Abstract
We have recently shown acetylation of tau at lysine residue 280 (AC-K280) to be a disease-specific modification in Alzheimer disease (AD), corticobasal degeneration, and progressive supranuclear palsy, likely representing a major regulatory tau modification. Herein, we extend our observations using IHC with a polyclonal antibody specific for AC-K280. Thirty brain regions were examined in argyrophilic grain disease (AGD; n = 5), tangle-predominant senile dementia (TPSD; n = 5), Pick disease (n = 4), familial AD (FAD; n = 2; PSEN1 p.G206A and p.S170P), and frontotemporal dementia with parkinsonism linked to chromosome-17 (FTDP-17; n = 2; MAPT p.P301L and IVS10 + 16). All AGD, TPSD, FAD, and FTDP-17 cases had significant AC-K280 reactivity that was similar in severity and distribution to phosphorylated tau. AC-K280 robustly labeled grain pathological characteristics in AGD and was predominantly associated with thioflavin-S–positive neurofibrillary tangles and less reactive in neuropil threads and extracellular tangles in TPSD and FAD. Thioflavin-S–negative neuronal and glial inclusions of patients with FTDP-17 were robustly AC-K280 reactive. A low degree of AC-K280 was found in a subset of 4-repeat tau-containing lesions in Pick disease. AC-K280 is a prominent feature of both neuronal and glial tau aggregations in tauopathies of various etiologies. The close association of AC-K280 with amyloid and pre-amyloid conformations of tau suggests a potential role in tangle maturation and, thus, could serve as a useful biomarker or therapeutic target in a variety of tauopathies.
Tauopathies are a heterogeneous group of neurodegenerative diseases characterized by abnormal conformations of tau proteins incorporated into inclusions and resulting in neuronal loss and gliosis. Pathogenic mutations in the MAPT tau gene causing frontotemporal dementia with parkinsonism linked to chromosome-17 (FTDP-17),1 with characteristic tau lesions at autopsy, provide compelling evidence of a central role of tau aggregation in disease pathogenesis.
Tau is subject to multiple post-translational modifications that, in disease states, may become dysregulated and contribute to pathological aggregation.2 Previously, our group3 and others4 have found that tau is modified by acetylation on lysine residues within the microtubule (MT)–binding domain (MTBD). Two main categories of tau isoforms exist, based on alternative splicing of exon 10 for inclusion or exclusion of the second MTBD for a total of 3 (3R) or 4 (4R) MTBD repeats. We found acetylation of lysine 280 (AC-K280) in the second MTBD in 4R tau to be disease specific, resulting in both decreased microtubule binding and increased fibrillization in vitro.3 Furthermore, AC-K280 is a prominent marker of pathological characteristics in Alzheimer disease (AD), corticobasal degeneration (CBD), and progressive supranuclear palsy (PSP).5 Herein, we further examine the AC-K280 modification in other sporadic and hereditary tauopathies characterized by insoluble 4R tau-predominant filaments, including argyrophilic grain disease (AGD), tangle-predominant senile dementia (TPSD), FTDP-17, and familial AD (FAD) cases caused by presenillin-1 (PSEN1) mutations. We also examined cases of the predominantly 3R tauopathy, Pick disease (PiD), because heterogeneity exists in the tau isoform profile in insoluble deposits of tau in the brains of patients with PiD.6–8
Materials and Methods
Patients
Patients were seen at the University of Pennsylvania Perelman School of Medicine (Philadelphia) Alzheimer's Disease Center or Frontotemporal Degeneration Center, and autopsies were performed at the Center for Neurodegenerative Disease Research. Autopsies were performed with informed consent, and all procedures were done in accordance with the local Institutional Review Board guidelines. Patient demographics were obtained from our integrated neurodegenerative disease database9 (Supplemental Table S1). Neuropathological assessment and diagnosis were performed as reported previously10 using established criteria.11,12 Tau and amyloid β pathological characteristics were staged according to Braak and Braak13 and the Consortium to Establish a Registry for Alzheimer's Disease14 criteria, respectively. AGD cases were staged according to Ferrer et al.15 Thirty areas were sampled and analyzed for five cases each of AGD and TPSD, four cases of PiD, and several hereditary FAD and FTDP-17 cases [n = 1: PSEN1 p.G206A,16 PSEN1 p.S170P, MAPT p.P301L,1 and MAPT IVS10 + 16 (c.915 + 16C>T)1]. We also examined available regions in two nonneurodegenerative control cases (Braak stage 0) (Supplemental Table S1).
The PSEN1 p.S170P mutation has not been previously reported, but it is likely pathogenic in view of the extremely young age of disease onset (27 years) in this patient and the family history of AD among the kindred of this patient (Supplemental Figure S1), as well as a similar reported pathogenic mutation at this residue (p.S170F).17
IHC Data
Fresh tissue was sampled at autopsy and fixed in 70% ethanol/150 mmol sodium chloride, embedded in paraffin, and cut into sections (6 μm thick). Immunohistochemistry (IHC) was performed, as previously described,5 using a rabbit-generated polyclonal antibody specific for tau modified with an acetyl group at the 280 lysine (K) residue3 (AC-K280; 1:250 to 1:1000 dilution) and an avidin-biotin complex detection system (VECTASTAIN ABC kit; Vector Laboratories, Burlingame, CA) with 3,3′-diaminobenzidine as the chromogen. AC-K280 specificity for the modified lysine residue has been described in detail.3 Mouse monoclonal antibodies (mAbs) specific for other tau epitopes were used herein, and they include phosphorylated tau [PHF-118; 1:500 to 1:1000; courtesy of Dr. Peter Davies (Albert Einstein College of Medicine, Department of Pathology, Bronx, NY)] and 3R (RD319; 1:2500 to 1:5000; Millipore, Billerica, MA) and 4R (RD419; 1:5000 to 1:10,000; Millipore) tau isoforms. Antigen retrieval was performed by using 88% formic acid for AC-K280 and RD3 or boiling with a citric acid unmasking solution (Vector Laboratories) for RD4.
