Abstract
AMP-activated protein kinase (AMPK) signaling is reported to protect neurons under pathologic conditions; however, its effect on oligodendrocytes (OLs) remains to be elucidated. We investigated whether AMPK signaling protects OLs to restore central nervous system (CNS) functions in an experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis. Increased inflammation and demyelination in the CNS and peripheral immune responses were consistent with the observed clinical impairments in EAE animals, which were attenuated by treatment with metformin compared with vehicle. In addition, expressions of neurotrophic factors and of signatory genes of OL lineages were increased in the CNS of metformin-treated EAE animals. Likewise, metformin attenuated inflammatory response and enhanced expressions of neurotrophic factors, thereby protecting OLs via AMPK activation in mixed glial cultures stimulated with lipopolysaccharide/interferon γ in vitro, as evidenced by analysis of the expression of signatory genes of O1+/MBP+ OLs and their cellular populations. Metformin also attenuated oxidative stress and malondialdehyde-containing protein levels, with corresponding induction of antioxidative defenses in OLs exposed to cytokines via AMPK activation. These effects of metformin were evident in the CNS of EAE animals. These data provide evidence that AMPK signaling is crucial to protect OLs and, thus, CNS functions in EAE animals. We conclude that AMPK activators, including metformin, have the potential to limit neurologic deficits in multiple sclerosis and related neurodegenerative disorders.
The critical role of oligodendrocytes (OLs) in producing and maintaining the myelin sheath around axons that supports rapid salutatory conduction in central nervous system (CNS) neurons is well established.1 In addition, OLs are reported to provide trophic factors and metabolic support for neurons.2–4 The CNS has the inherent capacity to generate myelin-forming OLs after episodes of demyelination under various pathologic conditions.5 Persistent demyelination is one of the pathologic hallmarks of multiple sclerosis (MS) and spinal cord (SC) trauma, which is responsible for neurologic deficits in affected individuals.6,7 Therefore, a major question in neurobiology today is how to protect OLs in CNS demyelinating diseases, including MS, to limit neurologic deficits in affected individuals.
Pro- and anti-inflammatory cytokines are known to play critical roles in the survival and generation of myelin-forming OLs that are lost after a demyelination attack.8,9 OLs are required for the induction of remyelination to restore the structural integrity of the axon-myelin unit and axonal conduction properties in the MS brain.9 Proinflammatory cytokine signaling is reported to induce OL death and is antagonized by anti-inflammatory cytokine signaling mechanisms.10–13 In MS brain lesions, spontaneous remyelination follows the demyelination attack in acute lesions.7 In contrast, remyelination fails in chronic lesions, irrespective of the presence of OL progenitor cells (OPCs).14 OPCs transplanted into demyelinated SC lesions, however, are reported to enhance remyelination in adult rats.1 Consistently, we earlier documented that lovastatin enhances the differentiation of OPCs into myelin-forming OLs that eventually enhance remyelination in experimental autoimmune encephalomyelitis (EAE), a murine model of MS.15,16 In light of this information, we assessed whether a pharmacologic agent that can influence OL survival holds promise to restore CNS integrity and functions in MS.
Accumulating evidence suggests that AMP-activated protein kinase (AMPK)–mediated restoration of the CNS energy balance is critical to protect the brain under pathologic conditions.17,18 We earlier documented that AMPK activators, ie, 5-amino-4-imidazole carboxamide riboside (AICAR) and metformin, attenuate clinical impairments in EAE animals.19,20 The protective effects of these AMPK activators were ascribed to their immunomodulatory activities and the restoration of blood-brain barrier integrity in EAE animals.19–21 AMPK activity was reduced in the brain at the onset and peak of EAE disease.22 Consistently, we earlier documented that the genetic ablation of AMPK causes severe clinical impairments in EAE animals.23 In addition, we documented that AICAR inhibits the expressions of inflammatory mediators in astrocytes, microglia, and peritoneal macrophages that are known to play critical roles in EAE/MS pathogenesis.24 Recently, AMPK signaling was reported to interfere with interferon γ (IFN-γ)–mediated activation of astrocytes and microglia22 as well as induction of the inflammatory response in liver cells.25,26 These studies provide evidence that CNS bioenergetics and inflammation are linked, thus understanding of their regulatory mechanisms has the scope to identify new therapeutic interventions for MS.
