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J reporter mouse,43 here represented as ZsGreen, a floxed stop cassette is excised, allowing for constitutive ZsGreen expression driven by a CAG promoter. Validation of the fidelity of this newly generated Ren1cCre×Rs-ZsGreen-R transgenic mouse in reporting cells of renin lineage was confirmed by visualizing adult kidney tissue (Supplemental Figure S2B). Ren1cCre×Rs-ZsGreen-R mice devoid of Cre were used as negative controls for labeling.
Ren1dCre×Z/EG
The existing Ren1dCre×Z/EG reporter mouse labels the Ren1d gene with GFP, as previously reported.34 The utility of this knock-in mouse is that all cells of renin lineage and their descendants permanently express GFP, even if renin expression is subsequently decreased or absent.44 Mice with Cre recombinase under control of the renin locus34 were crossed with Z/EG reporter mice.44 Z/EG reporter mice constitutively express lacZ under the control of the CMV enhancer/chicken actin promoter. When crossed with a Cre recombinase-expressing strain, lacZ expression is replaced with enhanced GFP expression in tissues expressing Cre.
RenGFP
The newly generated RenGFP reporter mouse serves to report renin promoter activity by the presence of a GFP reporter; that is, when renin is transcribed, GFP is expressed. Thus, to more comprehensively ensure control of reporter expression, homologous recombination was used to introduce a GFP cassette into exon one of the Ren1c gene residing within a 240-kb BAC to generate a construct that has GFP expression controlled from within the entire natural genomic sequence context for renin (Supplemental Figure S1). The insertion of GFP into the renin gene, which is centrally located within the BAC, allows large amounts of 5′- and 3′-flanking sequence to act on reporter expression. This is done in an effort to gain a more faithful representation of endogenous renin gene expression, as well as to insulate transgene expression from influences of surrounding sequence at the insertional site. The integration of GFP into the renin containing BAC to generate the RenGFP transgenic line has been described previously.42
Experimental Focal Segmental Glomerulosclerosis Model
Disease Model
The goal of these studies was to test the hypothesis that cells of renin lineage are progenitors for glomerular epithelial cells in glomerular disease characterized by an abrupt decline in podocyte number. Accordingly, an inducible model of experimental glomerular disease resembling classic clinical FSGS was induced with a cytotoxic antibody in all four strains of mice. We have previously reported that this experimental FSGS model induces an abrupt decline in podocyte number, which is associated clinically with the onset of proteinuria and histologically with focal and segmental glomerulosclerosis.30,45,46 This is not a model of crescentic glomerulonephritis, nor of podocyte proliferation, which is induced by a different antibody (as we have previously reported13,47–50).
Antibodies
Polyclonal antibodies were generated by immunizing sheep with whole rabbit glomeruli in Freund's adjuvant (Sigma-Aldrich). Antiserum was heat inactivated; IgG was isolated by caprylic acid (Sigma-Aldrich) precipitation of serum proteins. The retained IgG was then dialyzed against PBS sterile filtered.51 When injected, sheep anti-rabbit glomerular antibodies bind selectively to podocytes. This was verified by double-staining for sheep IgG deposition within the glomerulus and for the podocyte-specific proteins nephrin and synaptopodin. Sheep IgG staining in the glomerulus overlaps extensively with staining for both podocyte proteins, and does not bind to other cells in the glomerulus or beyond (Supplemental Figure S3). Antibody binding to podocytes induces rapid non–complement-mediated podocyte apoptosis and necrosis, leading to reduced podocyte number, proteinuria, and segmental glomerular scarring in fewer than 50% of glomeruli.30,45,46 Disease was induced in all four reporter mice by administering 12.5 mg/20 g body weight sheep anti-rabbit glomerular antibodies (two intraperitoneal injections, 24 hours apart), as previously reported.30,45,46 There was an increased incidence of mortality within 24 hours of antibody administration in the Ren1cCreER×Rs-tdTomato-R mouse strain. The dose was therefore decreased to 10 mg/20 g body weight (two intraperitoneal injection, 24 hours apart) in these mice. Control mice received vehicle only.
Time Points
The time points studied for each reporter strain after induction of experimental FSGS (Supplemental Figure S4) were as follows.
At 49 days after the last tamoxifen injection, a survival kidney biopsy was performed on all Ren1cCreER×Rs-tdTomato-R mice, to ensure that cells of renin lineage were labeled in individual mice given tamoxifen, but not in control mice. This biopsy specimen, weighing <3.5% of total kidney mass, served as the baseline (day 0) for each mouse. After the mice had recovered from surgery, disease was induced in all mice as described above. Diseased male and female Ren1cCreER×Rs-tdTomato-R mice aged 13 weeks were then sacrificed at day 7 (n = 5 per group) and day 14 (n = 5 per group) of disease. Male and female Ren1cCre×Rs-ZsGreen-R mice, aged 5 to 20 weeks, were sacrificed at day 0 (n = 3), day 3 (n = 3), day 7 (n = 3), or day 14 (n = 4) of disease.52 Male and female Ren1dCre×Z/EG mice, aged 8 to 15 weeks, were sacrificed at day 0 (n = 2), day 7 (n = 3), or day 14 (n = 3) of disease. Male and female RenGFP mice, aged 9 to 13 weeks, were sacrificed at day 0 (n = 6), day 14 (n = 3), day 21 (n = 3), or day 28 (n = 3) of disease.
Ethics
Experimental protocols were approved by the Animal Care committees of the University of Washington, Roswell Park Cancer Institute, Duke University Medical Center, and Durham Veterans Affairs Medical Center. All animal procedures were conducted in accordance with the respective Institutional Animal Care and Use Committee.
Immunostaining
Verifying Podocyte Specificity of Cytotoxic Antibody
To verify the binding of injected sheep anti-glomerular antibody to podocytes within the glomerulus, double-immunofluorescence staining for sheep IgG and nephrin or synaptopodin was performed. Rabbit anti-sheep IgG H&L (FITC) polyclonal antibody (Abcam, Cambridge, MA),47 guinea pig anti-nephrin polyclonal antibody (Fitzgerald Industries, Concord, MA), and mouse anti-synaptopodin monoclonal antibody (Fitzgerald Industries) were used. Nephrin and synaptopodin were visualized with Alexa Fluor 594 (Life Technologies–Invitrogen, Carlsbad, CA).
Identifying Cells of Renin Lineage
Cells of renin lineage were identified by inducible genetic fate mapping in Ren1cCreER×Rs-tdTomato-R mice by RFP and by genetic fate mapping in Ren1dCre×Z/EG mice with GFP, in Ren1cCre×Rs-ZsGreen-R mice with ZsGreen, and in RenGFP mice with GFP. To detect RFP and GFP, kidney biopsy specimens were fixed in neutralized formalin (Globe Scientific Inc., Paramus, NJ) and embedded in paraffin. For ZsGreen, kidneys were perfusion-fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.1 mol/L phosphate buffer, pH 7.4, followed by immersion fixation for 60 minutes. Antibodies were not needed to detect ZsGreen alone or RFP alone. Immunofluorescence staining for RFP and GFP was performed on 4-μm-thick sections, as previously reported.30,53
Determining Whether Cells of Renin Lineage Express Glomerular Cell Markers
We next determined whether labeled cells of renin lineage begin to express proteins considered to be unique to different glomerular cell types. Immunofluorescence double-staining was performed as we have previously reported54–56 for the cell of renin lineage reporters RFP, GFP, or ZsGreen with proteins considered to be specific for the podocyte markers WT-1, synaptopodin, and podocin (Abcam), as well as for PECs [PAX2 (Invitrogen, Carlsbad, CA) and claudin-1 (Zymed Laboratories, San Francisco, CA)], mesangial cells [PDGFR-β (R&D Systems Inc., Minneapolis, MN) and type IV collagen (Southern Biotechnology Associates, Inc., Birmingham, AL)], and glomerular endothelial cells (MECA-32; Novus Biologicals, Littleton, CO).
Measuring Podocyte Number
Because of concerns that WT-1 is not a reliable podocyte-specific protein, we used p57 staining to measure podocyte number. A previously published analysis comparing p57, WT-1, and podocyte-specific reporter mice substantiated the use of p57 to determine podocyte number.50 Immunostaining for p57 was performed with a rabbit p57 primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and then with a biotin-conjugated mouse anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA).
Measuring Renin Protein Expression
To ensure that renin protein expression was confined to cells in the JGC, and that renin expression did not occur in glomeruli de novo during disease, double-staining was performed for the reporters GFP, ZsGreen, and RFP with renin (biotinylated sheep antibody to renin; Innovative Research, Novi, MI), as described above.
Identifying Stem Cell Markers
Staining was performed for three markers that are considered markers of stem cells: CD24, CD44, and CD133 (all antibodies from Abcam).
Controls
Positive controls consisted of kidney tissue (from Ren1dCre×Z/EG and Ren1cCre×Rs-ZsGreen-R mice with and without disease) known to be positive for the proteins being stained. Immunostaining for renin,57 PAX2,58 claudin 1,59 WT-1,60 synaptopodin,61 GFP,62 and p5763 with the antibodies used in these studies has been verified previously. For negative controls, the primary antibody was omitted or Ren1dCre×Z/EG or Ren1cCre×Rs-ZsGreen-R mice devoid of Cre were used.
Quantification of Immunostaining
The number of cells expressing reporter alone and the number of cells double-staining for reporter and PAX2, claudin-1, WT-1, or synaptopodin were counted per glomerular cross section. An average of 76 ± 16 glomeruli from each animal were assessed for each strain. These cell numbers are presented as means ± SEM. One-way analysis of variance was calculated, and P < 0.05 was considered significant.
Assessment of Glomerular Injury
Glomerulosclerosis was measured and quantified on PAS-stained sections, as we have described previously.50,64,65 An average of 50 ± 10 glomeruli in each animal was graded quantitatively, as follows. Grade 0 was defined as 0% glomerular tuft area involvement (normal glomerulus with no abnormalities); grade 1, <1% to 25% (a few capillaries with dilation); grade 2, <26% to 50% (multiple capillaries with dilation); grade 3, <51% to 75% (multiple capillaries with dilation and some synechial attachments); and grade 4, <76% to 100% (multiple synechial attachments with focal segmental sclerosis).
Results
Characterization of Ren1cCreER×Rs-tdTomato-R Mice
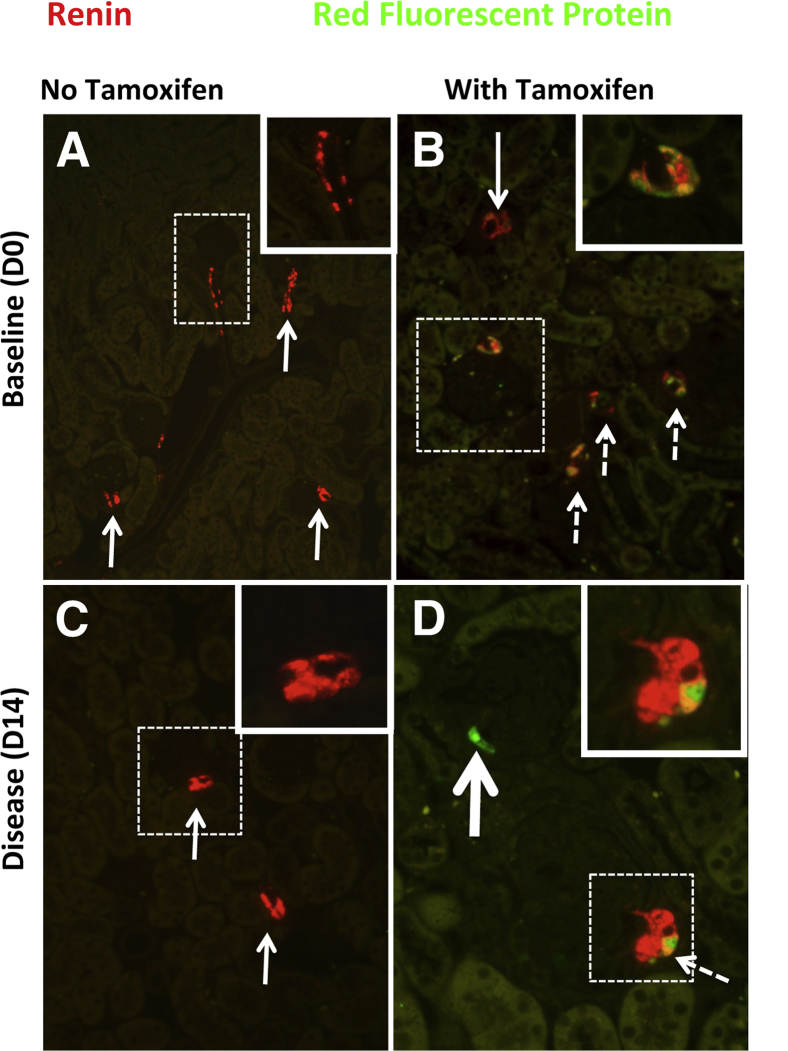
To ensure that the presence of labeled cells within the glomerulus was not due to increased de novo renin expression during disease, we generated Ren1cCreER×Rs-tdTomato-R mice, in which permanent labeling of renin-producing cells with the tdTomato Red reporter was induced in adult mice within specific temporal windows defined by administration of tamoxifen, thus allowing the fate of only these cells to be mapped. In normal Ren1cCreER×Rs-tdTomato-R mice given tamoxifen, staining for the tdTomato Red reporter with RFP antibody was detected only in cells restricted to the JGC; no labeled cells were detected in glomeruli (Figure 1, B and D). No staining for the reporter was detected when only vehicle was given (Figure 1, A and C). In normal mice, renin staining was restricted to cells in the JGC; no renin staining was detected in the glomeruli. These results validate the feasibility of genetically inducible fate mapping in Ren1cCreER×Rs-tdTomato-R mice to permanently label cells of renin lineage.
Figure 1.
Characterization of adult Ren1cCreER×Rs-tdTomato-R mice. Double-staining was performed for renin protein (red) and for the reporter for cells of renin lineage using a fluorescein-conjugated antibody to RFP (green). A merge of the two appears yellow. A: In baseline (day 0) mice not given tamoxifen, renin staining was restricted to the JGC (arrows). The boxed region is shown at higher magnification in the inset. Staining for the reporter was not detected. B: In baseline mice given tamoxifen, the majority of renin-staining cells costained for RFP (white; dashed arrows), and these costaining cells were confined to the JGC. Occasional JGC cells only stained for renin (arrow). The boxed region is shown at higher magnification in the inset, where costaining JGC are more clearly seen. C: In diseased Ren1cCreER×Rs-tdTomato-R mice not given tamoxifen (day 14), renin staining was restricted to the JGC (arrows). The boxed region is shown at higher magnification in the inset, where renin staining is seen to be restricted to the JGC. Staining for RFP was not detected. D: In diseased mice given tamoxifen (day 14), renin staining was restricted to the JGC and costained with RFP (white; dashed arrow). The boxed region is shown at higher magnification in the inset, where costaining JGC are more clearly seen. No renin was detected in the diseased glomerulus. A labeled cell of renin lineage in the glomerulus stains green (thick arrow). Original magnification: ×100 (main images); ×630 (insets).