Double-label immunofluorescence experiments were performed as described5 using AC-K280 and tau-specific mAbs or thioflavin-S (ThS) amyloid dye staining for fibrillar inclusions and Alexa Fluor 488 and 594 species-specific conjugated secondary antibodies (Invitrogen, Grand Island, NY). To confirm the 4R tau predominance in AGD grains and presence of 4R tau in PiD cases, serial sections and double-label experiments with AC-K280 and RD3/RD4 were performed on hippocampal sections of AGD (n = 4) and three areas of high AC-K280 reactivity in one PiD case (anterior cingulate gyrus, motor cortex, and temporal cortex). ThS and AC-K280 double labeling was also examined in these regions for AGD and PiD and, in addition, in TPSD hippocampus (n = 4), FAD hippocampus or frontal cortex (n = 2), and FTDP-17 hippocampus or temporal neocortex (n = 2). Digital images of immunofluorescence were obtained with an Olympus BX 51 microscope equipped with a bright-field and fluorescence light source with a DP-71 digital camera (Olympus, Center Valley, PA) and DP manager software version 3.1.1.208 (Olympus) and overlaid into a merge channel using Adobe Photoshop version 9.0.2 (Adobe Systems, San Jose, CA).
Genetic Analysis
For the hereditary cases in this study, DNA was extracted from peripheral blood samples or brain tissue using the manufacturer's protocol (Flexigene; Qiagen, Valencia, CA) or the Quick-Gene DNA whole blood kit (Autogen, Holliston, MA). DNA sequence analysis of the entire coding region of PSEN1 and targeted regions of MAPT known to harbor pathogenic mutations (exons 1 and 9 to 13) was performed as previously described.16,20 Data were analyzed for identification of mutations with Mutation Surveyor software version 4.0.7 (Soft Genetics, State College, PA).
Microscopic and Statistical Analysis
Regional distribution and severity of AC-K280 were compared with PHF-1 across 30 representative central nervous system regions and graded on a semiquantitative scale, as previously described5 (0, none or rare pathological feature; 1, weak; 2, moderate; and 3, strong). Median semiquantitative scores for grouped data were calculated for each region per disease group using SPSS, version 19.0 (SPSS Inc., Chicago, IL).
Results
Regional Distribution
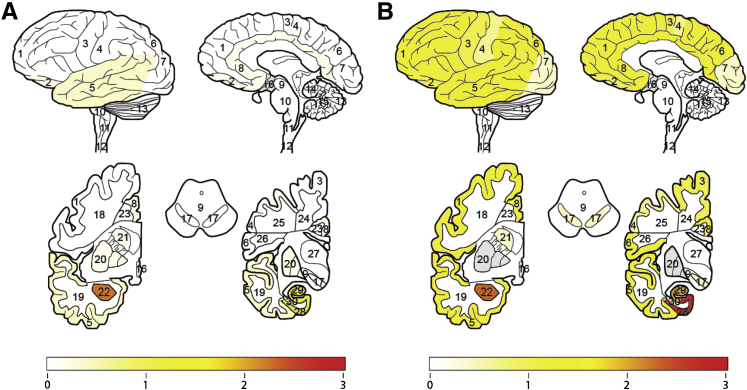
Detailed neuropathological assessment in 30 representative cortical and subcortical regions of the CNS revealed AC-K280 in a similar distribution and severity to the well-described PHF-1 phosphorylated-epitope of tau (Ser396/404)18 in AGD, TPSD, FAD, and FTDP-17 cases (Figure 1 and Supplemental Tables S2, S3, and S4). AC-K280 immunoreactivity was not found in areas negative for PHF-1, and, similarly, in regions with weak or rare PHF-1 pathological features, there was often no detectable AC-K280. This was also true in control cases, in which AC-K280 was limited to rare (one to three tangles) in the cornu ammonis in one case; these were also detected with PHF-1 in an adjacent section (Figure 2 and Supplemental Table S4). Only rarely in FTDP-17 did AC-K280 immunoreactivity appear slightly more prominent than PHF-1 (Supplemental Table S4). PiD cases were largely negative for AC-K280; however, a subset of regions in each PiD case contained a varying degree of AC-K280 immunoreactivity (Table 1). In most instances when AC-K280 immunoreactivity was present, it was found in rare (one to five per slide) glial inclusions or Pick bodies, although one case had three regions with an abundance of AC-K280 lesions. The fourth case had only rare AC-K280 tau pathological characteristics in the entorhinal cortex of the hippocampus.
Figure 1.
Regional distribution and severity of AC-K280–positive tau pathological characteristics in patients with AGD or TPSD. Heat map of AC-K280–positive tau pathological characteristics based on median semiquantitative score for AGD (n = 5) (A) and TPSD (n = 5) (B). Bar graph depicts color map of median severity score ranging from no pathological characteristics (0, white) to strong reactivity (3, red). Gray regions were not evaluated. Brain regions evaluated include the following: 1, midfrontal cortex gray matter; 2, oribitofrontal cortex gray matter; 3, motor cortex gray matter; 4, sensory cortex gray matter; 5, superior/midtemporal cortex gray matter; 6, angular cortex gray matter; 7, visual cortex gray matter; 8, anterior cingulate cortex gray matter; 9, midbrain; 10, pons; 11, medulla; 12, cervical spinal cord; 13, cerebellar cortex; 14, cerebellar white matter; 15, dentate nucleus; 16, hypothalamus; 17, substantia nigra; 18, midfrontal cortex white matter; 19, superior/midtemporal cortex white matter; 20, lentiform nucleus; 21, striatum; 22, amygdala; 23, anterior cingulate gyrus white matter; 24, motor cortex white matter; 25, sensory cortex white matter; 26, angular cortex white matter; 27, thalamus; 28, entorhinal cortex gray matter; 29, hippocampal formation (cornu ammonis 1 to 4/subiculum); and 30, entorhinal cortex white matter.