Recently, AMPK signaling was reported to protect neurons under pathologic conditions.27,28 In addition, we earlier documented that AMPK activity was reduced in OLs exposed to psychosine, a galactosylsphingosine lipid that accumulates in the Krabbe brain.29 Based on this knowledge, we investigated whether AMPK signaling can protect OLs in the CNS of EAE animals. By using in vitro and in vivo approaches, we demonstrated that AMPK signaling influences OL survival and, thereby, restores CNS integrity and functions in EAE animals treated with metformin (first-line drug of choice for type 2 diabetes mellitus).
Materials and Methods
Chemicals and Reagents
Unless otherwise stated, all the chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco's modified Eagle's medium (containing 4.5 g/L of glucose), fetal bovine serum, poly-d-lysine, rat IgG, and rabbit polyclonal IgG (control primary antibodies) and secondary antibodies, ie, Alexa Fluor conjugated with anti-rabbit IgG or anti-mouse IgG, were purchased from Life Technologies (Grand Island, NY). Recombinant platelet-derived growth factor (PDGF)-aa, basic fibroblast growth factor-2, ciliary neurotrophic factor (CNTF), tumor necrosis factor α (TNF-α) and IL-1β proteins were purchased from R&D Systems (Minneapolis, MN). Anti–myelin basic protein (MBP) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). Anti-A2B5 MicroBeads and MS columns were purchased from MACS, Miltenyi Biotec Inc. (Auburn, CA). Anti-O1, anti-A2B5, anti–TNF-α, anti–nitric oxide synthase-II (NOS-II), anti–heme oxygenase-1, anti–manganese superoxide dismutase 2 (SOD2), anti–phospho-AMPKα, and anti-AMPKα1 antibodies were purchased from Millipore (Billerica, MA). Rabbit anti-malondialdehyde (MDA) polyclonal antibodies were purchased from Cell Biolabs (San Diego, CA). Electrochemiluminescence-detecting reagents and nitrocellulose membranes were purchased from GE Healthcare Biosciences (Pittsburg, PA).
Animals
Adult female Lewis rats weighing 250 to 300 g or pregnant Sprague Dawley rats at embryonic gestation day 18 were purchased from Charles River Laboratories (Wilmington, MA) and were housed in the animal care facility at the Medical University of South Carolina (Charleston, SC) throughout the experiment and were provided with food and water ad libitum. All the animal experiments were conducted in accordance with accepted standards of humane care, as outlined in the ethical guidelines and approved by the Medical University of South Carolina's Animal Ethics Committee.
EAE Induction and Evaluation
The procedures used for induction of EAE are as described in previous publications with slight modifications.15,30 In brief, the rats received an s.c. injection of 25 μg of guinea pig MBP in 0.1 mL of PBS emulsified with an equal volume of complete Freund's adjuvent supplemented with 2 mg/mL of mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) in the hind limb foot pads on days 0 and 7. Immediately and again 24 hours later, the rats received 200 ng of pertussis toxin i.p. in 0.1 mL of PBS. Pertussis toxin was used as per the standardized protocol reported by us and other investigators for the induction of EAE. Similarly, healthy control group rats received an s.c. injection of PBS and complete Freund's adjuvant emulsion in the hind limb foot pads on days 0 and 7. The rats were examined for clinical scores by an experimentally blinded investigator (A.K.S.) daily. Clinical scores were assessed using a scale from 0 to 5: 0, no clinical disease; 1.0, piloerection; 2.0, loss in tail tonicity; 3.0, hind leg paralysis; 4.0, paraplegia, and 5.0, moribund or dead. At several times during the study, rats were weighed. The clinical data from rats with a clinical score >4.0 were not included in the statistical analysis. At the conclusion of the study, the rats were euthanized, perfusion was performed, and the lumbar SC was removed, snap frozen with liquid nitrogen, and stored at −70°C until use. Alternatively, the SC of each rat was cut into four small pieces and fixed in 4% paraformaldehyde for histopathologic analysis.
Drug Treatments
Metformin, 150 mg/mL, was suspended in PBS and administered by gavages every day in a 200-μL volume. Treatment was started in rats with established EAE (day 11 or 12 after immunization) and continued until the lessening of paralytic symptoms (day 22 after immunization; recovery). Control EAE rats without drug treatment received PBS once daily. Likewise, healthy control rats received vehicle or metformin once daily.