Cells of Renin Lineage Appear in Glomeruli of Ren1cCreER×Rs-tdTomato-R Mice with Experimental FSGS
After administration of the cytotoxic anti-podocyte antibody to induce experimental FSGS, sheep IgG staining was in a typical podocyte distribution, and was not detected in PECs or in the JGC (Supplemental Figure S5A). In diseased Ren1cCreER×Rs-tdTomato-R mice, podocyte number, measured by p57 staining,50 decreased relative to baseline (10.5 ± 0.45) by 32% at day 7 and by 33% at day 14 (7.1 ± 0.49 and 7.0 ± 0.56, respectively), (P < 0.001) (Supplemental Figure S5B). At days 7 and 14, however, the disease was focal, and typically between 10% and 40% of glomeruli had histological evidence of disease as previously shown.50,64 These data show that experimental FSGS was induced in Ren1cCreER×Rs-tdTomato-R mice.
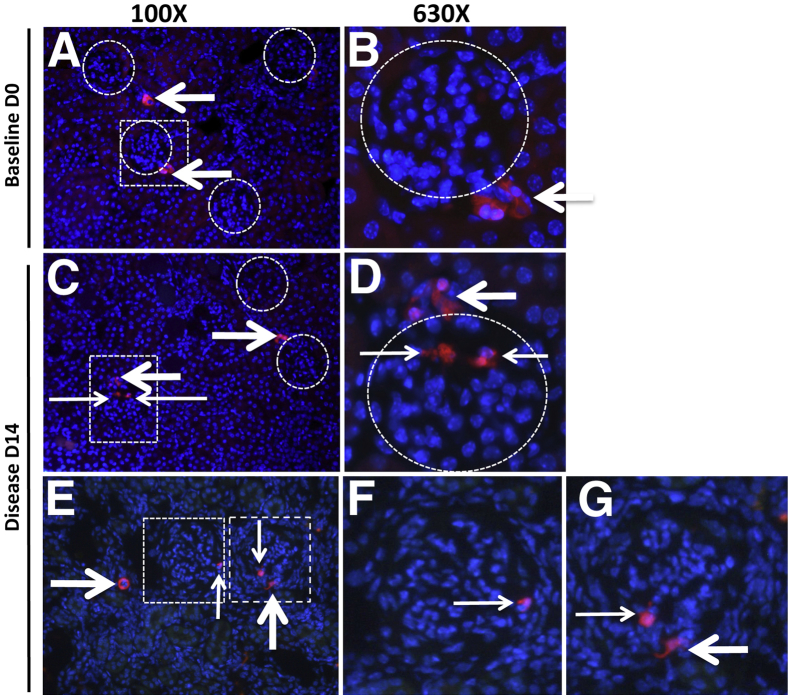
Cells of renin lineage, identified by the RFP reporter, were present in diseased glomeruli (Figure 2). After tamoxifen administration but before disease induction, a survival kidney biopsy was performed on all mice to ensure labeling; these biopsy data served as the baseline for individual mice. Labeled cells of renin lineage were restricted to the JGC in baseline survival kidney biopsy specimens. After disease induction, however, labeled cells of renin origin were detected in 13.8 ± 1.16% of glomeruli at day 7 and in 14.8 ± 1.78% of glomeruli at day 14 (both P < 0.0001 versus baseline) (Figure 2 and Supplemental Figure S5). In these focally involved glomeruli, labeled cells were detected either within the glomerular tuft or, to a lesser extent, along Bowman's capsule. Although reporter cells were detected in 14% to 15% of glomeruli, renin staining was localized to the cells outside the glomerulus; no de novo renin staining was detected within glomeruli of diseased mice (Figure 1). These data show that cells of renin lineage labeled with tomato red protein and derived from the JGC were detected de novo in glomeruli of diseased mice, and that these cells no longer expressed renin when located within the glomerulus.
Figure 2.
Cells of renin lineage are present in glomeruli of Ren1cCreER×Rs-tdTomato-R mice with experimental FSGS. Ren1cCreER×Rs-tdTomato-R mice were given tamoxifen for three alternate days to temporally and permanently label only cells of renin lineage. A survival biopsy was then performed (baseline, day 0), after which experimental FSGS was induced in each mouse. A: At day 0, labeled cells of renin lineage reporter (red) were restricted to the JGC (arrows). No labeled cells were detected in individual glomeruli (circles). B: A higher magnification view of the boxed region in panel A shows reporter-labeled cells of renin lineage in the JGC (arrow) but not within the glomerulus (circle). C: At day 14 of disease, reporter-labeled cells were detected in the JGC (thick arrows) and in a glomerulus (thin arrows). Labeled cells within glomeruli were focal in nature; fluorescence was absent in two glomeruli (circles). D: A higher magnification view of the boxed region in panel C shows reporter-labeled cells in the JGC (thick arrow) and within the glomerulus (thin arrows). E: At day 14 of disease, reporter-labeled cells were detected in the JGC (thick arrows) and in the glomerulus (thin arrows). F and G: Higher magnification views of the two boxed regions in panel E show reporter-labeled cells in the JGC (thick arrow) and within the glomerulus (thin arrows). Original magnification: ×100 (A, C, and E); ×630 (B, D, F, and G).
Cells of Renin Lineage within Glomeruli of Ren1cCreER×Rs-tdTomato-R Mice with Experimental FSGS Express Proteins Considered Specific to Podocytes and PECs
Podocyte Proteins
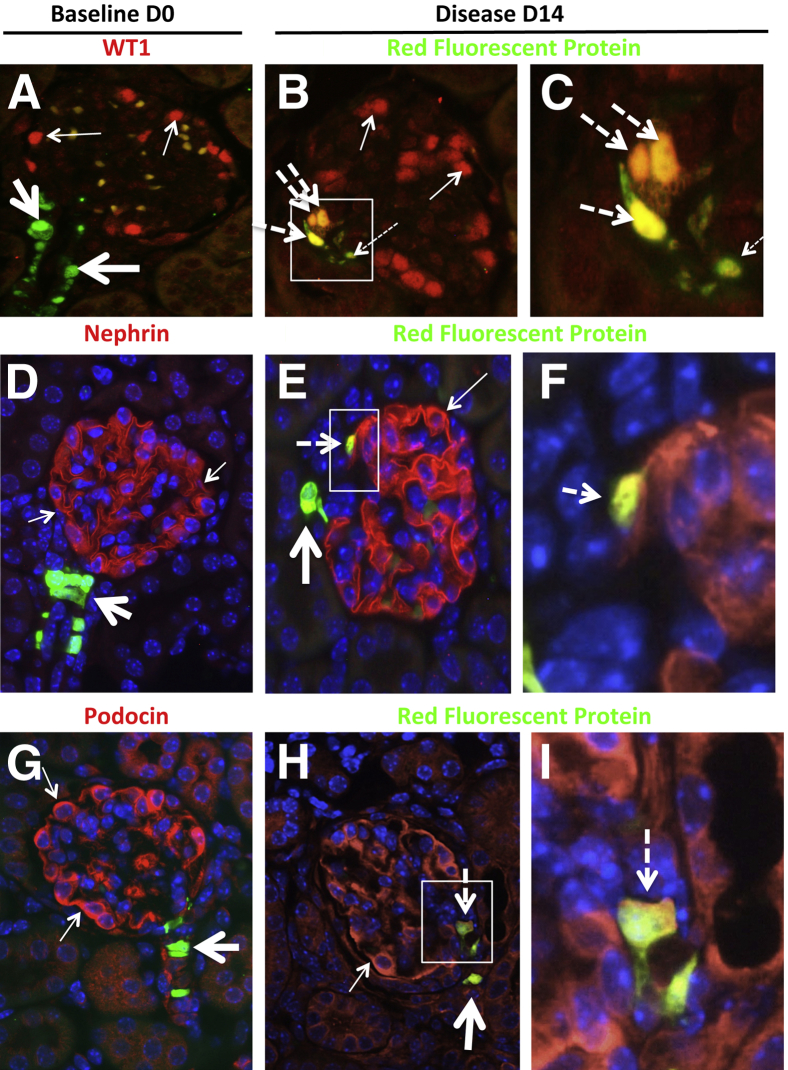
Having identified cells of renin lineage in glomeruli of diseased Ren1cCreER×Rs-tdTomato-R mice, we investigated whether these cells begin to express glomerular epithelial cell proteins. We performed double-staining for the renin lineage reporter and for three proteins traditionally considered to be podocyte specific: the transcriptional regulator WT-1, the transmembrane IgG superfamily protein nephrin, and the stomatin family protein podocin. Staining for the reporter and these podocyte proteins did not overlap in mice at baseline (they were in different kidney compartments). At days 7 and 14, however, the staining for the reporter and WT-1, nephrin, and podocin overlapped in glomeruli in diseased animals (Figure 3 and Supplemental Figure S6). The number of cells positive for both RFP and WT-1 represented 50 ± 10% of the RFP-positive cells in the glomerular tuft at day 14 of disease (0.074 ± 0.02 double-positive cells per glomerular cross section in disease versus 0 at baseline). Cells double-positive for both RFP and nephrin, or both RFP and podocin, represented 30 ± 8% of the RFP-positive cells in the glomerular tuft at day 14 of disease (0.04 ± 0.01 double-positive cells per glomerular cross section in disease versus 0 at baseline).
Figure 3.
Cells of renin lineage express proteins considered to be podocyte-specific in glomeruli of Ren1cCreER×Rs-tdTomato-R mice with experimental FSGS. Double-staining was performed for WT-1, nephrin, or podocin (red), and for the cells of renin lineage reporter with a fluorescein-conjugated antibody to RFP (green). A: Staining for WT-1 (red; thin arrows) was confined to the glomerular tuft at baseline (day 0). Staining for RFP was confined to the JGC (green; thick arrows). Red blood cell autofluorescence (yellow-orange) was noted in the glomerular tuft. B: Costaining for WT-1 and RFP (yellow-orange; thick dashed arrows) in three cells within the glomerulus at day 14 of disease. One reporter-labeled cell in the glomerulus did not costain for WT-1 (thin dashed arrow). Most glomerular cells stained for WT-1 alone (red; thin arrows). C: A higher magnification view of the boxed region in panel B. D: Staining for nephrin (red; thin arrows) was confined to the glomerular tuft at baseline (day 0). Staining for RFP was confined to the JGC (green; thick arrow). E: Costaining for nephrin and RFP (yellow-orange; dashed arrow) within the glomerulus at day 14 of disease. An RFP-labeled cell outside the glomerulus did not costain with nephrin (thick arrow). Ribbon-like Nephrin staining alone (red; thin arrow). F: A higher magnification view of the boxed region in panel E. G: Staining for podocin (red; thin arrows) was confined to the glomerular tuft at baseline (day 0). Staining for RFP was confined to the JGC (green; thick arrow). H: Costaining for podocin and RFP (yellow-orange; dashed arrow) within the glomerulus at day 14 of disease. An RFP-labeled cell outside the glomerulus did not costain with podocin (thick arrow). Cytoplasmic podocin staining alone (red; thin arrow). I: A higher magnification view of the boxed region in panel H. These representative examples show that a subset of cells of renin lineage that have entered the glomeruli of diseased Ren1cCreER×Rs-tdTomato-R mice begin to express WT-1, nephrin, and podocin, all of which are considered to be podocyte proteins. Original magnification: ×400 (A, B, D, E, G, and H); ×1000 (C, F, and I).
In both instances, labeled cells of renin lineage were typically detected in segments of glomeruli in which podocytes were depleted (as assessed by reduced WT-1, nephrin, or podocin staining), regardless of whether they coexpressed the marker protein.
Parietal Epithelial Cell Proteins
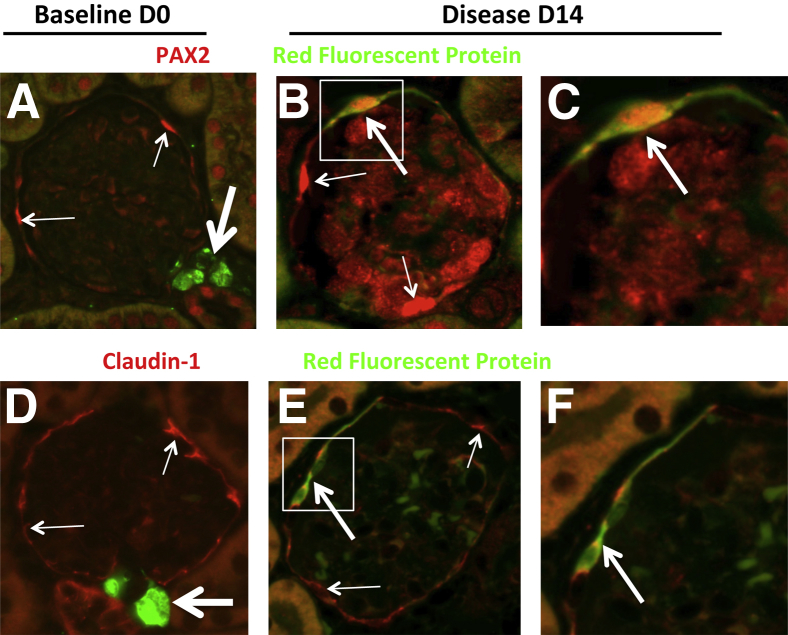
Double-staining was also performed for the renin lineage reporter and the nuclear transcription factor PAX2 or the tight junction protein claudin-1, both of which are expressed in glomerular PECs (Figure 4 and Supplemental Figure S7). At baseline before disease induction, staining for the reporter and the PEC markers did not overlap. In contrast, at days 7 and 14 of disease, overlapping staining was detected for the reporter and both PAX2 and claudin-1 in the majority (but not all) of the labeled cells in the glomerulus. Cells double-positive for both RFP and PAX2 or claudin-1 represented 60 ± 5% of the RFP-positive cells lining Bowman's capsule at day 14 of disease (0.03 ± 0.008 double-positive cells per glomerular cross section in disease versus 0 at baseline).
Figure 4.
Cells of renin lineage express glomerular PEC proteins in glomeruli of Ren1cCreER×Rs-tdTomato-R mice with experimental FSGS. Double-staining was performed for PEC proteins with antibodies to PAX2 or claudin-1 (red) and for the cells of renin lineage reporter with a fluorescein-conjugated antibody to RFP (green). A: At baseline (day 0) staining for PAX2 (thin arrows) was confined to Bowman's capsule, and did not overlap with staining for the reporter (thick arrow) restricted to the JGC. B: At day 14 of disease, a subset of cells lining Bowman's capsule within the glomerulus costained for PAX2 and the reporter (yellow; thick arrow). Other PAX2-staining cells did not costain with the reporter (thin arrows). C: A higher magnification view of the boxed region in panel B. D: In baseline mice, staining for claudin-1 (red; thin arrows) did not overlap with staining for the reporter (green; thick arrow), which was confined to the JGC. E: In diseased mice, RFP-labeled cells were detected lining Bowman's capsule; these cells costained for claudin-1 (yellow-orange; thick arrow). Staining for claudin-1 alone (red; thin arrows). Red blood cell autofluorescence (light green) was noted in the glomerular tuft. F: A higher magnification view of the boxed region in panel E. These representative examples show that, in diseased Ren1cCreER×Rs-tdTomato-R mice, a subset of cells of renin lineage that line Bowman's capsule express the PEC proteins PAX2 and claudin-1.