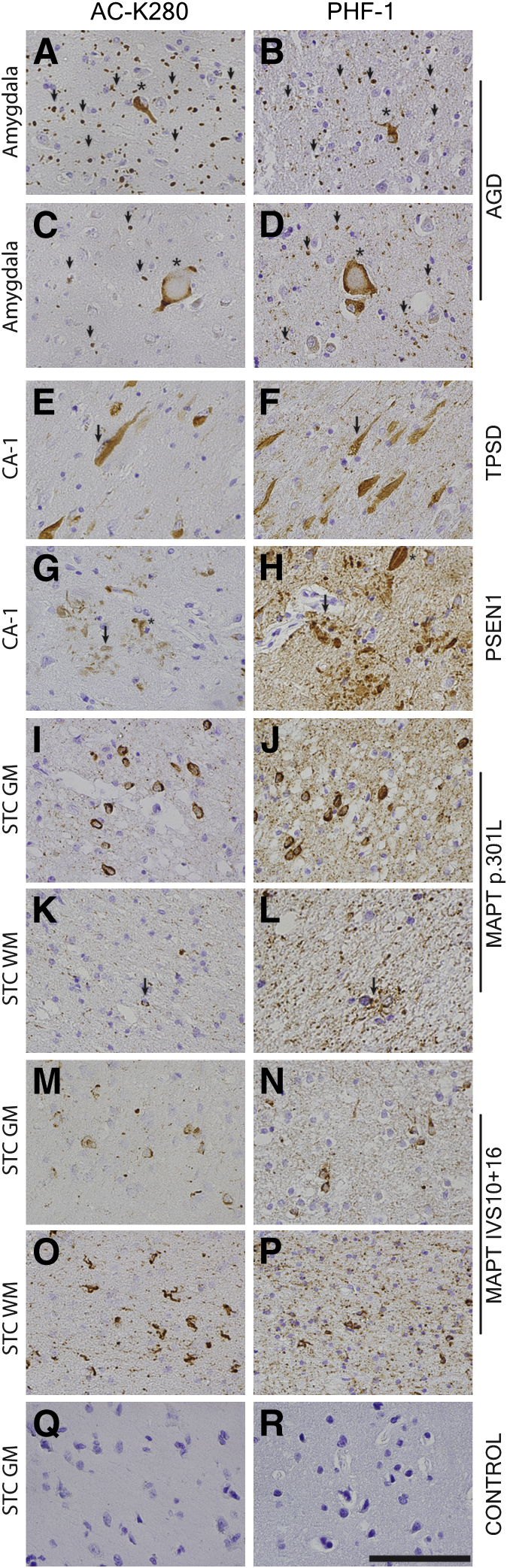
Figure 2.
Acetylated and hyperphosphorylated tau pathological characteristics in sporadic and hereditary tauopathies. A–P: Representative images of AC-K280 staining and B-R display PHF-1 staining. Sections from the amygdala in AGD show prominent AC-K280–reactive grains (arrowheads; A and C) and neurofibrillary tangles (NFTs; asterisk; A) and ballooned neurons (asterisk; C) similar to PHF-1 (B and D). AC-K280 reactivity in the cornu ammonis (CA-1) region of the hippocampus in TPSD is seen mostly in intracellular tangles (arrow; E), with less prominent staining of threads and extracellular ghost tangles labeled by PHF-1 (F). CA-1 region of the hippocampus of a PSEN1 case (p.S170P) displays typical AD pathological characteristics, with AC-K280 mainly in tangles (asterisk) and neuritic plaques (arrow; G) and less prominent in diffuse threads than PHF-1 (H). Superior temporal cortex (STC) of FTDP-17 cases (p.P301L and IVS10 + 16) have ACK280-reactive neuronal inclusions (I and M) and less prominent threads than PHF-1 (J and N) in gray matter (GM) and a similar burden of glial tau pathological features in white matter (WM; arrow; K and L). Nonneurodegnerative disease control superior temporal cortex showing an absence of AC-K280 (Q) and PHF-1 reactivity (R). Scale bar = 100 μm (R).
Table 1.
Summary of AC-K280 Reactivity in PiD Cases
| Region | Case no. |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Motor | +++ | Rare | 0 | NA |
| Striatum | 0 | 0 | ++ | 0 |
| Lentiform | 0 | 0 | Rare | 0 |
| Thalamus | 0 | 0 | Rare | NA |
| Hypothalamus | Rare | + | NA | NA |
| Midbrain | 0 | 0 | 0 | 0 |
| Substantia nigra | 0 | 0 | Rare | 0 |
| Pons | 0 | 0 | 0 | 0 |
| Locus coeruleus | 0 | 0 | Rare | 0 |
| Cerebellar cortex | 0 | 0 | 0 | 0 |
| Dentate nucleus or cerebellum | 0 | 0 | 0 | 0 |
| Medulla | 0 | 0 | 0 | 0 |
| Cervical spinal cord | 0 | 0 | 0 | 0 |
| Midfrontal cortex | Rare | Rare | + | 0 |
| Orbital frontal cortex | 0 | Rare | 0 | 0 |
| Anterior cingulate cortex | +++ | 0 | + | 0 |
| Sensory cortex | Rare | 0 | NA | 0 |
| Temporal cortex | +++ | 0 | 0 | 0 |
| Angular cortex | Rare | 0 | 0 | 0 |
| Visual cortex | 0 | 0 | NA | 0 |
| Entorhinal cortex or hippocampus | Rare | Rare | 0 | Rare |
| Cornu ammonis or hippocampus | 0 | 0 | 0 | 0 |
| Dentate gyrus or hippocampus | 0 | 0 | 0 | 0 |
| Amygdala | Rare | + | 0 | 0 |
+, weak; ++, moderate ; +++, strong.