Cultures and Treatments of OLs
Mixed glial cell cultures were generated from the brains of P1–P2 pups obtained from Sprague Dawley mothers as described earlier.31 Briefly, dissociated cortices in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 4 mmol/L l-glutamine were cultured on flasks coated with 50 μg/mL of poly-d-lysine before use. After 10 days, the cells were used directly for studies or the flasks were shaken (250 rpm) for 2 hours to remove microglia. The flasks were shaken for another 8 hours, and OPCs were dislodged from the astrocyte layer. Pure OPC cultures >99.9% were generated by using anti-A2B5 MicroBeads and passing through MS columns (MACS, Miltenyi Biotec). Purified OPCs (2000 cells/cm2) were plated on poly-d-lysine–coated culture dishes or glass coverslips in serum-free modified Bottenstein-Sato–based medium as described earlier.32 After 24 hours, Bottenstein-Sato–based medium supplemented with 10 ng/mL of CNTF was replaced, which showed the transformation of OPCs into OLs (O1+) at 48 hours of incubation. These OLs were grown in 24-well cell culture plates or 100 cm2 culture dishes and were exposed to 10 ng/mL of TNF-α and 20 ng/mL of IL-1β, simultaneously, in the presence or absence of metformin. OPC-enriched (20%) mixed glial cultures were plated in 6-well cell culture plates (2000 cells/cm2) in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum and 25 μg/mL of gentamicin (Nunc, Roskilde, Denmark) and were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cell cultures with 70% to 80% confluence were used to treat with 100 ng/mL of lipopolysaccharide (LPS) and 50 ng/mL of IFN-γ in the presence or absence of metformin or pharmacologic agents. Concentrations of the pharmacologic agents used in the study showed no cell toxicity as revealed by trypan blue exclusion and lactate dehydrogenase release assays.
Endogenous ROS Measurement
The reactive oxygen species (ROS) was determined using a microplate reader. Cells were plated in 24-well culture plates and were treated with cytokines in the presence or absence of metformin (Cultures and Treatment of OLs). For positive control, before performing the assay, cells were treated with 0.3% H2O2 suspended in culture medium and incubated for 30 minutes at 37°C. After 24 hours of incubation, the cells were washed with PBS (3×) and were incubated with 5 μmol/L cell-permeable fluorescent dye 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-DCFDA; Life Technologies) in serum-free medium as described previously.33 After incubating for 30 minutes, the cells were washed with PBS and lyzed in cell lysis buffer (0.1 N of NaOH in 50% methanol). From each well, 200 μL of solution was transferred to a 96-well black microplate. Changes in fluorescence were determined at excitation/emission = 485/530 nm using a SoftMax Pro spectrofluorometer (Molecular Devices, Sunnyvale, CA).
Measurement of Mitochondrial Membrane Potential
Changes in mitochondrial membrane potential were measured in cells using JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′tetraethylbenzimidazolylcarbocyanine iodide) dye. Cells were incubated with 1 μmol/L JC-1 dye for 15 minutes at 37°C. Then cells were rinsed in PBS (2×) and examined by fluorescence microscopy (Olympus BX60) and photographed using the attached Olympus digital camera (Optronics, Goleta, CA). Oxidative stress usually causes the disruption of mitochondrial membrane potential. JC-1 dye indicates mitochondria depolarization by a change from red to green fluorescence.
Immunocytochemical and Histopathologic Studies
For single labeling, a standard method was used. Briefly, slides were blocked by using an Image-iT fixation and permeabilization kit (Life Technologies) and were incubated with appropriately diluted primary antibody (dilution 1:100) at 4°C overnight followed by washing and further incubation with secondary antibodies, Alexa Fluor 488–conjugated goat anti-rabbit IgG antibodies (dilution 1:500) for 1 hour. For double labeling, slides were incubated simultaneously or separately with both types of primary antibodies after blocking with a serum-PBS solution at 4°C overnight as described for single labeling. Secondary antibodies Alexa Fluor 488–conjugated goat anti-rabbit IgG (CNTF) or Alexa Fluor 594–conjugated rabbit anti-mouse IgG [glial fibrillary acidic protein (GFAP)] antibodies were used. Slides were also incubated with Alexa Fluor–conjugated IgG antibodies without primary antibody as negative controls and an appropriate mouse IgG or rabbit polyclonal IgG as isotype controls. After thorough washings, slides were mounted using Fluoromount-G (Electron Microscopy Sciences, Hatfield, PA) containing Hoechst. Slides were examined using a fluorescence microscope (Olympus BX60), and images were captured at magnifications ×400 using an Olympus digital camera (Optronics) using a dual-bandpass filter. The contrast and brightness of images were processed using Adobe Photoshop CS5 software (Adobe Systems Inc., San Jose, CA).