Taken together, these data show that a subset of cells of renin lineage begin to express podocyte proteins and PEC proteins when in a glomerular location in diseased Ren1cCreER×Rs-tdTomato-R mice.
Podocyte Number Decreases in Ren1cCre×Rs-ZsGreen-R Mice with Experimental FSGS
To corroborate these findings, experimental FSGS was induced in Ren1cCre×Rs-ZsGreen-R mice, in which cells of renin lineage were labeled permanently by the Anthozoa coral reef protein ZsGreen.66 After the onset of disease, podocyte number (as judged by p57 immunostaining) decreased progressively relative to the baseline level (11.2 ± 0.29) by 15% at day 3 (9.5 ± 0.11), by 21% at day 7 (8.9 ± 0.27), and by 29% at day 14 (8.0 ± 0.31) (P = 0.002, days 3 and 7; P = 0.0004, day 14) (Supplemental Figure S8). Progressive segmental glomerular scarring and synechial attachments (glomerular tuft adherent to Bowman's capsule) increased relative to the baseline level (0.2 ± 0.013), reaching 0.5 ± 0.04 at day 3, 1.0 ± 0.05 at day 7, and 1.2 ± 0.15 at day 14 (P = 0.0008, days 3 and 7; P = 0.002, day 14) (Supplemental Figure S8). These data show that Ren1cCre×Rs-ZsGreen-R mice developed characteristic features of FSGS.
Experimental FSGS in Ren1cCre×Rs-ZsGreen-R Mice Is Accompanied by an Increase in Cells of Renin Lineage in Diseased Glomeruli
In Ren1cCre×Rs-ZsGreen-R mice, cells of renin lineage labeled with ZsGreen in the JGC (Figure 5 and Supplemental Figure S9), as expected. In normal adult reporter mice, ZsGreen-labeled cells were detected in 20 ± 1.98% of glomeruli at day 0 (baseline) (Figure 5). To determine whether this effect was due to age, younger mice were also studied. The percentage of glomeruli in which ZsGreen-labeled cells were detected was similar in younger mice (aged 7 or 28 days) and older mice (P > 0.05), which indicates that reporter labeling occurs early in development. In diseased Ren1cCre×Rs-ZsGreen-R mice, however, the percentage of glomeruli expressing at least one ZsGreen-labeled cell increased progressively, relative to baseline: 51 ± 4.2% at day 3 (P = 0.004), 59 ± 3.8% at day l7 (P = 0.007), and 61 ± 5.4% at day 14 (P = 0.009) (Figure 5).
Figure 5.
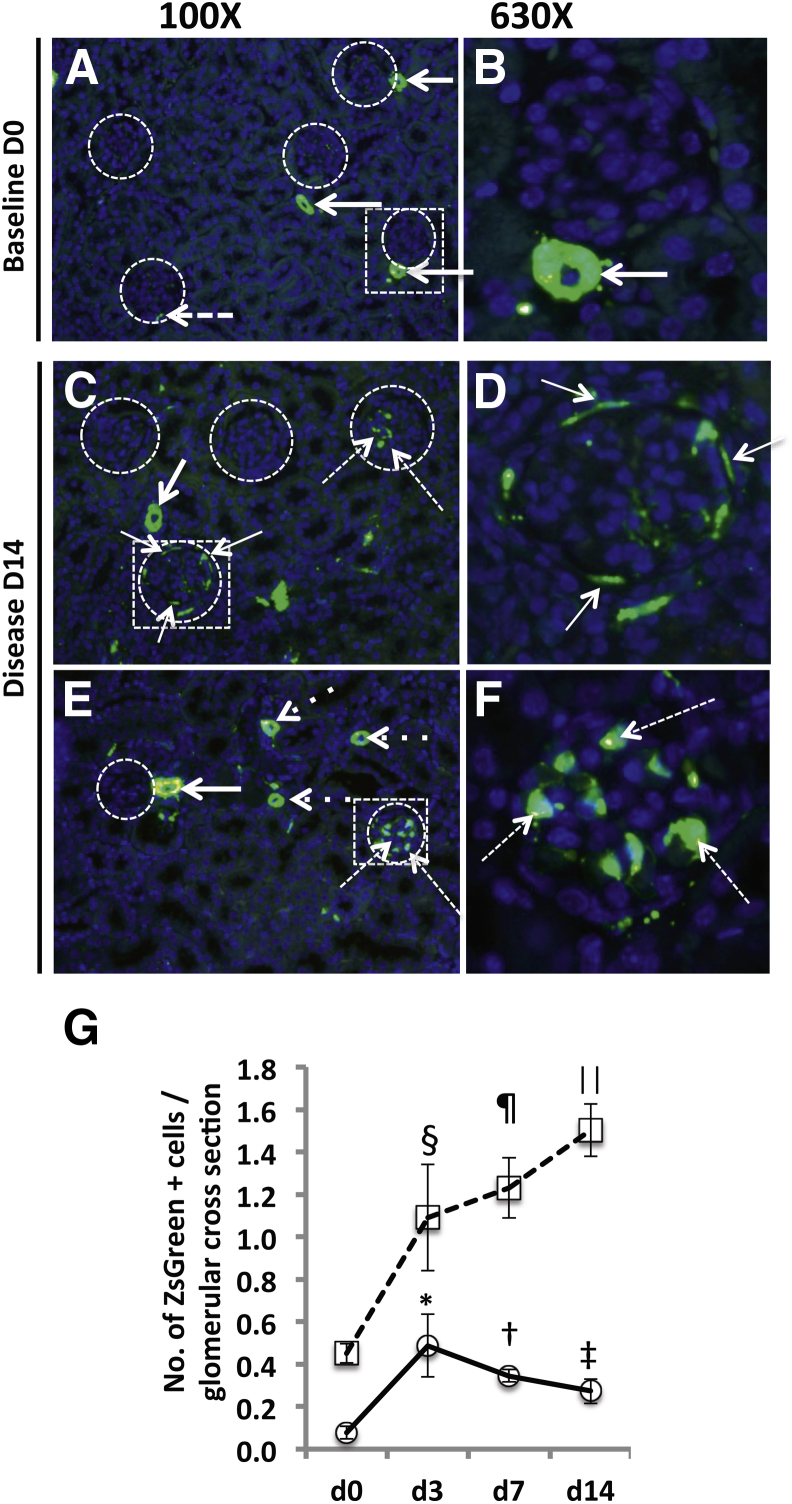
Cells of renin lineage increase in glomeruli of Ren1cCre×Rs-ZsGreen-R mice with experimental FSGS. Experimental disease was induced in Ren1cCre×Rs-ZsGreen-R mice to fate-map cells of renin lineage with ZsGreen reporter. No antibody was required to visualize ZsGreen, which stains green in both cytoplasm and nucleus; DAPI was used to counterstain nuclei blue. A and B: Representative ZsGreen labeling at baseline day 0. A: Low-power view of five glomeruli (circles). ZsGreen fluorescence is detected in cells within the JGC (arrows) alongside three of the glomeruli; only one cell is detected within a glomerulus (dashed arrow). B: A higher magnification view of the boxed region in panel A shows that the ZsGreen fluorescence (arrow) was confined to cells within the JGC. C–F: Representative ZsGreen labeling at day 14 of disease. C: Low-power view of four glomeruli (circles). The two glomeruli on the upper left are devoid of ZsGreen-labeled cells. The glomerulus on the upper right has several ZsGreen-labeled cells, which were predominantly located within the glomerular tuft (dashed arrows). In the circled glomerulus within the boxed region, ZsGreen-labeled cells are predominantly lining Bowman's capsule (thin arrows), but also appear in the JGC (thick arrow). D: A higher magnification view of the boxed region in panel C more clearly shows the ZsGreen-labeled cells lining Bowman's capsule (arrows). E: At day 14, one glomerulus (left circle) had no ZsGreen-labeled cells, but was accompanied by ZsGreen-labeled cells in the JGC (thick arrow) and in blood vessels (dotted arrows). Another glomerulus (right circle) had several ZsGreen-labeled cells (dashed arrows). F: A higher magnification view of the boxed region in panel E shows the ZsGreen-labeled cells as within the glomerular tuft (dashed arrows). G: Quantification of the number of ZsGreen-labeled cells. ZsGreen-positive cells lining Bowman's capsule (circles) were rarely detected at baseline (day 0). In disease, however, the numbers of these cells were significantly increased at all time points, relative to baseline. The number of ZsGreen-positive cells within the glomerular tuft (squares) had more than doubled at day 3, and continued to increase significantly at days 7 and 14. These data show an increase in the number of cells of renin lineage in glomeruli of mice with experimental FSGS. Labeled cells were detected either along Bowman's capsule, within the glomerular tuft, or at both locations. Data are expressed as means ± SEM. *P = 0.03, †P = 0.001, ‡P = 0.04 versus d0 Bowman's capsule; §P = 0.03, ¶P = 0.003, ⋮P = 0.002 versus d0 glomerular tuft.
To determine the location of labeled cells of renin lineage within glomeruli, the number of ZsGreen cells within the glomerular tuft and lining Bowman's capsule was quantified (Figure 5). ZsGreen-labeled cells lining Bowman's capsule per glomerular cross section increased in disease from 0.08 ± 0.03 at baseline (day 0) to 0.49 ± 0.15 at day 3 (P = 0.03), 0.35 ± 0.03 at day 7 (P = 0.003), and 0.27 ± 0.06 at day 14 (P = 0.002). The number of ZsGreen-labeled cells detected in the glomerular tuft increased from 0.45 ± 0.05 at day 0 to 1.09 ± 0.25 at day 3 (P = 0.03), 1.23 ± 0.14 at day 7 (P = 0.001), and 1.5 ± 0.12 at day 14 (P = 0.04) (Figure 5). Within individual glomeruli, there was no particular temporal preference for location within the glomerulus. As expected, ZsGreen labeling was not detected in kidneys of Ren1cCre×Rs-ZsGreen-R reporter mice devoid of Cre (used as negative control; data not shown).
To establish that the detection of labeled cells of renin lineage in glomeruli was not due to de novo renin protein expression in Ren1cCre×Rs-ZsGreen-R mice, double-staining was performed for ZsGreen and renin (Supplemental Figure S9). In both normal and diseased mice, renin immunostaining was restricted to cells in the characteristic JGC location. In the majority of these cells, renin staining overlapped with staining of ZsGreen-labeled cells of renin linage. Although ZsGreen cells were detected in a subset of glomeruli, renin staining was absent in all glomeruli of both normal and diseased mice. This was not a false negative, because cells in the adjacent JGC stained positive for renin.
Cells of Renin Lineage within Glomeruli of Ren1cCre×Rs-ZsGreen-R Mice with Experimental FSGS Express Proteins Considered Specific to Podocytes and PECs
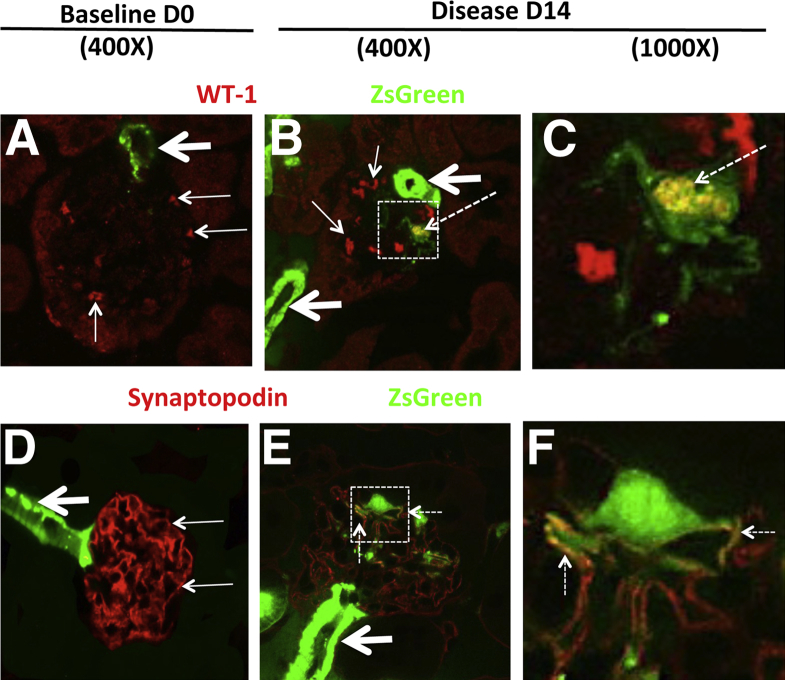
We next determined whether the cells of renin lineage detected within the glomerular tuft of Ren1cCre×Rs-ZsGreen-R mice express the podocyte proteins WT-1 and synaptopodin. In induction of experimental FSGS, a subset of ZsGreen-labeled cells within the glomerular tuft coexpressed WT-1 (Figure 6, A–C). The number of cells positive for both ZsGreen and WT-1 represented 64 ± 11% of the ZsGreen-positive cells in the glomerular tuft at day 14 of disease (0.89 ± 0.14 double-positive cells per glomerular cross section in disease versus 0.26 ± 0.01 at baseline; P = 0.007).
Figure 6.
In Ren1cCre×Rs-ZsGreen-R mice with experimental FSGS, cells of renin in glomeruli costain for podocyte proteins. To determine whether the cells of renin lineage express proteins considered to be podocyte specific, experimental FSGS was induced in Ren1cCre×Rs-ZsGreen-R mice; confocal microscopy was performed after staining for WT-1 or synaptopodin (used as podocyte markers). ZsGreen fluorescence, used to fate-map cells of renin lineage, does not require antibody detection. A–C: Double-staining for ZsGreen and WT-1. A: In a representative glomerulus at baseline (day 0), ZsGreen-labeled cells were detected outside the glomerulus, in the JGC (green; thick arrow); ZsGreen staining did not overlap with WT-1 staining (red; thin arrows) within the glomerulus. B: At day 14 of disease, ZsGreen fluorescence was detected in the JGC (thick arrows) and also in a cell within the glomerular tuft (dashed arrow), where it overlapped with WT-1, appearing yellow. Nuclear staining for WT-1 alone (red; thin arrows). C: A higher magnification view of the boxed region in panel B more clearly shows the coexpression overlap of ZsGreen and WT-1 (yellow; dashed arrow). D–F: Double-staining for ZsGreen and synaptopodin. D: At day 0, ZsGreen fluorescence was seen in the afferent arteriole (thick arrow); ZsGreen staining did not overlap with synaptopodin staining (red; thin arrows) in the adjoining glomerulus. E: At day 14, ZsGreen fluorescence was still seen in the afferent arteriole (thick arrow), and an overlap of ZsGreen and synaptopodin staining (yellow; dashed arrows) was seen in a cell within the glomerular tuft. F: A higher magnification view of the boxed region in panel B more clearly shows the coexpression overlap of ZsGreen and synaptopodin (dashed arrows). These representative examples show that, in Ren1cCre×Rs-ZsGreen-R mice with experimental FSGS, a subset of cells of renin lineage labeled by ZsGreen within the glomerulus express the podocyte proteins WT-1 and synaptopodin.