NA, tissue not available.
Morphological Assessment
Argyrophilic Grain Disease
AC-K280 was robust in tau-positive comma-shaped grains in all five AGD cases studied herein (Figure 2), which were largely restricted to limbic areas (one stage II and four stage III). PHF-1–positive intracellular pretangles and neurofibrillary tangles (NFTs), in addition to lightly tau-reactive swollen achromatic ballooned neurons and coiled bodies in adjacent white matter, were also labeled by AC-K280 (Figure 2). AC-K280 was found in predominantly ThS-positive NFTs and variably ThS-positive grains (Figure 3). Comparison with isoform-specific tau mAbs confirmed the predominance of 4R tau in grains, because the 4R-specific tau mAb (RD4) displayed a similar predominance of intracellular NFTs and grains to AC-K280, whereas a 3R-specific mAb (RD3) mainly labeled AD-associated extracellular ghost tangles and diffuse neuropil threads (NTs) that were not well labeled by the AC-K280 antibody (Supplemental Figure S2). AC-K280 colocalized well with RD4 in grains that were largely RD3 negative, and colocalized with both isoform-specific mAbs in intracellular NFTs (Supplemental Figure S2).
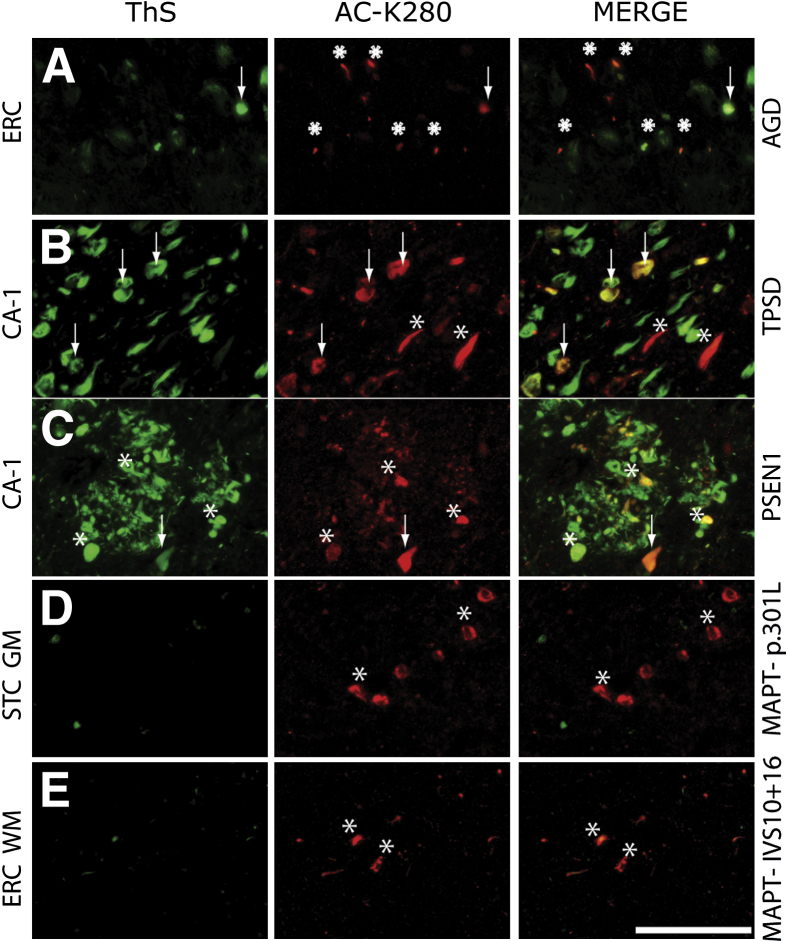
Figure 3.
Colocalization of acetylated tau with fibrillar (ThS +) and nonfibrillar (ThS −) tau inclusions. A: AGD entorhinal cortex (ERC) shows AC-K280–reactive grains that are largely ThS negative (asterisks) and a ThS-positive AD-associated tangle (arrow). B: Cornu ammonis region (CA-1) of TPSD showing mostly AC-K280 in ThS-positive intracellular tangles (arrows) and a subset of ThS-negative pretangles (asterisks). C: CA-1 region of FAD (S170P) showing AC-K280–reactive tangle (arrows) and large dystrophic neurites (asterisks) associated with a neuritic plaque. Tau lesions robustly positive for AC-K280 in the temporal cortex of p.P301L (D) and IVS10 + 16 FTDP-17 (E) cases were minimally reactive to ThS (asterisks). Scale bar = 100 μm (E).
Tangle-Predominant Senile Dementia
TPSD cases had a predominance of PHF-1–reactive NFTs similar to AD in the absence of significant amyloid β deposits (Supplemental Table S1). AC-K280 was a prominent feature of intracellular NFTs and less conspicuous in extracellular ghost tangles and diffuse scattered NTs (Figure 2). Most AC-280–reactive NFTs in CA-1 were ThS positive (Figure 3). A subset of cases had more widespread tau pathological characteristics outside of the medial temporal lobe structures (Braak V to VI, n = 2). These cases had abundant tau-positive tangles and threads in the medial and lateral temporal lobe cortical regions, with occasional tau-positive glial inclusions largely restricted to gray matter, with some compact lesions reminiscent of tufted astrocytes in PSP and other, more diffuse, lesions resembling astrocytic plaques of CBD. However, the lack of significant white matter tau pathological characteristics and infrequent tau inclusions in the brainstem precluded these diagnoses and, thus, these findings were most consistent with a diagnosis of TPSD. AC-K280 immunoreactivity appeared similar in these cases, with prominent deposition in neuronal tangles and glial lesions, with minimal positivity in NTs or extracellular ghost tangles.