Fixed SC sections of 5 μm were stained with Luxol fast blue and H&E (Sigma-Aldrich) and were examined using light microscopy (Olympus BX60). Images were acquired using an Olympus digital camera (Optronics). The region of SC depicting >10 nuclei was considered as lesion. The meanings, gray matter, and white matter of the sections were scored in a blinded manner based on the presence or absence of infiltrating cells in each region of the SC. Histopathology scores were recorded as the number of lesions in the SC of each group of rats that showed a readily identifiable inflammatory cell infiltrate. Similarly, demyelination in the white matter of the SC was examined and scored. The demyelination degree was scored as grade 0, no disease; grade 1, foci of demyelination/axonal loss that is superficial and involves <25% of the lateral columns; grade 2, deep foci that involve >25% of the lateral columns; and grade 3, diffuse and widespread demyelination and axonal loss. Histologic data are from five rats per group, where three to four sections per rat were examined for the presence of lesions.
RNA Preparation, cDNA Synthesis, and Real-Time PCR Analysis
Cells or tissues were carefully processed for RNA isolation using TRIzol reagent (Invitrogen, Carlsbad, CA) followed by cDNA synthesis and real-time PCR analysis using a Bio-Rad CFX96 system (Bio-Rad Laboratories, Hercules, CA). The primer sets used in the study (Table 1) were designed using PrimerQuest and were synthesized by Integrated DNA Technologies (Coralville, IA). iQ SYBR Green supermix was purchased from Bio-Rad Laboratories. Thermal cycling conditions were as follows: activation of iTaq DNA polymerase (Bio-Rad Laboratories) at 95°C for 10 minutes followed by 40 cycles of amplification at 95°C for 30 seconds and 59°C to 60°C for 30 seconds. The specificity and detection methods for data analysis are as described earlier.31 In brief, real-time PCR specificity for each analysis was determined by melting curve analysis of the amplified product. The level of target gene transcripts was calculated relative to the expression of reference gene, β-actin, in each sample. The detection of threshold was set above the mean baseline fluorescence determined by the first 20 cycles. A standard curve for each template was generated with a serial dilution of the template (cDNA). Likewise, cytokine and chemokine expressions were measured by using cytokine and chemokine PCR arrays (PARN-150ZD-2; Qiagen Inc., Valencia, CA) with cDNA of each sample, using real-time PCR at conditions described in the product manual. Data were analyzed using website-based RT2 Profiler PCR data analysis software version 3.5 (SABiosciences, a QIAGEN company, Valencia, CA).
Table 1.
Primers Used for Quantitative Real-Time PCR Analysis
| Gene name | Primer sequence |
|---|---|
| β-actin | Forward: 5′-AGCTGTGCTATGTTGCCCTAGACT-3′Reverse: 5′-ACCGCTCATTGCCGATAGTGATGA-3′ |
| TNF-α | Forward: 5′-CTTCTGTCTACTGAACTTCGGGGT-3′Reverse: 5′-TGGAACTGATGAGAGGGAGCC-3′ |
| NOS-II | Forward: 5′-GGAAGAGGAACAACTACTGCTGGT-3′Reverse: 5′-GAACTGAGGGTACATGCTGGAGC-3′ |
| IFN-γ | Forward: 5′-TTTGAGGTCAACAACCCACAGGTC-3′Reverse: 5′-TTTCCGCTTCCTGAGGCTGGATT-3′ |
| IL-17A | Forward: 5′-TCTGAGCCAGCCAAGAAGAAGTGT-3′Reverse: 5′-TTCCAACCCAAACATAGGCAC-3′ |
| ROR-γt | Forward: 5′-AGGCCATTCAGTATGTGGTGGAGT-3′Reverse: 5′-TGTGTGGTTGTTGGCATTGTAGGC-3′ |