At day 14 of disease, the number of ZsGreen-labeled cells that coexpressed synaptopodin increased significantly (0.72 ± 0.11 double-positive cells per glomerular cross section versus 0.38 ± 0.09 at baseline; P = 0.038) (Figure 6, D–F); within the glomerular tuft, 89 ± 2% of the ZsGreen-labeled cells coexpressed synaptopodin at day 14 of disease. WT-1 and synaptopodin staining were absent when the primary antibodies were omitted (data not shown), and ZsGreen labeling was not detected in the Cre-negative control mice (data not shown).
Taken together, these data show that when genetically labeled cells of renin lineage move from the JGC to the glomerular tuft in Ren1cCre×Rs-ZsGreen-R mice with experimental FSGS, they express the podocyte proteins WT-1 and synaptopodin, and they do not express renin de novo.
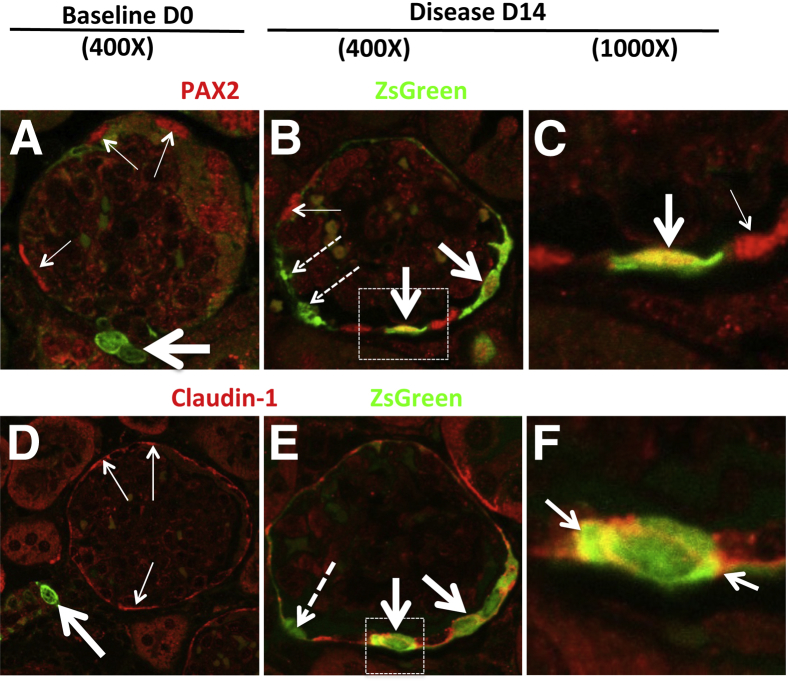
We next determined whether cells of renin lineage stain for proteins expressed by PECs in diseased Ren1cCre×Rs-ZsGreen-R mice by double-staining for ZsGreen with PAX2 or claudin-1 (Figure 7). The number of cells double-positive for ZsGreen and PAX2 cells increased significantly along Bowman's capsule at day 14 of disease (0.6 ± 0.06 double-positive cells per glomerular cross section versus 0.18 ± 0.03 at baseline; P = 0.0006). The number of cells double-positive for ZsGreen and claudin-1 increased more than twofold at day 14 (0.56 ± 0.13 double-positive cells per glomerular cross section versus 0.24 ± 0.03 at baseline; P = 0.0048). These findings indicate that, although at baseline occasional cells stained for both PAX2 and claudin-1 in Ren1cCre×Rs-ZsGreen-R mice, the number of double-positive cells increased significantly in disease. Taken together, these data show that in Ren1cCre×Rs-ZsGreen-R mice with disease, labeled cells of renin lineage lining Bowman's capsule increase expression of the PEC proteins PAX2 and claudin-1.
Figure 7.
In Ren1cCre×Rs-ZsGreen-R mice with experimental FSGS, cells of renin lineage that have entered the glomerulus coexpress PEC proteins. Experimental FSGS was induced in Ren1cCre×Rs-ZsGreen-R mice and confocal microscopy was performed after staining with antibodies to PAX2 and claudin-1 (used as PEC markers). ZsGreen fluorescence, used to fate-map cells of renin lineage, does not require antibody detection. A–C: Double-staining for ZsGreen and PAX2. A: In a representative glomerulus at baseline (day 0), ZsGreen-labeled cells were detected in cells outside the glomerulus in the JGC (green; thick arrow); within the glomerulus, ZsGreen staining did not overlap with PAX2 staining (red; thin arrows). Red blood cell autofluorescence (light green) was noted in the glomerular tuft. B: At day 14, in a subset of cells along Bowman's capsule, ZsGreen staining overlapped with PAX2 staining (yellow; thick arrows). In another subset, ZsGreen-labeled cells lining Bowman's capsule did not express PAX2 (dashed arrows), and for some PAX2-positive cells the staining did not overlap with ZsGreen (thin arrow). C: A higher magnification view of the boxed region in panel B more clearly shows the coexpression overlap of ZsGreen and PAX2 staining (thick arrow), along with single PAX2 stain (thin arrow). D–F: Double-staining for ZsGreen and claudin-1. D: At baseline, ZsGreen fluorescence in the afferent arteriole (thick arrow) did not overlap with claudin-1 staining (red; thin arrows) in the adjoining glomerulus. E: At day 14, in two cells lining Bowman's capsule ZsGreen staining overlapped with claudin-1 staining (yellow; thick arrows). One ZsGreen-labeled cell lining Bowman's capsule did not stain for claudin-1 (dashed arrow). F: A higher magnification view of the boxed region in panel E more clearly shows the coexpression overlap of ZsGreen and claudin-1 (arrows). These representative examples show that, in Ren1cCre×Rs-ZsGreen-R mice with experimental FSGS, a subset of cells of renin lineage labeled by ZsGreen within the glomerulus express the PEC proteins PAX2 and claudin-1 when these cells line Bowman's capsule.
Experimental FSGS in Ren1dCre×Z/EG Mice
To further validate the findings, experimental FSGS was induced in a third renin cell lineage reporter strain, Ren1dCre×Z/EG, in which cells of renin lineage are genetically labeled with GFP. Podocyte number, as measured by p57 staining,50 decreased in Ren1dCre×Z/EG mice relative to baseline (10.6 ± 0.43) by 25% after 7 days (7.9 ± 0.11; P = 0.008) and by 34% after 14 days (7.0 ± 0.17; P = 0.0003) (Supplemental Figure S10). Mice also developed progressive focal and segmental glomerular scarring with synechial attachments: mean glomerulosclerosis grade of 1.2 ± 0.04 at day 7 and 1.4 ± 0.15 at day 14, versus 0.2 ± 0.004 at baseline (P = 0.0001, day 7; P = 0.004, day 14) (Supplemental Figure S10). These data showed that experimental FSGS was successfully induced in Ren1dCre×Z/EG mice, and that the extent of podocyte loss and glomerular scarring was highest in this reporter strain.
Cells of Renin Lineage Increase in Glomeruli of Ren1dCre×Z/EG Mice with Experimental FSGS
Cells of renin lineage are permanently labeled with GFP in Ren1dCre×Z/EG mice; these cells were not detected in the kidneys of the mice devoid of Cre used as negative controls, nor if the primary antibody was omitted for GFP staining. At baseline (day 0), only 5 ± 1.3% of glomeruli contained one or more GFP-labeled cell. In disease, the percentage of glomeruli with GFP-labeled cells increased significantly and progressively, relative to baseline: 38 ± 2% at day 7 (P = 0.003) and 43 ± 5% at day 14 (P = 0.009) (Supplemental Figure S11). As with the other reporter mice, renin staining was limited to the JGC, where it costained with GFP (data not shown). No renin staining was detected in normal or diseased glomeruli.
In diseased mice, a progressive increase in GFP-labeled cells along Bowman's capsule was detected, relative to baseline (0.03 ± 0.02 GFP-labeled cells per glomerular cross section): 0.74 ± 0.22 at day 7 (P = 0.05) and 1.8 ± 0.08 at day 14 (P = 0.0006) (Supplemental Figure S11). The number of GFP-labeled cells in the glomerular tuft also increased, relative to zero at baseline: 0.1 ± 0.0003 GFP-labeled cells per glomerular cross section at day 7 (P < 0.05) and 0.4 ± 0.04 at day 14 (P = 0.004) (Supplemental Figure S11). Within individual glomeruli, GFP-labeled cells were detected either along Bowman's capsule, within the glomerular tuft, or in both locations. There was no particular temporal preference for location within a glomerulus. These data in Ren1dCre×Z/EG mice are consistent with labeled cells of renin lineage having moved after the onset of experimental FSGS from the JGC to the glomerular compartment, where they line Bowman's capsule and the glomerular tuft.
Cells of Renin Lineage Express Glomerular Epithelial Cell Proteins in Diseased Ren1dCre×Z/EG Mice
At baseline in Ren1dCre×Z/EG reporter mice, WT-1 staining was restricted to podocytes, and GFP labeling was mainly restricted to cells of renin lineage in the JGC. WT-1 and GFP staining did not overlap at baseline (zero double-positive cells). In diseased Ren1dCre×Z/EG mice, occasional GFP-labeled cells double-stained for WT-1 (0.1 ± 0.01 double-positive cells per glomerular cross section; P < 0.05). By day 14, the number of cells coexpressing GFP and WT-1 in the glomerular tuft increased significantly (0.37 ± 0.1; P = 0.03). It is noteworthy that, in the glomerular tuft, 95 ± 2% of the GFP-labeled cells coexpressed WT-1 by day 14.
No costaining for GFP and synaptopodin was detected in the glomerular tuft at baseline. In experimental FSGS, GFP-labeled cells in the glomerular tuft that coexpressed synaptopodin increased slightly, relative to zero at baseline: 0.07 ± 0.001 double-positive cells per glomerular cross section at day 7 (P = 0.08) and 0.27 ± 0.01 at day 14 (P = 0.005). In the glomerular tuft, 89 ± 2% of GFP-labeled cells coexpressed synaptopodin by day 14 of disease.
Although occasional GFP-labeled cells were detected in less than 5% of glomeruli, none of them coexpressed PAX2 at baseline. In experimental FSGS, a subpopulation of cells along Bowman's capsule stained for both GFP and PAX2 at both day 7 (0.45 ± 0.03 double-positive cells per glomerular cross section; P = 0.05) and day 14 (0.55 ± 0.13; P = 0.03). Of the GFP-labeled cells lining Bowman's capsule, 65 ± 15% of GFP-labeled cells coexpressed PAX2 on day 7, which decreased to 31 ± 7% on day 14 of disease. Thus, a subpopulation of GFP-labeled cells lining Bowman's capsule did not express PAX2.
No cells costained for claudin-1 and GFP at baseline. The number of GFP-labeled cells that coexpressed claudin-1 increased significantly in diseased mice at both day 7 (0.62 ± 0.06 double-positive cells per glomerular cross section; P = 0.008) and day 14 (0.81 ± 0.1; P = 0.005).
Taken together, these data show that, during experimental FSGS in Ren1dCre×Z/EG reporter mice, labeled cells of renin lineage increase in number within glomeruli, where they do not express renin but begin to express proteins considered to be unique to both types of glomerular epithelial cells (ie, both podocytes and PECs).
Cells of Renin Lineage Do Not Express Mesangial or Glomerular Endothelial Cell Markers
Double-staining for cells of renin lineage with markers for mesangial cells (PDGFR-β and type IV collagen) or endothelial cells (MECA-32) did not increase in glomeruli in this model (data not shown).
Cells of Renin Lineage Do Not Express Certain Stem Cell Markers
Cells of renin lineage did not stain for CD24, CD44, or CD133 in normal or diseased states (data not shown).
Renin Transcripts Are Not Detected in Diseased Glomeruli
Expression of renin transcripts may not result in protein expression. We therefore generated RenGFP transgenic reporter mice using a BAC transgenic method similar to that which drives mouse renin Cre (RenCre), in which a GFP reporter is driven directly by the renin promoter and all its regulatory elements in a 227-kb segment of chromosome 140 (Supplemental Figure S1). Any cell actively transcribing renin mRNA will express GFP protein. At baseline, GFP staining was readily detected, but restricted only to cells in the JGC that precisely stained for renin protein (Supplemental Figure S12). An average of 50 glomeruli from each mouse were observed at days 14, 21, and 28 of disease. No GFP or renin staining was detected within any glomeruli of RenGFP mice with experimental FSGS (Supplemental Figure S12). This indicates that the renin promoter was not active in cells within the glomerulus during disease (no mRNA transcribed) and also explains why renin protein was not detected in diseased glomeruli (Supplemental Figure S12). These data indicate that the appearance of genetically labeled cells of renin lineage in glomeruli during experimental FSGS is likely due to migration of cells from the JGC into the glomerulus, and not due to DNA recombination in injured glomerular cells because of activation of the renin gene.
Discussion
The leading causes of progressive kidney disease with proteinuria are diabetic nephropathy, FSGS, and membranous nephropathy. These glomerular diseases are characterized by injury to glomerular podocytes. Injury-induced apoptosis, necrosis, autophagy, and detachment lead to progressive podocyte loss. Although many other cells in the kidney are replaced after loss by proliferation of surviving cells, total podocyte number per glomerulus frequently decreases after loss, because of impaired capacity of surviving podocytes to successfully undergo cell division.11–17 Until recently, little has been understood about the kinetics of podocyte turnover and attrition in chronic disease.
Recent seminal studies have shed light on neighboring glomerular PECs as a likely source of podocyte progenitors. A subset of PECs near the tubular pole express progenitor markers.27,32,67,68 In diseased states characterized by podocyte injury, PECs can activate cellular protein markers normally considered to be restricted to podocytes,27,29,30,68 and fate-mapping experiments suggest that PECs may become podocytes during postnatal kidney growth.24 Although still speculative, these findings suggest that PECs may subsume some of the functions ascribed to podocytes, particularly in response to disease. At present, however, it remains unclear whether there is a common pool of precursor cells that can replenish podocytes and PECs, and whether replacement of podocytes by PECs is reparative or is, alternatively, part of an ongoing process of injury. The present results indicate that cells of renin lineage have the capacity to serve as progenitors for both PECs and podocytes in an experimental model characterized by reduced podocyte number.