Familial AD
Both the PSEN1 p.G206A and p.S170P FAD cases displayed typical AD tau pathological features, with widespread NTs, NFTs, and neuritic plaques throughout cortical and, to a lesser degree, subcortical structures (Supplemental Table S4). Similar to sporadic AD,5 AC-K280 stained all forms of neurofibrillary pathological features but was most prominent in ThS-positive intracellular NFTs and large dystrophic neurites associated with neuritic plaques and was less reactive to other NTs and extracellular NFTs (Figures 2 and 3).
Frontotemporal Dementia with Parkinsonism Linked to Chromosome-17
FTDP-17 cases had microscopic findings consistent with previous reports of abundant and widespread tau pathological characteristics, albeit in different distributions in cases that resembled PSP or CBD versus other phenotypic manifestations of MAPT gene mutations.21,22 The p.P301L case showed widespread strong immunoreactivity in tau deposits throughout cortical and subcortical gray matter and, to a lesser extent, white matter (Supplemental Table S4). Pleomorphic neuronal tangles were prominent in superficial and deep cortical layers and were robustly reactive with AC-K280, whereas associated diffuse threads seen with PHF-1 were less evident in AC-K280–stained sections (Figure 2). Pick-like bodies were rarely observed with either epitope. Glial pathological features were moderate and mainly found in coiled bodies and astrocytic lesions in white matter, both of which were reactive to AC-K280 (Figure 2). In contrast, the IVS10 + 16 case had less prominent neuronal tau inclusions and threads in gray matter and, instead, strong immunostaining in white matter glial pathological features throughout the neocortex and limbic cortices (Figure 2). Glial pathological features were robustly positive for AC-K280 and, in rare instances, slightly more prominent than PHF-1 (Supplemental Table S4). AC-K280–reactive pathological characteristics in FTDP-17 cases were largely negative for ThS (Figure 3). In summary, AC-K280 was a prominent feature of the varied glial and neuronal inclusions and less common in diffuse threads seen in these FTDP-17 cases.
Pick Disease
Although PHF-1 and RD3 staining revealed numerous tau-positive inclusions and diffusely distributed NTs, only a minimal subset of tau lesions in the PiD cases was AC-K280 immunoreactive, and these included Pick bodies and compact astrocytic inclusions (Supplemental Figure S3). Areas with strong AC-K280 immunostaining in neuronal Pick body and glial inclusions were confirmed to contain 4R tau through comparison of serial sections and double labeling with RD4. The morphological characteristics of the AC-K280–positive inclusions were similar to the RD4-stained inclusions and both colocalized in neuronal and glial lesions, whereas double labeling with RD3 demonstrated that some RD3-labeled inclusions colocalized in the AC-K280–positive tau pathological characteristics, but most often RD3 positivity did not colocalize in AC-K280 (Supplemental Figure S3). PiD inclusions, including those reactive to AC-K280, were weakly reactive for ThS (Supplemental Figure S3).
Discussion
We extend our previous studies of acetylated tau pathological characteristics by documenting the presence of AC-K280 immunoreactivity in the hallmark tau lesions of AGD, TPSD, FAD, and FTDP-17 in a similar pattern and severity to a well-annotated phosphorylated-epitope (Ser396/404). Furthermore, we have also demonstrated the disease-specific nature of the AC-K280 modification because little or no AC-K280 reactivity was found in the control cases examined herein (Supplemental Table S4) or in previous studies.3,5 AC-K280 also detected tau pathological characteristics in PiD, although to a much lesser extent and more variable degree, reflecting the low burden of 4R tau inclusions in most PiD cases. PiD is considered to be a 3R tauopathy, although some cases have been found to have a variable, but significant, burden of 4R tau-positive lesions or insoluble 4R species of pathological tau.6–8 Indeed, we found minimal AC-K280 immunoreactvity in PiD, which corresponded to 4R-containing lesions within individual cases. Interestingly, AC-K280 was found in both 3R-positive and 3R-negative tau inclusions, which probably reflects the presence of low levels of 4R tau in some of these inclusions. These results highlight the heterogeneity of pathological features within and between individuals with PiD and further implicate the AC-K280 modification in pathological tau aggregations of multiple etiologies.
AGD is a sporadic tauopathy associated with advanced aging23,24 and cognitive impairment,25,26 by which tau-positive grains have been localized to apical and basal dendrites27 and contain a predominance of 4R tau.28–30 Indeed, we found robust reactivity for AC-K280 in grain pathological features of all five AGD brains examined herein, which exclusively colocalized with another 4R-specific epitope detected by the RD4 mAb. We found AC-K280 reactivity in AD-associated intracellular tangles as well, and the common association of AD pathological characteristics and AGD suggests that formation of neurofibrillary and grain pathological characteristics may be mechanistically linked.26 Indeed, grains contain many of the same phosphorylation epitopes as AD NFTs,31 and herein we find that AGD grain pathological features and AD-like NFTs share the AC-K280 modification as well. The explanation for the discrepancy between robust AC-K280 in grains and weak NT staining is not clear, but this may reflect differences in deacetylase activity within the cytosolic compartments of neurons, or differing mechanisms of inclusion formation in grains versus NTs.
These findings are in contrast to a recent report by Grinberg et al,32 who found a conspicuous absence of AC-tau in grain pathological features in AGD, despite reactivity in AD-type NFTs in these cases, using a novel monoclonal antibody specific for acetylation of tau at the nearby lysine residue 274 (AC-K274).32 One possibility for this discrepancy is the effect of fixatives used for tissue preparation, because other tau antibodies for epitopes in the MTBD label varying morphological characteristics of tau inclusions, depending on tissue fixation.33 Indeed, we have found AC-K280 to be sensitive to formalin fixation, requiring the use of tissue fixed in 70% ethanol with 150 mmol/L NaCl for visualization of pathological characteristics in our studies (DJ Irwin, TJ Cohen, VMY Lee, JQ Trojanowski, unpublished observations). Notably, the study of Grinberg et al32 did test the temporal effects of formalin fixation on AC-K274 and found that the formalin fixation longer than 72 hours resulted in decreased signal, but they did not examine other fixatives. Thus, these two epitopes could be differentially exposed in grain pathological characteristics when AGD tissues are fixed in formalin versus ethanol. Another possibility for these discrepancies is that AC-K274 is located in the first MTBD, which is present in both 3R and 4R tau isoforms, whereas AC-K280 is found exclusively in 4R tau because of its presence in the second MTBD; thus, the difference in specific AC tau epitopes detected by these antibodies could be responsible for these observations as well.