| IL-4 | Forward: 5′-GGTATCCACGGATGTAACGACAGC-3′Reverse: 5′-CCGTGGTGTTCCTTGTTGCCGTAA-3′ |
| IL-10 | Forward: 5′-CTGTCATCGATTTCTCCCTGTGAG-3′Reverse: 5′-TGAGTGTCACGTAGGCTTTCATGC-3′ |
| FoxP3 | Forward: 5′-AGAGTTTCTCAAGCACTGCCAAGC-3′Reverse: 5′-TGCATAGCTCCCAGCTTCTCCTTT-3′ |
| CD4 | Forward: 5′-AGGTCTCCCTTCAGTTTGCTGGTT-3′Reverse: 5′-TCACCACCAGGTTCACTTCCTGAT-3′ |
| CD8 | Forward: 5′-AAAGCAAGACCTGGACCAACGAGA-3′Reverse: 5′-TAACGTGCCTGACCATTCACAGGA-3′ |
| CD11b | Forward: 5′- GGGAGATGTGAATGGAGACAAA-3′Reverse: 5′- GTACTGATGCTGGCTACTGATG-3′ |
| CCL3 | Forward: 5′-TCCTATGGACGGCAAATTCCACGA-3′Reverse: 5′-AGATCTGCCGGTTTCTCTTGGTCA-3′ |
| CCL5 | Forward: 5′-TGTGTGCCAACCCAGAGAAGAAGT-3′Reverse: 5′-TAGCATCTATGCCCTCCCAGGAAT-3′ |
| CXCL10 | Forward: 5′-TGCAAGTCTATCCTGTCCGCATGT-3′Reverse: 5′-CCTTCTTTGGCTCACCGCTTTCAA-3′ |
| SOD2 | Forward: 5′-AAACTGACAGCTGTGTCTGTGGGA-3′Reverse: 5′-TTGCAGTGGGTCCTGATTAGAGCA-3′ |
| PDGF-αR | Forward: 5′-CAGACATTGACCCTGTTCCAGAGG-3′Reverse: 5′-GAATCTATGCCAATATCATCCATC-3′ |
| Olig2 | Forward: 5′-TGCGCAAGCTCTCCAAGATCG-3′Reverse: 5′-TCTCGCTCACCAGTCTCTTCATCT-3′ |
| MBP | Forward: 5′-CTCTGGCAAGGACTCACACAC-3′Reverse: 5′-TCTGCTGAGGGACAGGCCTCTC-3′ |
| SOX10 | Forward: 5′-TCTACACGGCCATCTCTGACC-3′Reverse: 5′-GTCGTATATACTGGCTGTTCCCAGTG-3′ |
| PDGF | Forward: 5′-AGGATGCCTTGGAGACAAACCTGA-3′Reverse: 5′-ACTGCGGGAATGGCTTCCTCAATA-3′ |
| CNTF | Forward: 5′-CTTCAAGAGCTCTCACAGTG-3′Reverse: 5′-TGCTTATCTTTGGCCCCATAAT-3′ |
Enzyme Assays and ELISA Methods
Extracts of tissues or cells were collected by homogenization in PBS and centrifugation at 14,000 rpm for 15 minutes at 4°C. Protein, 50 to 100 μg per sample, in extract was used for AMPK activity measurement using AMPK KinEASE FP fluorescein green assay kit (32-007; Millipore) as instructed in the product manual. Data are presented as percentage of controls. Likewise, SOD activity in the extracts was measured using SOD determination kits (19160-1KT-F; Sigma-Aldrich) as instructed in the product manual. Data are presented as percentage of inhibition that reflects SOD activity. CNTF levels in the culture supernatants or tissue extracts were measured by using an enzyme-linked immunosorbent assay (ELISA) kit as per instructions in the product manual (R&D Systems). Anti-MBP–specific IgG isotypes were detected in serum samples by solid-phase ELISA as described earlier,34 where Luminata Forte substrate (Millipore) was used for horseradish peroxidase enzyme assay.
Detection of Nitrite Levels
Nitrite levels in the culture supernatants were measured by using Griess reagent as described elsewhere.35
Western Blot Analysis
Total cell lysates were prepared in ice-cold lysis buffer (radioimmunoprecipitation assay buffer) and were used for Western blot analysis as described elsewhere.32 Autoradiographs were scanned and the band intensity was quantified by using ImageJ software (NIH, Bethesda, MD).
Detection of Cell Viability and Cell Death
Cell viability was determined by trypan blue exclusion assay by mixing 10 μL of 0.4% trypan blue solution with 10 μL of cell suspension and reading the results using a TC10 automated cell counter (Bio-Rad Laboratories). Cell death was analyzed by measuring lactate dehydrogenase enzyme activity in the culture supernatants using a lactate dehydrogenase release assay kit (Roche Diagnostics Corp., Indianapolis, IN).