The central approach used in the present studies was genetic fate mapping of cells of renin lineage in three newly generated and one existing reporter transgenic mouse strain. The advantage of the newly developed Ren1cCreER×Rs-tdTomato-R mouse is that a cohort of cells of renin lineage is genetically labeled with tomato red protein in an inducible manner only during the specific time period when the mice are given tamoxifen. This enables definitive and permanent cell fate mapping by measuring RFP. In contrast, the newly developed Ren1cCre×Rs-ZsGreen-R and the previously reported Ren1dCre×Z/EG mice rely on constitutive reporter systems; thus, the entire complement of cells that express renin during ontogeny permanently express ZsGreen and GFP in the two strains, respectively, including both cells of renin lineage and cells derived from their lineage. In the newly developed RenGFP mice, GFP labels cells in which renin mRNA is actively being transcribed.
Renin Lineage Cells Exist at Different Numbers within the Glomerulus at Baseline
The first major finding in the present study was a differential number of labeled cells of renin lineage in the glomeruli of normal baseline reporter mice (ie, before disease induction). In baseline adult Ren1cCreER×Rs-tdTomato-R mice given tamoxifen, RFP was not detected in any glomeruli. In baseline Ren1dCre×Z/EG and Ren1cCre×Rs-ZsGreen-R mice, however, less than 5% of glomeruli expressed at least one GFP-labeled cell and less than 20% of glomeruli expressed at least one ZsGreen-labeled cell. Several explanations are possible for the differences between transgenic strains in the number of labeled cells within the glomerulus at baseline. First, by administering tamoxifen in normal Ren1cCreER×Rs-tdTomato-R adult mice (well before injury induction), we ensured that only cells in the JGC were labeled. The more extensive preglomerular vascular wall population that expresses renin during renal ontogeny and is captured by RenCre were therefore not marked in this strain, although they are marked are in Ren1dCre×Z/EG and Ren1cCre×Rs-ZsGreen-R mice.
Another possible explanation is that the two reporter systems use highly similar but distinct promoters, Ren1d and Ren1c, to drive Cre expression. Prior assessments of Ren1c and Ren1d expression suggest that the genes exhibit distinct, but highly overlapping, tissue-specific and developmental expression patterns. The Ren1d allele is found in association with another renin gene locus, Ren2. We developed a methodology for distinguishing the highly similar transcripts generated by the individual alleles at the Ren1 locus or at the Ren1 and Ren2 loci.69 Ren1c was observed to accumulate in normal kidney to twice the steady-state level of Ren1d in F1 animals (hybrids of the one- and two-locus strains), and Ren1d was observed to accumulate to slightly higher levels than Ren2 in kidney.69 The latter result was also observed by Smithies and colleagues.70 The best available evidence from all investigators suggests that, in normal animals, all three transcripts are localized to similar cells in kidney. More marked, gene-specific variation in expression is observed in other tissues.69 We used both strains because they differ in allelic reporting potential and also in other important characteristics. The Ren1dCre strain is constructed by gene targeting on a two-renin-locus strain background. It is normally used as a heterozygote, to avoid disruption of both alleles at the Ren locus. Although Ren2 can compensate for homozygous loss of Ren1d as regards observable renal pathology, there is presumably diminished output from the Ren1d promoter and unknown drive as regards compensatory cell recruitment.70 The Ren1cCre strain was constructed by homologously recombining the Cre cassette in phase with the Ren1c promoter within a large 227-kb C57BL/6 BAC and constructing transgenic mice. In consequence, the Ren gene is surrounded by a large normal flanking chromosome environment, but there is no compromising of expression from the endogenous Ren loci. In effect, it is more of a reporter. We submit that at this juncture it remains to be elucidated whether we are dealing with variable fate sorting during normal development or possibly even in response to reparative action during normal development. It is quite conceivable that relative sensitivity or compensatory drive also come into play. What is more important at this point, in our opinion, is that the different types of Cre constructs are reporting similar results qualitatively, albeit not identical results quantitatively. Most importantly, some of these issues necessarily reflect the fact that Cre is reporting, at some level of sensitivity, the entire history of expression during kidney development, whereas the RenCreERt2 construct permits capturing the response of a potentially more restricted juxtaglomerular population from adult animals.
As a third explanation, it is possible that the floxed ZsGreen reporter is more sensitive in response or intensity of expression, facilitating earlier or more robust detection. Fourth, it is also conceivable that any natural replacement being reported is highly stochastic between individual mice, or is influenced by environmental differences in vivarium conditions. Finally, to explore the possibility that the number of labeled cells might reflect age differences in Ren1cCre×Rs-ZsGreen-R mice, we also studied young mice, during the final phase of kidney development (1 week of age) and in adolescence (4 weeks), and compared these to mature mice (>10 weeks). The number of glomerular cells expressing ZsGreen label were similar across all time points, suggesting that labeled cells were normally present in glomeruli starting at a young age and thus are not a function of age per se. The fact that the adolescent and mature patterns were similar suggests that the observed events arise early. These results support the findings of Gomez and colleagues,34 who detected the presence of cells of renin lineage in adult Ren1dCre×Z/EG mice. This is consistent with our observation that the Ren1cCreER×Rs-tdTomato-R reporter had a zero baseline level. It is noteworthy that that renin cells are very few in number, and account for only 0.01% to 0.001% of the total kidney cell mass.34
Numbers of Renin Lineage Cells Increase in Glomeruli after Disease
A second major finding from the present study is that genetically fate-mapped cells of renin lineage were detected in significantly more glomeruli of all three reporter mouse strains after the development of experimental FSGS. An increase in labeled cells was detected as early as day 3 of disease in Ren1cCre×Rs-ZsGreen-R mice. In this strain, and also in the Ren1dCre×Z/EG mice, there was a progressive and significant increase in number of fate-mapped cells of renin lineage at each time point. It is noteworthy that the number of labeled cells in the glomerulus temporally correlated with a progressive decline in podocyte number during disease. We have previously reported that the model used in these studies has many features characteristic of clinical FSGS (ie, reduced podocyte number, proteinuria, and glomerulosclerosis).30,45,46 One explanation for the differences in the absolute numbers of labeled cells in the glomeruli of the three different reporters with disease is that, despite similar deposition of disease-inducing cytotoxic anti-podocyte antibody, the intensity of disease differed by strain. It is not surprising, therefore, that the number of cells of renin lineage detected in glomeruli differ by strain. Because Ren1cCreER×Rs-tdTomato-R mice were given tamoxifen only on three occasions during their sixth week of life, one possible explanation for their relatively lower number of labeled cells in disease glomeruli, compared with the constitutive reporter strains, is that the reservoir or pool of labeled cells of renin lineage was simply insufficient to capture the magnitude of cells that might enter the glomerulus from generations before or after the labeling process. Although cells of renin lineage were detected both lining Bowman's capsule and within the glomerular tuft, there was no obvious pattern, either temporally or spatially, of labeled cells in individual glomeruli. Noteworthy is the location of cells of renin lineage within glomeruli. Romagnani and colleagues27,68 have elegantly shown that a subpopulation of PECs that serve as progenitors are located predominantly at the tubular pole. Cells of renin lineage appear to enter the glomerulus at the vascular pole, but thereafter are not restricted in location.
Renin Lineage Cells Express Markers of Glomerular Epithelial Cells
The third major finding in the present study was that, on entering the glomerulus, a subset of cells of renin lineage begins to express proteins considered to be specific for glomerular epithelial cells (ie, podocytes and PECs). In diseased Ren1cCreER×Rs-tdTomato-R, Ren1cCre×Rs-ZsGreen-R, and Ren1dCre×Z/EG mice, a significant subpopulation of labeled cells in the glomerular tuft coexpressed WT-1, nephrin, podocin, or synaptopodin, which are considered markers of podocytes. Additionally, after the onset of experimental FSGS in all three reporter mice, a significant subpopulation of labeled cells lining the Bowman's capsule, coexpressed PAX2 or claudin-1, both considered markers of PECs. There was no obvious pattern of cells of renin lineage expressing either podocyte or PEC markers.
One might ask whether the numbers of labeled cells expressing either podocyte or PEC proteins is a meaningful measure. In this model, the decrease in podocyte number after the onset of disease was typically three to four cells per glomerular cross section. Also, labeled cells were found in 0% to 20% of glomeruli before disease induction in the constitutive reporter mice, and this increased to between 41% and 61% of glomeruli after disease induction (P < 0.001). These data give an indication of how many glomeruli contained positive cells. The mean number of labeled cells per glomerular cross section was also quantified. An average of 76 glomeruli were counted, so on average this results in between 0.3 and 0.63 cells along Bowman's capsule per glomerular cross section and 0.1 to 1.71 cells within the glomerular tuft per glomerular cross section after disease. Because FSGS is a focal disease, it is not surprising that many glomeruli have no labeled cells, some have only a few, and some have many. Finally, glomerular cross sections were analyzed, rather than the three-dimensional structure of the entire glomerulus; the latter would of course enhance the overall number of labeled cells. It is noteworthy that, although some have reported that PEC progenitors appear evident predominantly at the tubular pole,68 others report them at the vascular pole.24,28 It is not an anomaly, therefore, that our studies show the cells of renin lineage deriving from the vascular pole.
Several lines of evidence in the present study indicate that the detection of cells of renin lineage within glomeruli does not represent false positives simply due to the expression or re-expression of renin protein and/or mRNA. First, in Ren1cCreER×Rs-tdTomato-R reporter mice, cells of renin lineage are labeled only after the administration of tamoxifen. Also, tamoxifen was withdrawn well before disease induction, and therefore all glomerular-associated reporting is attributable only to pre-existing tdTomato Red–labeled cells (exclusively of juxtaglomerular origin) in this reporter mouse. These cells may later change phenotype, migrate, or divide, but they will have initially arisen as cells of renin lineage from the JGC. Second, no immunostaining with an antibody recognizing renin and prorenin was detected in any glomeruli in any of the four reporters at any time in health or disease; rather, such immunostaining was confined entirely to the JGC. Third, double-staining for each reporter (RFP, ZsGreen, and GFP) with renin showed coexpression only in the JGC, not in glomeruli. Fourth, we used a RenGFP reporter mouse, which we generated specifically to report contemporaneous renin promoter activity by GFP expression. As expected, renin mRNA was restricted to cells of the JGC that stained for renin. Finally, another point to keep in mind when looking at RenCre lineage in glomeruli is that cells of renin lineage could arise from other than the JGC (eg, cells from larger arterioles). Moreover, we know that RenCre is expressed in other locations, such as bone marrow (B cells), and thus one could argue that these cells are infiltrating the glomerulus. The Ren1cCreER×Rs-tdTomato-R experiments makes for a slightly cleaner model with regard to kidney reporting, but one that is not necessarily infallible with regard to bone marrow infiltrate. Taken together, these data show that the genetic cell fate-mapping approach to studying cells of renin lineage in experimental FSGS is accurate and the findings are not due to de novo expression or re-expression of renin mRNA or protein, nor are they an artifact of the Cre system used.
Conclusions
Our findings suggest a number of critical implications. Renin-producing cells of the juxtaglomerulus are modified smooth muscle cells at the tip of the afferent arteriole supplying the glomerulus; they are derived from metanephric mesenchyme fated to become kidney stroma, not epithelium. Our data show that, under the conditions studied, podocytes and PECs might originate from cells of renin lineage, a finding that could shed light on novel functions previously not considered. Because podocytes and PECs exhibit structural and molecular features similar to epithelial cells, glomerular cells derived de novo from cells of renin lineage may be functionally discrete from the glomerular cells. These studies also beg the question of whether a low-level replacement of podocytes and PECs, from cells of renin lineage, occurs in homeostasis. Although it has been assumed that the podocyte population is not replenished, it is more likely that a cell so critical to survival can somehow be replaced. Thus, it is quite possible that podocytes are steadily replenished throughout life. Our findings also raise critical questions about whether PECs are an intermediate for podocytes that arise from cells of renin lineage and whether PECs not derived from cells of renin lineage can also become podocytes. The present studies were not designed to determine whether cells of renin lineage first become differentiated PECs and subsequently become podocytes; further detailed fate mapping would be required to ascertain this sequence.
We have reflected a great deal on the movement of cells of renin lineage from their juxtaglomerular origin to the glomerulus, which likely includes the crossing of either the parietal and/or the glomerular basement membrane. Although we did not perform formal migration studies, we believe that such migration is entirely possible, based on literature supporting the notion that cells routinely cross basement membranes.71–75 A minor population of renin lineage cells are pericytes of the peritubular capillaries and are migratory. Vascular smooth muscle cells in other arterioles have been shown to migrate from the vessel wall to the adventitia and back, breaching membranes and, in artery walls, vascular smooth muscle cells have been shown to migrate to cover atheromatous plaque, which also involves breaching membranes. It is therefore likely that these cells can pass through membranes. Also, when epithelial-derived tumors metastasize, the first barrier that invading cells breach is the basement membrane underlying the epithelium. Freed cells then traverse the stromal connective tissue, a gelatinous extracellular matrix rich in proteoglycans that surrounds glands and blood vessels, and then again cross the basement membranes of blood or lymphatic vessels to disperse into these vessels. During extravasation, the invasive cells cross back through the vascular basement membrane into new tissues where they can form micrometastases.71 Other examples include cells routinely crossing basement membranes during normal development and cells crossing membrane to perform immune-system functions. Moreover, red blood cells have been shown by transmission electron microscope to cross the glomerular basement membrane in thin basement membrane nephropathy72 and in minimal change disease.73 Podocytes have been shown by multiphoton excitation fluorescence microscopy to cross Bowman's capsule.74 Finally, careful examination of published reports of transmission electron microscope studies reveals that the structure of the area where the afferent arteriole, Bowman's capsule, and glomerular basement membrane converge is highly layered (many thin layers of basement membrane), rather than a single thick layer.75 The layering likely enables cells to get across. Taken together, these various studies suggest that cells of renin lineage crossing one or more membranes to get to the glomerulus is a physiologically plausible notion.
Although the present studies provide evidence for new podocytes and PECs arising from cells of renin lineage, functional data are lacking. The critical functions of podocytes are to maintain the slit diaphragm, maintain the glomerular basement membrane, and to maintain endothelial cell integrity. Further studies would be required to determine whether podocytes derived from cells of renin lineage can perform these critical tasks. Currently, the molecular programs that generate podocytes are poorly understood. We were unable to detect a limited number of stem cell and progenitor cell markers (CD24, CD44, and CD133) in cells of renin lineage during disease. This of course does not rule out stemness, given the large number of potential markers that might be considered. Further studies are needed to dissect the transcriptional program for the differentiation of cells of renin lineage into podocytes and PECs.