Notably, we recently demonstrated that tau possesses a novel enzymatic activity as a acetyltransferase with an amino acid sequence and functional similarities to members of the MYST family of enzymes.34 In this study, we proposed that tau acetylates itself (autoacetylation) as part of an autoinhibitory signaling mechanism to prevent tau-MT interactions, and we also demonstrated that tau phosphorylation within the repeat regions could enhance tau autoacetylation at K280.34 Future studies examining the effect of different phospho-tau epitopes on tau acetylation are needed to elucidate the biochemical interplay between these modifications and the complex combinatorial set of modifications that affect normal tau functions and contribute to the pathogenesis of tau pathological characteristics. Finally, we speculate that the detection of diverse disease-specific tau modifications in cerebral spinal fluid of patients could aid in the antemortem diagnosis of tauopathies.
TPSD is associated with advanced aging and shows a distribution of neurofibrillary tau pathological characteristics that are similar to AD, and these pathological characteristics contain both 3R and 4R tau isoforms.35,36 Indeed, AC-K280 was highly associated with, but not exclusive to, ThS-positive intracellular NFTs in TPSD and FAD and, less commonly, in NTs and extracellular ghost tangles similar to AD. In contrast, AC-K280 was also prominent in ThS-negative neuronal pretangles and glial inclusions of FTDP-17 (Figure 3). Notably, the MAPT p.P301L mutation is thought to enhance 4R aggregation,37 whereas the IVS10 + 16 mutation is thought to increase splicing to generate more 4R tau isoforms.1
These results are similar to observations of relative minimal AC-K274 reactivity in diffuse NTs in AD and prominence of AC-274 in two cases of PSP and CBD and one case of FTDP-1732; they also agree with our previous observations of AC-K280 in sporadic AD and the nonamyloid lesions seen in the 4R tauopathies, CBD and PSP.5 In light of the findings shown in this study, together with our recent observations of tau autoacetylation,34 it is plausible to infer that prominent K280-positive immunoreactivity detected in neuronal and glial tau aggregates results from abnormal tau enzymatic activity, leading to the accumulation of autoacetylated tau proteins that no longer efficiently bind MTs. Whether aberrant tau autoacetylation is triggered by tau phosphorylation within MTBD regions remains to be determined. Indeed, we speculate that autoacetylation likely drives the formation of predominantly thioflavin-positive tau inclusions, explaining the striking correlation between K280 immunoreactivity and tau amyloid deposits in AD.5 However, given that we observed K280-positive inclusions that were not thioflavin positive in CBD, PSP,5 and FTDP-17, K280 acetylation also can occur independent from the formation of tau amyloid. Further studies in animal and cell models are needed to clarify the role of AC-K280 and tau autoacetylation in tangle formation, including possible interplay with phospho-, neo-, and other acetyl-epitopes of tau, based on these morphological observations. Nevertheless, AC-K280 represents a significant marker of tau deposition in these diverse 4R-containing tauopathies resulting from multiple pathogenic mechanisms, including intrinsic mutations in MAPT, mutations in Aβ-associated PSEN1, and age-associated neurodegenerative conditions, thereby demonstrating the importance of this AC-K280 modification of tau in disease pathogenesis.
Acknowledgments
We thank the patients and their families. If not for their meaningful contribution, this work would not be possible. We also thank Young Baek, Curtis Tsai, Frank Smith, and Jonathan Bekisz for their technical assistance and Dr. Peter Davies for his generous contribution of the PHF-1 mAb.
Footnotes
Supported by NIH grants P30 AG10124, AG17586, and T32-AG000255 (D.J.I. received the last grant only); an American College of Radiology Imaging Network grant (S.A.E.); a Pfizer grant (S.A.E.); Alzheimer's Disease Neuroimaging Initiative grant (S.A.E.); Johnson & Johnson grant (S.A.E.); a National Institute of Drug Abuse grant (S.A.E.); a Neuronetrix grant (S.A.E.); a National Institute of Mental Health grant (S.A.E.); an Eli Lilly grant (S.A.E.); AstraZeneca (V.M.-Y.L. and J.Q.T.); and BMS (V.M.-Y.L. and J.Q.T.).
Disclosures: S.A.E. has board membership at Cowan Group, Eli Lilly, and BMS, provides consulting services for a Philadelphia district attorney's office and Bonner Kiernan Trebach & Cociata LLP, and received payments for lectures at Harvard University, Rush University Medical Center, Trinitas Regional Medical Center, and University of Puerto Rico. V.M.-Y.L. and J.Q.T. report single consulting services to Pfizer, J&J, MetLife, and BMS, and receive royalty payments through University of Pennsylvania licenses.
Supplemental Data
Pedigree of PSEN1 p.P170S mutation case. N, an unspecified number of unaffected children.
Morphological characteristics of acetylated tau in AGD. A: Serial sections of the cornu ammonis (CA-1) region of the hippocampus show more prominent diffusely distributed neuropil threads (NTs) and extracellular ghost tangles (asterisks) with the 3R-specific antibody RD3, whereas both ACK280 and the 4R-specific antibody, RD4, prominently label grains (arrowheads). B: Double labeling of the entorhinal cortex (ERC) of the hippocampus showing that AC-K280 colocalizes with RD3 in intracellular tangles (arrows) but not grains, whereas ACK280 detects a large subset of grains visualized with RD4 (C). Scale bar = 100 μm (C).