Statistical Analysis
Data are given as means ± SEM and were analyzed by using Student's t-test for two-group comparisons or one-way multiple-range analysis of variance for multiple-column comparisons, followed by a Bonferroni posttest. P values were determined for three to four separate samples in each experiment using GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA). P < 0.05 was considered significant.
Results
Metformin Attenuates Inflammation in the Peripheral and CNS Compartments, Limiting Clinical Impairments in EAE Animals
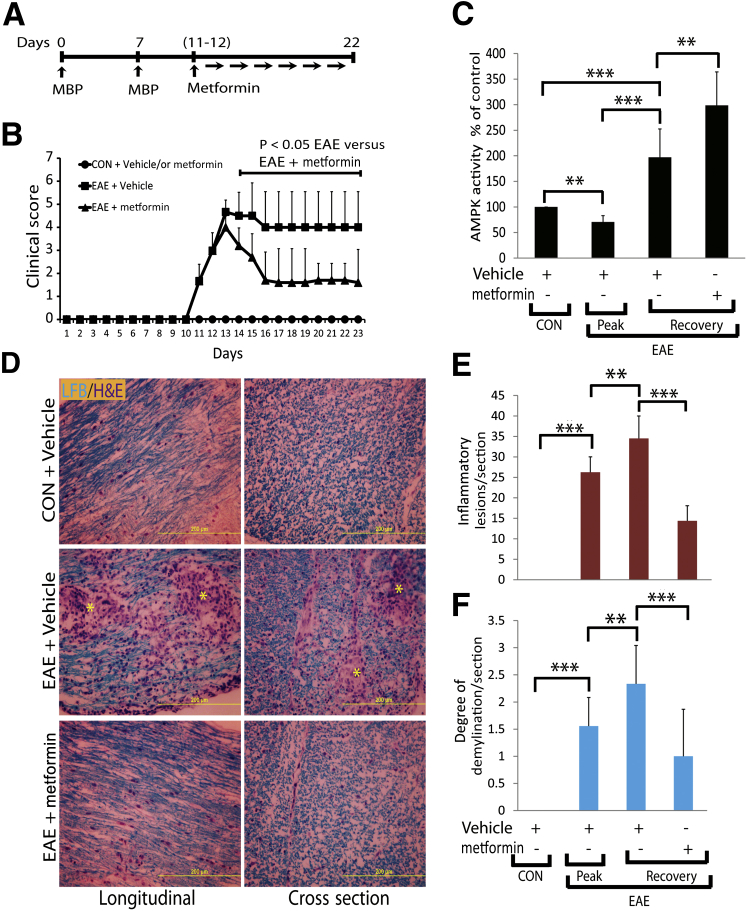
In the experiments described herein, we used metformin to treat female Lewis rats with established EAE exhibiting clinical scores ≥3.0 (Figure 1A). The observed higher clinical scores in EAE rats were reduced significantly by treatment with metformin compared with vehicle (Figure 1B). This lessening of clinical disease in metformin-treated EAE rats was accompanied by a reduction of cellular infiltration and demyelination in their SCs (Figure 1, D–F). For example, the mean ± SD number of inflammatory lesions was increased in the SCs of EAE rats treated with vehicle (33.5 ± 9.9) compared with controls and were reduced significantly (10.4 ± 4.4; P < 0.01) by treatment with metformin (Figure 1E). In addition, metformin attenuated the mean ± SD degree of demyelination in the SCs of EAE rats compared with vehicle treatment, ie, 1.0 ± 0.7 versus 2.5 ± 0.67, respectively (Figure 1F). Note that the number of inflammatory lesions and the degree of demyelination were significantly low in the SCs of EAE rats on the peak clinical day compared with the lessening of symptoms (recovery phase) (Figure 1, E and F). A recent study reported that AMPK activity was reduced in the brain at the onset and peak of EAE disease.22 Therefore, we next assessed the status of AMPK activity in similarly treated EAE rats. As expected, AMPK activity was significantly reduced in the SCs of EAE rats on the peak clinical day compared with healthy controls, and that was reversed on recovery (Figure 1C). Importantly, AMPK activity was remarkably increased in the SCs of EAE rats treated with metformin compared with vehicle on recovery (Figure 1C). These data imply that metformin protects CNS integrity, thereby limiting clinical impairments in EAE rats via AMPK-dependent mechanisms.
Figure 1.