Our data in the present study strongly support the notion that, in this experimental disease model of FSGS, cells of renin lineage do not likely give rise to mesangial and glomerular endothelial cells. The availability of models of podocyte disease is limited in mice, being restricted by strain and sex, among other determinants.76 Our present model relies on an antibody that recognizes one or more as yet unidentified podocyte antigens. However, as we have reported for several other transgenic mouse strains,30,45,46 the antibody reliably reduces podocyte number, which leads to segmental glomerular scarring in less than half of all glomeruli and to synechial attachment in approximately 30% to 35% of glomeruli. These findings resemble classic clinical FSGS, and therefore the model was appropriate for the purposes of the present study. This model is distinct from other models in mice induced by anti-glomerular antibodies leading to pseudo-crescents.13,47–50 Because cells of renin lineage no longer express renin in the glomerulus during disease, this limits validation in other rodent models or in human disease until such time as a specific cell of renin lineage marker is identified. Additional experimental models in reporter mice are needed. It is unclear why cells of renin lineage no longer express renin. Reports indicate that WT-1 can regulate renin,77 but further studies are needed to determine the events involved. The lack of renin staining does provide a challenge for studies in nonreporter mice and until such time as another marker of cells of renin lineage can be identified.
In summary, we propose the emergence of a novel paradigm in which cells of renin lineage serve as progenitor cells for both glomerular PECs and podocytes in experimental glomerular disease. We suggest that further study and exploration are merited.
Acknowledgments
We thank Ronald Krofft, Geoffrey Linn, Caretta Reese, Greg Martin, and Mary K. Ellsworth for their technical contributions and Dr. R. Ariel Gomez (University of Virginia) for providing the Ren1d Cre mice used in these studies.
Footnotes
Supported in part by NIH grants R01-HL048459R21-CA121212, and P30-CA016056 (K.G.); R01 DK87389, RC1 DK84077, and R24 DK94768 (J.S.D.); and R01-DK056799 and R21-DK081835 (SJS).
See related Commentary on page 333.
Supplemental Data
Generation of Ren1c reporter constructs. The RP23-88k7 BAC was from a library (C57Bl/6 mice) and represents a segment of chromosome 1. The renin gene (Ren1c) is centrally located and is flanked by Pepp3, Kiss1, Etnk2, and Sox13. Using homologous recombination, either a Cre, CreERt2, or GFP cassette was inserted at exon 1 of Ren1c. Transgenic mice were generated by zygotic microinjection of this construct.
Renin expression in kidney in the Ren1cCre×Rs-ZsGreen-R transgenic line. A: The Ren1cCreER×Rs-ZsGreen-R transgenic line tags the renin-expressing cell in a specific temporal window dependent on tamoxifen induction. An adult 6-week-old Ren1cCreER×Rs-ZsGreen-R mouse was administered 1 mg/day tamoxifen for 4 days; the animal was sacrificed at 1 week after the last injection and whole kidney tissue was examined under a dissecting microscope. Only juxtaglomerular cells (sites of adult renin expression) are tagged with ZsGreen. Note one large nontagged vessel (arrow). B: The Ren1cCre×Rs-ZsGreen-R transgenic line faithfully reports natural renin expression in the kidney. Whole kidney tissue from a 6-week-old Ren1cCre×Rs-ZsGreen-R mouse shows lineal descendants of renin-expressing cells in the renal vasculature. Original magnification, ×12.
Sheep anti-rabbit glomerular antibodies bind selectively to podocytes. To demonstrate that the antibody used to induce experimental FSGS binds to podocytes, double-staining for sheep IgG (green) and the podocyte-specific proteins nephrin and synaptopodin (red) was used. A–F: Only nephrin and synaptopodin staining were detected in normal mice. G–L: Sheep IgG was detected in mice given disease-inducing antibody, which costained with podocyte proteins (yellow). M–O: No staining overlap was detected when the primary nephrin or synaptopodin antibodies were omitted as controls. AB, antibody. Arrows indicate location of JGC where no staining for sheep IgG was observed.
Study design. Four reporter mice that genetically fate-map cells of renin lineage with different labels were given cytotoxic anti-podocyte antibody to induce experimental FSGS. Mice were sacrificed at different days of disease. Tamoxifen was administered to 5-week-old Ren1cCre_Rs-ZsGreen-R mice and experimental FSGS was induced 49 days later at 12 weeks of age. BW, body weight; d, day; IP, intraperitoneal injection.
Characterization of experimental FSGS in Ren1cCreER×Rs-tdTomato-R mice. Disease was induced with a cytotoxic anti-podocyte antibody. A: After injection of cytotoxic antibody, sheep IgG staining was detected within glomeruli by immunofluorescence staining in a podocyte distribution. B: Quantification of podocyte number, measured by the number of cells staining for p57 per glomerular cross section, shows a progressive decrease at days 7 and 14 of disease. C: Quantification of the percentage of glomeruli with one or more RFP-labeled cells increased significantly at days 7 and 14 of disease. Data are expressed as means ± SEM.
Cells of renin lineage express WT-1, nephrin, and podocin in glomeruli of Ren1cCreER×Rs-tdTomato-R mice with experimental FSGS. A–H: Double-staining was performed at baseline (day 0) (A), day 7 (B and C), and day 14 (D–H) of disease with antibody to WT-1 (red; thin arrows) and for the reporter with an antibody to RFP (green; thick arrows). Overlapping stains appear yellow-orange (thick dashed arrows) in merged images. WT-1 and the reporter staining did not overlap in baseline mice; in diseased mice, they overlapped in some (B–E) but not in all (F–H) glomeruli. I and J: Double-staining was performed for nephrin (red; thin arrow) and RFP (green; thick arrow). Nephrin staining overlapped with RFP (yellow; dashed arrow) in some (I) but not in all (J) glomeruli. K and L: Double-staining was performed for podocin (red; thin arrows) and RFP (green; thick arrows) at day 14 of disease. Podocin staining overlapped with RFP (yellow; dashed arrows) in some (K) but not in all (L) glomeruli. M–R: No staining for WT-1 (M), nephrin (O), or podocin (Q) was detected when the primary antibodies were omitted. No staining for the reporter was detected when the RFP antibody was omitted in sections double-stained for WT-1 (N), nephrin (P), or podocin (R). Circles indicate glomeruli. Boxed regions indicate JGCs.
Cells of renin lineage express PEC proteins in glomeruli of Ren1cCreER×Rs-tdTomato-R mice with experimental FSGS. A–D: Double-staining was performed at baseline (day 0) (A) and day 14 (B–D) of disease with an antibody to PAX2 (red; thin arrows), and for the reporter with an antibody to RFP (green; thick arrows). Overlapping stains appear yellow-orange (thick dashed arrows) in merged images. PAX2 and reporter staining did not overlap in baseline mice (A); in diseased mice, they overlapped in some (B and C) but not in all (D, thin dashed arrows) glomeruli. E–H: Double-staining was performed at baseline (day 0) (E) and day 14 (F–H) of disease with an antibody to claudin-1 (red; thin arrows) and for the reporter with an antibody to RFP (green; thick arrows). Overlapping stains appear yellow-orange (thick dashed arrows) in merged images. Claudin-1 and reporter staining did not overlap in baseline mice (E); in diseased mice, they overlapped in some (F and G) but not in all (H, thin dashed arrows) glomeruli. I and J: Staining for the reporter was not detected when the RFP antibody was omitted in sections double-stained for PAX2 (I) and claudin-1 (J). The JGC is indicated by a square.
Podocyte depletion and glomerular scarring characterize experimental FSGS in Ren1cCre×Rs-ZsGreen-R mice. Disease was induced with a cytotoxic anti-podocyte antibody. A: Quantification of podocyte number measured by the number of cells staining for p57 per glomerular cross section showed a progressive decrease at days 3, 7, and 14 of disease. B: Quantification of glomerular scarring, measured by a glomerular sclerosis score, showed a progressive increase in mice with disease at all time points. C and D: Podocytes were identified by p57 staining. C: p57 staining in podocytes (arrows) in a representative glomerulus at baseline. D: At day 14, p57 staining was reduced. E and F: PAS staining was used to assess glomerular histology. E: Glomerular histology was normal at baseline. F: At day 14, a representative glomerulus in a reporter mouse shows segmental scarring (dashed arrow), and the glomerular tuft forms an adhesion with Bowman's capsule (arrows). These data show that the administration of an anti-podocyte antibody to Ren1cCre×Rs-ZsGreen-R mice caused a decrease in podocyte number accompanied by progressive glomerular scarring, features characteristic of clinical FSGS. Data are expressed as means ± SEM. Original magnification, ×630.
Renin protein was restricted to cells in the JGC and was not detected in diseased glomeruli. Experimental FSGS was induced in Ren1cCre×Rs-ZsGreen-R mice. Double-staining was performed for renin and ZsGreen at baseline (A–F) and at 14 days of disease (G–L). A: At baseline, renin staining (red; arrows) was restricted to cells in the JGC; renin was not detected in the four glomeruli (circles). B: At baseline, ZsGreen-labeled cells (dashed arrows) were restricted to cells in the JGC and were not detected in glomeruli (circle). C: At baseline, ZsGreen labeling and renin staining overlapped (yellow; white arrows) in the JGC; no such costaining was detected in glomeruli (circles). Some ZsGreen-labeled cells do not express renin (yellow arrows). D–F: Higher magnification views of the boxed region in panels A–C, respectively. G–I: G: At day 14, renin staining was restricted to cells in the JGC (red; arrows); renin was not detected in the four glomeruli (dashed arrows). H: At day 14, ZsGreen-labeled cells were detected in three glomeruli (dashed circle), but not in a fourth glomerulus (solid circle). ZsGreen-labeled cells were also detected in the JGC (dashed arrows). I: At day 14 of disease, ZsGreen label overlapped with renin staining (yellow; white arrows). Some ZsGreen-labeled cells do not express renin (yellow arrows). J–L: Higher magnification views of the boxed region in panels G–I, respectively. In one of the glomeruli (K), ZsGreen-labeled cells were detected within the glomerular tuft (thin arrows), as well as in the JGC (thick arrow). Taken together, these representative examples show that, at both baseline and 14 days, renin staining was not detected in the glomeruli Ren1cCre×Rs-ZsGreen-R mice and was restricted to cells of the JGC only. This is consistent with the notion that the appearance of ZsGreen cells in glomeruli is not due to renin protein expression or re-expression.
Reduced podocyte number and glomerular scarring characterize experimental FSGS in Ren1dCre×Z/EG mice. Disease was induced in Ren1dCre×Z/EG mice with a cytotoxic anti-podocyte antibody. A: Quantification of podocyte number, measured by the number of cells staining for p57 per glomerular cross section, shows a progressive and significant decrease at days 7 and 14 of disease. B: Quantification of glomerular scarring, measured by a glomerular sclerosis score, shows a progressive and significant increase in diseased mice at all time points. C and D: p57 staining identified podocytes. C: A representative glomerulus in baseline mice shows p57 staining in podocytes (arrows). D: p57 staining was reduced at day 14 of disease. E and F: PAS staining was used to assess glomerular histology. E: Glomerular histology was normal at baseline. F: A representative image of a glomerulus in a mouse with experimental FSGS at day 14 shows segmental scarring (dashed arrow); the glomerular tuft forms an adhesion with Bowman's capsule (arrows). These data show that the administration of an anti-podocyte antibody to Ren1dCre×Z/EG mice causes a progressive decrease in podocyte number, accompanied by progressive glomerular scarring; both features are characteristic of clinical FSGS. Data are expressed as means ± SEM. Original magnification, ×630.
Cells of renin lineage increase in glomeruli of Ren1dCre×Z/EG mice with experimental FSGS. Disease was induced in Ren1dCre×Z/EG mice with an anti-podocyte antibody, to fate-map cells of renin lineage by permanent labeling with GFP; nuclei were counterstained with DAPI. A and B: Quantification of GFP-positive cells at baseline (day 0) and at days 7 and 14 of disease. A: At day 0, at least one GFP-positive cell was detected in only 5% of glomeruli. At days 7 and 14 of disease, the number of glomeruli expressing GFP-positive cells increased significantly and progressively. B: Quantification of the number of GFP-labeled cells lining Bowman's capsule showed that GFP-labeled cells were rarely detected lining Bowman's capsule at day 0, but their numbers increased significantly at days 7 and 14 of disease. Within the glomerular tuft, GFP-labeled cells were barely detected at baseline, but increased progressively and significantly at days 7 and 14 of disease. C: After injection of cytotoxic antibody, sheep IgG staining was detected within glomeruli by immunofluorescence staining in a podocyte distribution. Data are expressed as means ± SEM. Original magnification, ×200.
Renin mRNA and protein is restricted to cells in the JGC at baseline and in diseased RenGFP reporter mice. Experimental FSGS was induced in RenGFP reporter mice, in which GFP represents renin promoter activity and thus also renin transcription. Double-staining was performed at baseline and in diseased mice with renin (red) and GFP (green) for renin protein and renin mRNA, respectively. A: Positive renin staining (red; arrows) indicates that renin protein was restricted to the JGC in baseline RenGFP mice. Renin staining was absent in glomeruli (circles). B: Positive GFP staining (green; arrows) indicates that renin mRNA expression was restricted to the JGC in baseline RenGFP mice. GFP staining was absent in glomeruli (circles). C: Cells double-positive for both GFP and renin (yellow; arrows) were restricted to the JGC. No coexpression staining overlap was noted within glomeruli. D–F: Higher magnification views of the boxed regions in panels A–C. G: Positive renin staining (red; arrows) indicates that renin protein was restricted to the JGC in diseased RenGFP mice at day 14 of disease. Renin staining was absent in glomeruli (circles). H: Positive GFP staining (green; arrows) in the JGC in diseased RenGFP mice. GFP staining was absent in glomeruli (circles). Some green autofluorescence is seen in red blood cells within the glomerular capillary loops. I: Cells double-positive for both GFP and renin (yellow; arrows) were restricted to the JGC. No staining coexpression overlap was detected within glomeruli (circles). J–L: Higher magnification views of the boxed regions in panels G–I. These data show that, in RenGFP reporter mice, renin promoter activity was limited to cells in the JGC in both control and diseased mice. Thus, labeled cells derived from renin lineage that appear in the glomerulus are due neither to renin promoter activity nor to renin protein expression. Original magnification: ×100 (A–C and G–I); ×630 (D–F and J–L).