Morphological characteristics of acetylated tau in PiD. A: Serial sections of anterior cingulate gyrus in a PiD case with heavy ACK280-positive pathological characteristics showing both astrocytic (asterisks) and neuronal Pick body (arrows) tau pathological characteristics with less prominent threads for both the AC-K280 and RD4 antibodies. Double labeling of AC-K280 in this region reveals some modest colocalization with RD3 (arrows) and many AC-K280–labeled inclusions that are RD3 negative (asterisks; B), whereas there was complete colocalization with RD4 (C). PiD tau lesions, including ACK280 pathological features, were weakly reactive to ThS (D). Scale bar = 100 μm (D).
References
- 1.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 2.Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 3.Cohen T.J., Guo J.L., Hurtado D.E., Kwong L.K., Mills I.P., Trojanowski J.Q., Lee V.M. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min S.W., Cho S.H., Zhou Y., Schroeder S., Haroutunian V., Seeley W.W., Huang E.J., Shen Y., Masliah E., Mukherjee C., Meyers D., Cole P.A., Ott M., Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin D.J., Cohen T.J., Grossman M., Arnold S.E., Xie S.X., Lee V.M., Trojanowski J.Q. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain. 2012;135:807–818. doi: 10.1093/brain/aws013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhukareva V., Mann D., Pickering-Brown S., Uryu K., Shuck T., Shah K., Grossman M., Miller B.L., Hulette C.M., Feinstein S.C., Trojanowski J.Q., Lee V.M. Sporadic Pick's disease: a tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter. Ann Neurol. 2002;51:730–739. doi: 10.1002/ana.10222. [DOI] [PubMed] [Google Scholar]
- 7.Arai T., Ikeda K., Akiyama H., Shikamoto Y., Tsuchiya K., Yagishita S., Beach T., Rogers J., Schwab C., McGeer P.L. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick's disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 2001;101:167–173. doi: 10.1007/s004010000283. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida M. Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology. 2006;26:457–470. doi: 10.1111/j.1440-1789.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 9.Xie S.X., Baek Y., Grossman M., Arnold S.E., Karlawish J., Siderowf A., Hurtig H., Elman L., McCluskey L., Van Deerlin V., Lee V.M., Trojanowski J.Q. Building an integrated neurodegenerative disease database at an academic health center. Alzheimers Dement. 2011;7:e84–e93. doi: 10.1016/j.jalz.2010.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman M.S., Farmer J., Johnson J.K., Clark C.M., Arnold S.E., Coslett H.B., Chatterjee A., Hurtig H.I., Karlawish J.H., Rosen H.J., Van Deerlin V., Lee V.M., Miller B.L., Trojanowski J.Q., Grossman M. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie I.R., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J., Kovacs G.G., Ghetti B., Halliday G., Holm I.E., Ince P.G., Kamphorst W., Revesz T., Rozemuller A.J., Kumar-Singh S., Akiyama H., Baborie A., Spina S., Dickson D.W., Trojanowski J.Q., Mann D.M. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., Mirra S.S., Nelson P.T., Schneider J.A., Thal D.R., Trojanowski J.Q., Vinters H.V., Hyman B.T. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 14.Mirra S.S., Heyman A., McKeel D., Sumi S.M., Crain B.J., Brownlee L.M., Vogel F.S., Hughes J.P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer I., Santpere G., van Leeuwen F.W. Argyrophilic grain disease. Brain. 2008;131:1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 16.Arnold S.E., Vega I.E., Karlawish J.H., Wolk D.A., Nunez J., Negron M., Xie S.X., Wang L.S., Dubroff J.G., McCarty-Wood E., Trojanowski J.Q., Van Deerlin V. Frequency and clinicopathological characteristics of presenilin 1 Gly206Ala mutation in Puerto Rican Hispanics with dementia. J Alzheimers Dis. 2013;33:1089–1095. doi: 10.3233/JAD-2012-121570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snider B.J., Norton J., Coats M.A., Chakraverty S., Hou C.E., Jervis R., Lendon C.L., Goate A.M., McKeel D.W., Jr., Morris J.C. Novel presenilin 1 mutation (S170F) causing Alzheimer disease with Lewy bodies in the third decade of life. Arch Neurol. 2005;62:1821–1830. doi: 10.1001/archneur.62.12.1821. [DOI] [PubMed] [Google Scholar]
- 18.Otvos L., Jr., Feiner L., Lang E., Szendrei G.I., Goedert M., Lee V.M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- 19.de Silva R., Lashley T., Gibb G., Hanger D., Hope A., Reid A., Bandopadhyay R., Utton M., Strand C., Jowett T., Khan N., Anderton B., Wood N., Holton J., Revesz T., Lees A. Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol. 2003;29:288–302. doi: 10.1046/j.1365-2990.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Deerlin V.M., Gill L.H., Farmer J.M., Trojanowski J.Q., Lee V.M. Familial frontotemporal dementia: from gene discovery to clinical molecular diagnostics. Clin Chem. 2003;49:1717–1725. doi: 10.1373/49.10.1717. [DOI] [PubMed] [Google Scholar]
- 21.Lantos P.L., Cairns N.J., Khan M.N., King A., Revesz T., Janssen J.C., Morris H., Rossor M.N. Neuropathologic variation in frontotemporal dementia due to the intronic tau 10(+16) mutation. Neurology. 2002;58:1169–1175. doi: 10.1212/wnl.58.8.1169. [DOI] [PubMed] [Google Scholar]