References
- 1.Foley R.N., Collins A.J. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–2648. doi: 10.1681/ASN.2007020220. [DOI] [PubMed] [Google Scholar]
- 2.Pagtalunan M.E., Miller P.L., Jumping-Eagle S., Nelson R.G., Myers B.D., Rennke H.G., Coplon N.S., Sun L., Meyer T.W. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura T., Ushiyama C., Suzuki S., Hara M., Shimada N., Ebihara I., Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 4.Petermann A.T., Pippin J., Krofft R., Blonski M., Griffin S., Durvasula R., Shankland S.J. Viable podocytes detach in experimental diabetic nephropathy: potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol. 2004;98:e114–e123. doi: 10.1159/000081555. [DOI] [PubMed] [Google Scholar]
- 5.Lerco M.M., Macedo C.S., Silva R.J., Pinheiro Dde O., Spadella C.T. The number of podocyte and slit diaphragm is decreased in experimental diabetic nephropathy. Acta Cir Bras. 2006;21:87–91. doi: 10.1590/s0102-86502006000200006. [DOI] [PubMed] [Google Scholar]
- 6.Petermann A.T., Krofft R., Blonski M., Hiromura K., Vaughn M., Pichler R., Griffin S., Wada T., Pippin J., Durvasula R., Shankland S.J. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int. 2003;64:1222–1231. doi: 10.1046/j.1523-1755.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiggins R.C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 8.Hara M., Yanagihara T., Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron. 2001;89:342–347. doi: 10.1159/000046097. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y.H., Goyal M., Kurnit D., Wharram B., Wiggins J., Holzman L., Kershaw D., Wiggins R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 10.Wharram B.L., Goyal M., Wiggins J.E., Sanden S.K., Hussain S., Filipiak W.E., Saunders T.L., Dysko R.C., Kohno K., Holzman L.B., Wiggins R.C. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 11.Shankland S.J. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 12.Kuan C.J., al-Douahji M., Shankland S.J. The cyclin kinase inhibitor p21WAF1, CIP1 is increased in experimental diabetic nephropathy: potential role in glomerular hypertrophy. J Am Soc Nephrol. 1998;9:986–993. doi: 10.1681/ASN.V96986. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.G., Alpers C.E., Brugarolas J., Johnson R.J., Couser W.G., Shankland S.J. The cyclin kinase inhibitor p21CIP1/WAF1 limits glomerular epithelial cell proliferation in experimental glomerulonephritis. Kidney Int. 1999;55:2349–2361. doi: 10.1046/j.1523-1755.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 14.Petermann A.T., Pippin J., Hiromura K., Monkawa T., Durvasula R., Couser W.G., Kopp J., Shankland S.J. Mitotic cell cycle proteins increase in podocytes despite lack of proliferation. Kidney Int. 2003;63:113–122. doi: 10.1046/j.1523-1755.2003.00723.x. [DOI] [PubMed] [Google Scholar]
- 15.Petermann A., Hiromura K., Pippin J., Blonski M., Couser W.G., Kopp J., Mundel P., Shankland S.J. Differential expression of d-type cyclins in podocytes in vitro and in vivo. Am J Pathol. 2004;164:1417–1424. doi: 10.1016/S0002-9440(10)63228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagata M., Nakayama K., Terada Y., Hoshi S., Watanabe T. Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol. 1998;153:1511–1520. doi: 10.1016/s0002-9440(10)65739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata M., Yamaguchi Y., Ito K. Loss of mitotic activity and the expression of vimentin in glomerular epithelial cells of developing human kidneys. Anat Embryol (Berl) 1993;187:275–279. doi: 10.1007/BF00195765. [DOI] [PubMed] [Google Scholar]
- 18.Lemley K.V., Lafayette R.A., Safai M., Derby G., Blouch K., Squarer A., Myers B.D. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- 19.Macconi D., Bonomelli M., Benigni A., Plati T., Sangalli F., Longaretti L., Conti S., Kawachi H., Hill P., Remuzzi G., Remuzzi A. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42–54. doi: 10.2353/ajpath.2006.050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki T., Matsusaka T., Nakayama M., Asano T., Watanabe T., Ichikawa I., Nagata M. Genetic podocyte lineage reveals progressive podocytopenia with parietal cell hyperplasia in a murine model of cellular/collapsing focal segmental glomerulosclerosis. Am J Pathol. 2009;174:1675–1682. doi: 10.2353/ajpath.2009.080789. [Erratum appeared in Am J Pathol 2009, 175:1349] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamczak M., Gross M.L., Krtil J., Koch A., Tyralla K., Amann K., Ritz E. Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats. J Am Soc Nephrol. 2003;14:2833–2842. doi: 10.1097/01.asn.0000095248.91994.d3. [DOI] [PubMed] [Google Scholar]
- 22.Macconi D., Sangalli F., Bonomelli M., Conti S., Condorelli L., Gagliardini E., Remuzzi G., Remuzzi A. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol. 2009;174:797–807. doi: 10.2353/ajpath.2009.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemeth Z., Kokeny G., Godo M., Mózes M., Rosivall L., Gross M.L., Ritz E., Hamar P. Increased renoprotection with ACE inhibitor plus aldosterone antagonist as compared to monotherapies–the effect on podocytes. Nephrol Dial Transplant. 2009;24:3640–3651. doi: 10.1093/ndt/gfp371. [DOI] [PubMed] [Google Scholar]
- 24.Appel D., Kershaw D.B., Smeets B., Yuan G., Fuss A., Frye B., Elger M., Kriz W., Floege J., Moeller M.J. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swetha G., Chandra V., Phadnis S., Bhonde R. Glomerular parietal epithelial cells of adult murine kidney undergo EMT to generate cells with traits of renal progenitors. J Cell Mol Med. 2011;15:396–413. doi: 10.1111/j.1582-4934.2009.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poulsom R., Little M.H. Parietal epithelial cells regenerate podocytes. J Am Soc Nephrol. 2009;20:231–233. doi: 10.1681/ASN.2008121279. [DOI] [PubMed] [Google Scholar]
- 27.Sagrinati C., Netti G.S., Mazzinghi B., Lazzeri E., Liotta F., Frosali F., Ronconi E., Meini C., Gacci M., Squecco R., Carini M., Gesualdo L., Francini F., Maggi E., Annunziato F., Lasagni L., Serio M., Romagnani S., Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 28.Bariety J., Mandet C., Hill G.S., Bruneval P. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol. 2006;17:2770–2780. doi: 10.1681/ASN.2006040325. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Hansen K.M., Pippin J.W., Chang A.M., Taniguchi Y., Krofft R.D., Pickering S.G., Liu Z., Abrass C.K., Shankland S.J. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol. 2012;302:F571–F580. doi: 10.1152/ajprenal.00516.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohse T., Vaughan M.R., Kopp J.B., Krofft R.D., Marshall C.B., Chang A.M., Hudkins K.L., Alpers C.E., Pippin J.W., Shankland S.J. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol. 2010;298:F702–F711. doi: 10.1152/ajprenal.00428.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romagnani P. Parietal epithelial cells: their role in health and disease. Contrib Nephrol. 2011;169:23–36. doi: 10.1159/000313943. [DOI] [PubMed] [Google Scholar]
- 32.Lasagni L., Romagnani P. Glomerular epithelial stem cells: the good, the bad, and the ugly. J Am Soc Nephrol. 2010;21:1612–1619. doi: 10.1681/ASN.2010010048. [DOI] [PubMed] [Google Scholar]
- 33.Romagnani P., Remuzzi G. Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol Metab. 2013;24:13–20. doi: 10.1016/j.tem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Sequeira López M.L.S., Pentz E.S., Nomasa T., Smithies O., Gomez R.A. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 35.Hugo C., Shankland S.J., Bowen-Pope D.F., Couser W.G., Johnson R.J. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest. 1997;100:786–794. doi: 10.1172/JCI119592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barajas L. Anatomy of the juxtaglomerular apparatus. Am J Physiol. 1979;237:F333–F343. doi: 10.1152/ajprenal.1979.237.5.F333. [DOI] [PubMed] [Google Scholar]
- 37.Celio M.R., Inagami T. Renin in the human kidney. Immunohistochemical localization. Histochemistry. 1981;72:1–10. doi: 10.1007/BF00496773. [DOI] [PubMed] [Google Scholar]
- 38.Faraggiana T., Gresik E., Tanaka T., Inagami T., Lupo A. Immunohistochemical localization of renin in the human kidney. J Histochem Cytochem. 1982;30:459–465. doi: 10.1177/30.5.7042817. [DOI] [PubMed] [Google Scholar]
- 39.Herrera M., Coffman T.M. The kidney and hypertension: novel insights from transgenic models. Curr Opin Nephrol Hypertens. 2012;21:171–178. doi: 10.1097/MNH.0b013e3283503068. [DOI] [PubMed] [Google Scholar]
- 40.Feil R., Brocard J., Mascrez B., LeMeur M., Metzger D., Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sparwasser T., Gong S., Li J.Y., Eberl G. General method for the modification of different BAC types and the rapid generation of BAC transgenic mice. Genesis. 2004;38:39–50. doi: 10.1002/gene.10249. [DOI] [PubMed] [Google Scholar]
- 42.Glenn S.T., Jones C.A., Pan L., Gross K.W. In vivo analysis of key elements within the renin regulatory region. Physiol Genomics. 2008;35:243–253. doi: 10.1152/physiolgenomics.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., Lein E.S., Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novak A., Guo C., Yang W., Nagy A., Lobe C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 45.Zhang J., Pippin J.W., Vaughan M.R., Krofft R.D., Taniguchi Y., Romagnani P., Nelson P.J., Liu Z.H., Shankland S.J. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol. 2012;121:e23–e37. doi: 10.1159/000342808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi Y., Pippin J.W., Hagmann H., Krofft R.D., Chang A.M., Zhang J., Terada Y., Brinkkoetter P., Shankland S.J. Both cyclin I and p35 are required for maximal survival benefit of cyclin-dependent kinase 5 in kidney podocytes. Am J Physiol Renal Physiol. 2012;302:F1161–F1171. doi: 10.1152/ajprenal.00614.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ophascharoensuk V., Pippin J.W., Gordon K.L., Shankland S.J., Couser W.G., Johnson R.J. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int. 1998;54:416–425. doi: 10.1046/j.1523-1755.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan M.R., Pippin J.W., Griffin S.V., Krofft R., Fleet M., Haseley L., Shankland S.J. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int. 2005;68:133–144. doi: 10.1111/j.1523-1755.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 49.Griffin S.V., Hiromura K., Pippin J., Petermann A.T., Blonski M.J., Krofft R., Takahashi S., Kulkarni A.B., Shankland S.J. Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am J Pathol. 2004;165:1175–1185. doi: 10.1016/S0002-9440(10)63378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin S.V., Krofft R.D., Pippin J.W., Shankland S.J. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int. 2005;67:977–986. doi: 10.1111/j.1523-1755.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 51.Jefferson J.A., Pippin J.W., Shankland S.J. Experimental models of membranous nephropathy. Drug Discov Today Dis Models. 2010;7:27–33. doi: 10.1016/j.ddmod.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones C.A., Hurley M.I., Black T.A., Kane C.M., Pan L., Pruitt S.C., Gross K.W. Expression of a renin/GFP transgene in mouse embryonic, extra-embryonic, and adult tissues. Physiol Genomics. 2000;4:75–81. doi: 10.1152/physiolgenomics.2000.4.1.75. [DOI] [PubMed] [Google Scholar]
- 53.Ohse T., Chang A.M., Pippin J.W., Jarad G., Hudkins K.L., Alpers C.E., Miner J.H., Shankland S.J. A new function for parietal epithelial cells: a second glomerular barrier. Am J Physiol Renal Physiol. 2009;297:F1566–F1574. doi: 10.1152/ajprenal.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burnworth B., Pippin J., Karna P., Akakura S., Krofft R., Zhang G., Hudkins K., Alpers C.E., Smith K., Shankland S.J., Gelman I.H., Nelson P.J. SSeCKS sequesters cyclin D1 in glomerular parietal epithelial cells and influences proliferative injury in the glomerulus. Lab Invest. 2012;92:499–510. doi: 10.1038/labinvest.2011.199. [DOI] [PubMed] [Google Scholar]
- 55.Chang A.M., Ohse T., Krofft R.D., Wu J.S., Eddy A.A., Pippin J.W., Shankland S.J. Albumin-induced apoptosis of glomerular parietal epithelial cells is modulated by extracellular signal-regulated kinase 1/2. Nephrol Dial Transplant. 2012;27:1330–1343. doi: 10.1093/ndt/gfr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin S.V., Olivier J.P., Pippin J.W., Roberts J.M., Shankland S.J. Cyclin I protects podocytes from apoptosis. J Biol Chem. 2006;281:28048–28057. doi: 10.1074/jbc.M513336200. [DOI] [PubMed] [Google Scholar]
- 57.Mercure C., Lacombe M.J., Khazaie K., Reudelhuber T.L. Cathepsin B is not the processing enzyme for mouse prorenin. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1212–R1216. doi: 10.1152/ajpregu.00830.2009. [DOI] [PubMed] [Google Scholar]
- 58.Ryan G., Steele-Perkins V., Morris J.F., Rauscher F.J., 3rd, Dressler G.R. Repression of Pax-2 by WT1 during normal kidney development. Development. 1995;121:867–875. doi: 10.1242/dev.121.3.867. [DOI] [PubMed] [Google Scholar]
- 59.Kiuchi-Saishin Y., Gotoh S., Furuse M., Takasuga A., Tano Y., Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 60.Ghanem M.A., Van der Kwast T.H., Den Hollander J.C., Sudaryo M.K., Oomen M.H., Noordzij M.A., Van den Heuvel M.M., Nassef S.M., Nijman R.M., Van Steenbrugge G.J. Expression and prognostic value of Wilms' tumor 1 and early growth response 1 proteins in nephroblastoma. Clin Cancer Res. 2000;6:4265–4271. [PubMed] [Google Scholar]
- 61.Barisoni L., Kriz W., Mundel P., D'Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka D.H., Mikami S., Nagasawa T., Miyazaki J., Nakajima K., Murakami F. CXCR4 is required for proper regional and laminar distribution of cortical somatostatin-, calretinin-, and neuropeptide Y-expressing GABAergic interneurons. Cereb Cortex. 2010;20:2810–2817. doi: 10.1093/cercor/bhq027. [DOI] [PubMed] [Google Scholar]
- 63.Haley S.A., Zhao T., Zou L., Klysik J.E., Padbury J.F., Kochilas L.K. Forced expression of the cell cycle inhibitor p57Kip2 in cardiomyocytes attenuates ischemia-reperfusion injury in the mouse heart. BMC Physiol. 2008;8:4. doi: 10.1186/1472-6793-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jarad G., Pippin J.W., Shankland S.J., Kreidberg J.A., Miner J.H. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol Renal Physiol. 2011;300:F811–F820. doi: 10.1152/ajprenal.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brinkkoetter P.T., Wu J.S., Ohse T., Krofft R.D., Schermer B., Benzing T., Pippin J.W., Shankland S.J. p35, the non-cyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int. 2010;77:690–699. doi: 10.1038/ki.2009.548. [DOI] [PubMed] [Google Scholar]
- 66.Matz M.V., Fradkov A.F., Labas Y.A., Savitsky A.P., Zaraisky A.G., Markelov M.L., Lukyanov S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol. 1999;17:969–973. doi: 10.1038/13657. [Erratum appeared in Nat Biotechnol 1999, 17:1227] [DOI] [PubMed] [Google Scholar]
- 67.Romagnani P. Toward the identification of a “renopoietic system”? Stem Cells. 2009;27:2247–2253. doi: 10.1002/stem.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ronconi E., Sagrinati C., Angelotti M.L., Lazzeri E., Mazzinghi B., Ballerini L., Parente E., Becherucci F., Gacci M., Carini M., Maggi E., Serio M., Vannelli G.B., Lasagni L., Romagnani S., Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fabian J.R., Field L.J., McGowan R.A., Mullins J.J., Sigmund C.D., Gross K.W. Allele-specific expression of the murine Ren-1 genes. J Biol Chem. 1989;264:17589–17594. [PubMed] [Google Scholar]
- 70.Kim H.S., Maeda N., Oh G.T., Fernandez L.G., Gomez R.A., Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210–14217. doi: 10.1074/jbc.274.20.14210. [DOI] [PubMed] [Google Scholar]
- 71.Sherwood D.R. Cell invasion through basement membranes: an anchor of understanding. Trends Cell Biol. 2006;16:250–256. doi: 10.1016/j.tcb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Collar J.E., Ladva S., Cairns T.D., Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney Int. 2001;59:2069–2072. doi: 10.1046/j.1523-1755.2001.00721.x. [DOI] [PubMed] [Google Scholar]
- 73.Liapis H., Foster K., Miner J.H. Red cell traverse through thin glomerular basement membrane. Kidney Int. 2002;61:762–763. doi: 10.1046/j.1523-1755.2002.00181.x. [Comment] [DOI] [PubMed] [Google Scholar]
- 74.Peti-Peterdi J., Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21:1835–1841. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elger M., Sakai T., Kriz W. The vascular pole of the renal glomerulus of rat. Adv Anat Embryol Cell Biol. 1998;139:1–98. doi: 10.1007/978-3-642-80449-6. [DOI] [PubMed] [Google Scholar]
- 76.Pippin J.W., Brinkkoetter P.T., Cormack-Aboud F.C., Durvasula R.V., Hauser P.V., Kowalewska J., Krofft R.D., Logar C.M., Marshall C.B., Ohse T., Shankland S.J. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296:F213–F229. doi: 10.1152/ajprenal.90421.2008. [DOI] [PubMed] [Google Scholar]
- 77.Steege A., Fähling M., Paliege A., Bondke A., Kirschner K.M., Martinka P., Kaps C., Patzak A., Persson P.B., Thiele B.J., Scholz H., Mrowka R. Wilms' tumor protein (-KTS) modulates renin gene transcription. Kidney Int. 2008;74:458–466. doi: 10.1038/ki.2008.194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of Ren1c reporter constructs. The RP23-88k7 BAC was from a library (C57Bl/6 mice) and represents a segment of chromosome 1. The renin gene (Ren1c) is centrally located and is flanked by Pepp3, Kiss1, Etnk2, and Sox13. Using homologous recombination, either a Cre, CreERt2, or GFP cassette was inserted at exon 1 of Ren1c. Transgenic mice were generated by zygotic microinjection of this construct.
Renin expression in kidney in the Ren1cCre×Rs-ZsGreen-R transgenic line. A: The Ren1cCreER×Rs-ZsGreen-R transgenic line tags the renin-expressing cell in a specific temporal window dependent on tamoxifen induction. An adult 6-week-old Ren1cCreER×Rs-ZsGreen-R mouse was administered 1 mg/day tamoxifen for 4 days; the animal was sacrificed at 1 week after the last injection and whole kidney tissue was examined under a dissecting microscope. Only juxtaglomerular cells (sites of adult renin expression) are tagged with ZsGreen. Note one large nontagged vessel (arrow). B: The Ren1cCre×Rs-ZsGreen-R transgenic line faithfully reports natural renin expression in the kidney. Whole kidney tissue from a 6-week-old Ren1cCre×Rs-ZsGreen-R mouse shows lineal descendants of renin-expressing cells in the renal vasculature. Original magnification, ×12.
Sheep anti-rabbit glomerular antibodies bind selectively to podocytes. To demonstrate that the antibody used to induce experimental FSGS binds to podocytes, double-staining for sheep IgG (green) and the podocyte-specific proteins nephrin and synaptopodin (red) was used. A–F: Only nephrin and synaptopodin staining were detected in normal mice. G–L: Sheep IgG was detected in mice given disease-inducing antibody, which costained with podocyte proteins (yellow). M–O: No staining overlap was detected when the primary nephrin or synaptopodin antibodies were omitted as controls. AB, antibody. Arrows indicate location of JGC where no staining for sheep IgG was observed.
Study design. Four reporter mice that genetically fate-map cells of renin lineage with different labels were given cytotoxic anti-podocyte antibody to induce experimental FSGS. Mice were sacrificed at different days of disease. Tamoxifen was administered to 5-week-old Ren1cCre_Rs-ZsGreen-R mice and experimental FSGS was induced 49 days later at 12 weeks of age. BW, body weight; d, day; IP, intraperitoneal injection.
Characterization of experimental FSGS in Ren1cCreER×Rs-tdTomato-R mice. Disease was induced with a cytotoxic anti-podocyte antibody. A: After injection of cytotoxic antibody, sheep IgG staining was detected within glomeruli by immunofluorescence staining in a podocyte distribution. B: Quantification of podocyte number, measured by the number of cells staining for p57 per glomerular cross section, shows a progressive decrease at days 7 and 14 of disease. C: Quantification of the percentage of glomeruli with one or more RFP-labeled cells increased significantly at days 7 and 14 of disease. Data are expressed as means ± SEM.
Cells of renin lineage express WT-1, nephrin, and podocin in glomeruli of Ren1cCreER×Rs-tdTomato-R mice with experimental FSGS. A–H: Double-staining was performed at baseline (day 0) (A), day 7 (B and C), and day 14 (D–H) of disease with antibody to WT-1 (red; thin arrows) and for the reporter with an antibody to RFP (green; thick arrows). Overlapping stains appear yellow-orange (thick dashed arrows) in merged images. WT-1 and the reporter staining did not overlap in baseline mice; in diseased mice, they overlapped in some (B–E) but not in all (F–H) glomeruli. I and J: Double-staining was performed for nephrin (red; thin arrow) and RFP (green; thick arrow). Nephrin staining overlapped with RFP (yellow; dashed arrow) in some (I) but not in all (J) glomeruli. K and L: Double-staining was performed for podocin (red; thin arrows) and RFP (green; thick arrows) at day 14 of disease. Podocin staining overlapped with RFP (yellow; dashed arrows) in some (K) but not in all (L) glomeruli. M–R: No staining for WT-1 (M), nephrin (O), or podocin (Q) was detected when the primary antibodies were omitted. No staining for the reporter was detected when the RFP antibody was omitted in sections double-stained for WT-1 (N), nephrin (P), or podocin (R). Circles indicate glomeruli. Boxed regions indicate JGCs.
Cells of renin lineage express PEC proteins in glomeruli of Ren1cCreER×Rs-tdTomato-R mice with experimental FSGS. A–D: Double-staining was performed at baseline (day 0) (A) and day 14 (B–D) of disease with an antibody to PAX2 (red; thin arrows), and for the reporter with an antibody to RFP (green; thick arrows). Overlapping stains appear yellow-orange (thick dashed arrows) in merged images. PAX2 and reporter staining did not overlap in baseline mice (A); in diseased mice, they overlapped in some (B and C) but not in all (D, thin dashed arrows) glomeruli. E–H: Double-staining was performed at baseline (day 0) (E) and day 14 (F–H) of disease with an antibody to claudin-1 (red; thin arrows) and for the reporter with an antibody to RFP (green; thick arrows). Overlapping stains appear yellow-orange (thick dashed arrows) in merged images. Claudin-1 and reporter staining did not overlap in baseline mice (E); in diseased mice, they overlapped in some (F and G) but not in all (H, thin dashed arrows) glomeruli. I and J: Staining for the reporter was not detected when the RFP antibody was omitted in sections double-stained for PAX2 (I) and claudin-1 (J). The JGC is indicated by a square.
Podocyte depletion and glomerular scarring characterize experimental FSGS in Ren1cCre×Rs-ZsGreen-R mice. Disease was induced with a cytotoxic anti-podocyte antibody. A: Quantification of podocyte number measured by the number of cells staining for p57 per glomerular cross section showed a progressive decrease at days 3, 7, and 14 of disease. B: Quantification of glomerular scarring, measured by a glomerular sclerosis score, showed a progressive increase in mice with disease at all time points. C and D: Podocytes were identified by p57 staining. C: p57 staining in podocytes (arrows) in a representative glomerulus at baseline. D: At day 14, p57 staining was reduced. E and F: PAS staining was used to assess glomerular histology. E: Glomerular histology was normal at baseline. F: At day 14, a representative glomerulus in a reporter mouse shows segmental scarring (dashed arrow), and the glomerular tuft forms an adhesion with Bowman's capsule (arrows). These data show that the administration of an anti-podocyte antibody to Ren1cCre×Rs-ZsGreen-R mice caused a decrease in podocyte number accompanied by progressive glomerular scarring, features characteristic of clinical FSGS. Data are expressed as means ± SEM. Original magnification, ×630.
Renin protein was restricted to cells in the JGC and was not detected in diseased glomeruli. Experimental FSGS was induced in Ren1cCre×Rs-ZsGreen-R mice. Double-staining was performed for renin and ZsGreen at baseline (A–F) and at 14 days of disease (G–L). A: At baseline, renin staining (red; arrows) was restricted to cells in the JGC; renin was not detected in the four glomeruli (circles). B: At baseline, ZsGreen-labeled cells (dashed arrows) were restricted to cells in the JGC and were not detected in glomeruli (circle). C: At baseline, ZsGreen labeling and renin staining overlapped (yellow; white arrows) in the JGC; no such costaining was detected in glomeruli (circles). Some ZsGreen-labeled cells do not express renin (yellow arrows). D–F: Higher magnification views of the boxed region in panels A–C, respectively. G–I: G: At day 14, renin staining was restricted to cells in the JGC (red; arrows); renin was not detected in the four glomeruli (dashed arrows). H: At day 14, ZsGreen-labeled cells were detected in three glomeruli (dashed circle), but not in a fourth glomerulus (solid circle). ZsGreen-labeled cells were also detected in the JGC (dashed arrows). I: At day 14 of disease, ZsGreen label overlapped with renin staining (yellow; white arrows). Some ZsGreen-labeled cells do not express renin (yellow arrows). J–L: Higher magnification views of the boxed region in panels G–I, respectively. In one of the glomeruli (K), ZsGreen-labeled cells were detected within the glomerular tuft (thin arrows), as well as in the JGC (thick arrow). Taken together, these representative examples show that, at both baseline and 14 days, renin staining was not detected in the glomeruli Ren1cCre×Rs-ZsGreen-R mice and was restricted to cells of the JGC only. This is consistent with the notion that the appearance of ZsGreen cells in glomeruli is not due to renin protein expression or re-expression.
Reduced podocyte number and glomerular scarring characterize experimental FSGS in Ren1dCre×Z/EG mice. Disease was induced in Ren1dCre×Z/EG mice with a cytotoxic anti-podocyte antibody. A: Quantification of podocyte number, measured by the number of cells staining for p57 per glomerular cross section, shows a progressive and significant decrease at days 7 and 14 of disease. B: Quantification of glomerular scarring, measured by a glomerular sclerosis score, shows a progressive and significant increase in diseased mice at all time points. C and D: p57 staining identified podocytes. C: A representative glomerulus in baseline mice shows p57 staining in podocytes (arrows). D: p57 staining was reduced at day 14 of disease. E and F: PAS staining was used to assess glomerular histology. E: Glomerular histology was normal at baseline. F: A representative image of a glomerulus in a mouse with experimental FSGS at day 14 shows segmental scarring (dashed arrow); the glomerular tuft forms an adhesion with Bowman's capsule (arrows). These data show that the administration of an anti-podocyte antibody to Ren1dCre×Z/EG mice causes a progressive decrease in podocyte number, accompanied by progressive glomerular scarring; both features are characteristic of clinical FSGS. Data are expressed as means ± SEM. Original magnification, ×630.
Cells of renin lineage increase in glomeruli of Ren1dCre×Z/EG mice with experimental FSGS. Disease was induced in Ren1dCre×Z/EG mice with an anti-podocyte antibody, to fate-map cells of renin lineage by permanent labeling with GFP; nuclei were counterstained with DAPI. A and B: Quantification of GFP-positive cells at baseline (day 0) and at days 7 and 14 of disease. A: At day 0, at least one GFP-positive cell was detected in only 5% of glomeruli. At days 7 and 14 of disease, the number of glomeruli expressing GFP-positive cells increased significantly and progressively. B: Quantification of the number of GFP-labeled cells lining Bowman's capsule showed that GFP-labeled cells were rarely detected lining Bowman's capsule at day 0, but their numbers increased significantly at days 7 and 14 of disease. Within the glomerular tuft, GFP-labeled cells were barely detected at baseline, but increased progressively and significantly at days 7 and 14 of disease. C: After injection of cytotoxic antibody, sheep IgG staining was detected within glomeruli by immunofluorescence staining in a podocyte distribution. Data are expressed as means ± SEM. Original magnification, ×200.
Renin mRNA and protein is restricted to cells in the JGC at baseline and in diseased RenGFP reporter mice. Experimental FSGS was induced in RenGFP reporter mice, in which GFP represents renin promoter activity and thus also renin transcription. Double-staining was performed at baseline and in diseased mice with renin (red) and GFP (green) for renin protein and renin mRNA, respectively. A: Positive renin staining (red; arrows) indicates that renin protein was restricted to the JGC in baseline RenGFP mice. Renin staining was absent in glomeruli (circles). B: Positive GFP staining (green; arrows) indicates that renin mRNA expression was restricted to the JGC in baseline RenGFP mice. GFP staining was absent in glomeruli (circles). C: Cells double-positive for both GFP and renin (yellow; arrows) were restricted to the JGC. No coexpression staining overlap was noted within glomeruli. D–F: Higher magnification views of the boxed regions in panels A–C. G: Positive renin staining (red; arrows) indicates that renin protein was restricted to the JGC in diseased RenGFP mice at day 14 of disease. Renin staining was absent in glomeruli (circles). H: Positive GFP staining (green; arrows) in the JGC in diseased RenGFP mice. GFP staining was absent in glomeruli (circles). Some green autofluorescence is seen in red blood cells within the glomerular capillary loops. I: Cells double-positive for both GFP and renin (yellow; arrows) were restricted to the JGC. No staining coexpression overlap was detected within glomeruli (circles). J–L: Higher magnification views of the boxed regions in panels G–I. These data show that, in RenGFP reporter mice, renin promoter activity was limited to cells in the JGC in both control and diseased mice. Thus, labeled cells derived from renin lineage that appear in the glomerulus are due neither to renin promoter activity nor to renin protein expression. Original magnification: ×100 (A–C and G–I); ×630 (D–F and J–L).