- 22.Nasreddine Z.S., Loginov M., Clark L.N., Lamarche J., Miller B.L., Lamontagne A., Zhukareva V., Lee V.M., Wilhelmsen K.C., Geschwind D.H. From genotype to phenotype: a clinical pathological, and biochemical investigation of frontotemporal dementia and parkinsonism (FTDP-17) caused by the P301L tau mutation. Ann Neurol. 1999;45:704–715. doi: 10.1002/1531-8249(199906)45:6<704::aid-ana4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Pham C.T., de Silva R., Haik S., Verny M., Sachet A., Forette B., Lees A., Hauw J.J., Duyckaerts C. Tau-positive grains are constant in centenarians' hippocampus. Neurobiol Aging. 2011;32:1296–1303. doi: 10.1016/j.neurobiolaging.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Ding Z.T., Wang Y., Jiang Y.P., Yoshida M., Mimuro M., Inagaki T., Iwase T., Hashizume Y. Argyrophilic grain disease: frequency and neuropathology in centenarians. Acta Neuropathol. 2006;111:320–328. doi: 10.1007/s00401-006-0043-2. [DOI] [PubMed] [Google Scholar]
- 25.Jicha G.A., Petersen R.C., Knopman D.S., Boeve B.F., Smith G.E., Geda Y.E., Johnson K.A., Cha R., Delucia M.W., Braak H., Dickson D.W., Parisi J.E. Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:602–609. doi: 10.1097/01.jnen.0000225312.11858.57. [DOI] [PubMed] [Google Scholar]
- 26.Thal D.R., Schultz C., Botez G., Del Tredici K., Mrak R.E., Griffin W.S., Wiestler O.D., Braak H., Ghebremedhin E. The impact of argyrophilic grain disease on the development of dementia and its relationship to concurrent Alzheimer's disease-related pathology. Neuropathol Appl Neurobiol. 2005;31:270–279. doi: 10.1111/j.1365-2990.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K., Akiyama H., Kondo H., Haga C. A study of dementia with argyrophilic grains: possible cytoskeletal abnormality in dendrospinal portion of neurons and oligodendroglia. Acta Neuropathol. 1995;89:409–414. doi: 10.1007/BF00307644. [DOI] [PubMed] [Google Scholar]
- 28.Zhukareva V., Shah K., Uryu K., Braak H., Del Tredici K., Sundarraj S., Clark C., Trojanowski J.Q., Lee V.M. Biochemical analysis of tau proteins in argyrophilic grain disease, Alzheimer's disease, and Pick's disease: a comparative study. Am J Pathol. 2002;161:1135–1141. doi: 10.1016/s0002-9440(10)64390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togo T., Sahara N., Yen S.H., Cookson N., Ishizawa T., Hutton M., de Silva R., Lees A., Dickson D.W. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol. 2002;61:547–556. doi: 10.1093/jnen/61.6.547. [DOI] [PubMed] [Google Scholar]
- 30.Fujino Y., Wang D.S., Thomas N., Espinoza M., Davies P., Dickson D.W. Increased frequency of argyrophilic grain disease in Alzheimer disease with 4R tau-specific immunohistochemistry. J Neuropathol Exp Neurol. 2005;64:209–214. doi: 10.1093/jnen/64.3.209. [DOI] [PubMed] [Google Scholar]
- 31.Tolnay M., Spillantini M.G., Goedert M., Ulrich J., Langui D., Probst A. Argyrophilic grain disease: widespread hyperphosphorylation of tau protein in limbic neurons. Acta Neuropathol. 1997;93:477–484. doi: 10.1007/s004010050642. [DOI] [PubMed] [Google Scholar]
- 32.Grinberg L.T., Wang X., Wang C., Sohn P.D., Theofilas P., Sidhu M., Arevalo J.B., Heinsen H., Huang E.J., Rosen H., Miller B.L., Gan L., Seeley W.W. Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol. 2013;125:581–593. doi: 10.1007/s00401-013-1080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bondareff W., Wischik C.M., Novak M., Amos W.B., Klug A., Roth M. Molecular analysis of neurofibrillary degeneration in Alzheimer's disease: an immunohistochemical study. Am J Pathol. 1990;137:711–723. [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen T.J., Friedmann D., Hwang A.W., Marmorstein R., Lee V.M. The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat Struct Mol Biol. 2013;20:756–762. doi: 10.1038/nsmb.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jellinger K.A., Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia) Brain Pathol. 1998;8:367–376. doi: 10.1111/j.1750-3639.1998.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jellinger K.A., Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007;113:107–117. doi: 10.1007/s00401-006-0156-7. [DOI] [PubMed] [Google Scholar]
- 37.von Bergen M., Barghorn S., Li L., Marx A., Biernat J., Mandelkow E.M., Mandelkow E. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem. 2001;276:48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pedigree of PSEN1 p.P170S mutation case. N, an unspecified number of unaffected children.
Morphological characteristics of acetylated tau in AGD. A: Serial sections of the cornu ammonis (CA-1) region of the hippocampus show more prominent diffusely distributed neuropil threads (NTs) and extracellular ghost tangles (asterisks) with the 3R-specific antibody RD3, whereas both ACK280 and the 4R-specific antibody, RD4, prominently label grains (arrowheads). B: Double labeling of the entorhinal cortex (ERC) of the hippocampus showing that AC-K280 colocalizes with RD3 in intracellular tangles (arrows) but not grains, whereas ACK280 detects a large subset of grains visualized with RD4 (C). Scale bar = 100 μm (C).
Morphological characteristics of acetylated tau in PiD. A: Serial sections of anterior cingulate gyrus in a PiD case with heavy ACK280-positive pathological characteristics showing both astrocytic (asterisks) and neuronal Pick body (arrows) tau pathological characteristics with less prominent threads for both the AC-K280 and RD4 antibodies. Double labeling of AC-K280 in this region reveals some modest colocalization with RD3 (arrows) and many AC-K280–labeled inclusions that are RD3 negative (asterisks; B), whereas there was complete colocalization with RD4 (C). PiD tau lesions, including ACK280 pathological features, were weakly reactive to ThS (D). Scale bar = 100 μm (D).