Metformin limits clinical impairments with reduction of inflammation and demyelination in the SCs of EAE rats. A: EAE was induced with 25 μg per rat guinea pig MBP in female Lewis rats, and 150 mg/kg of metformin or vehicle treatments were started orally by gavages in rats with established EAE and healthy controls (CON). B: Composite means ± SEM of the clinical scores in rats (n = 9 per group) evaluated in three separate experiments. C: Composite means ± SEM of three to four samples per group analyzed for AMPK activity in the SCs of EAE rats. D: Representative images of the longitudinal and cross section of lateral funiculi of the SCs of rats (n = 6 per group) stained with Luxol fast blue (LFB) plus H&E depict cellular infiltration and demyelination. Asterisks in D depict cellular infiltration and demyelination in the white mater. Composite means ± SEM of the number of inflammatory lesions (E) and degree of demyelination (F) in the SCs of EAE rats (n = 6 per group) on day 23 after immunization. Recovery indicates lessening of clinical symptoms. ∗∗P < 0.01, ∗∗∗P < 0.001.
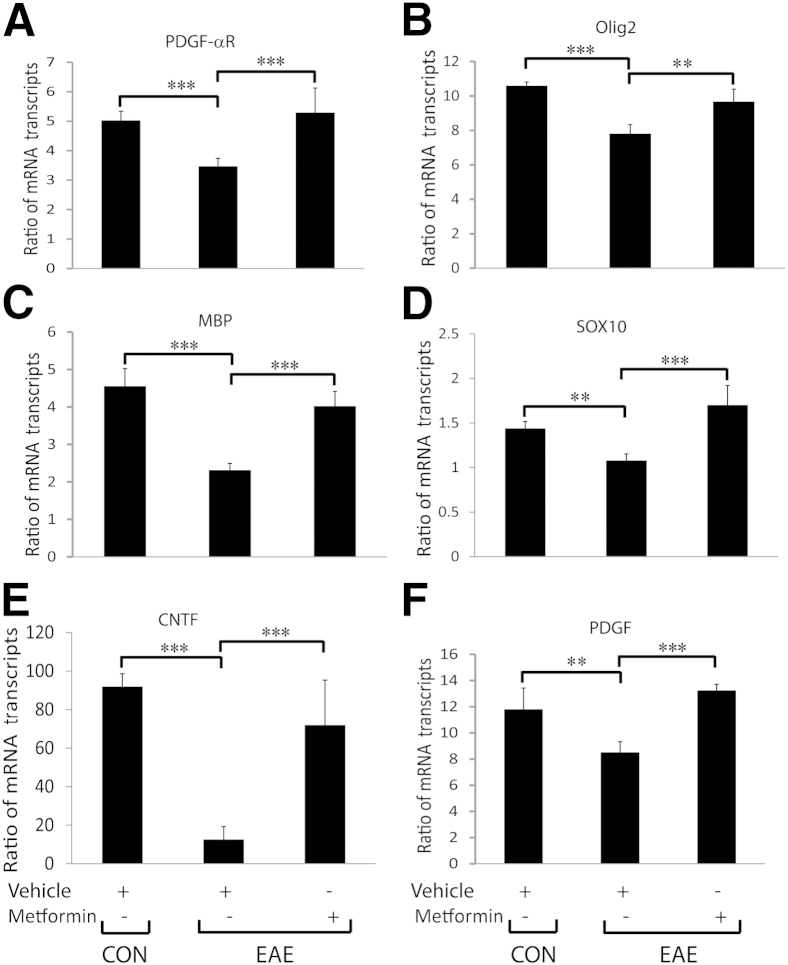
To further confirm that metformin restores CNS integrity and functions in EAE animals, we measured expressions of the signatory genes of OL lineage cells and of neurotrophic factors in the SCs of similarly treated EAE rats. As expected, levels of the mRNA transcripts of the signatory genes of OPCs, ie, PDGF-αR and Olig2 (Figure 2, A and B), and of OLs, ie, MBP and SOX10 (Figure 2, C and D), were significantly reduced, suggestive of OL loss in the SCs of EAE rats treated with vehicle compared with healthy controls, and were rescued by treatment with metformin. Accordingly, levels of the mRNA transcripts of CNTF and PDGF were reduced in the SCs of EAE rats treated with vehicle compared with healthy controls and were abolished by treatment with metformin (Figure 2, E and F). Consistent with RNA data, the mean ± SD CNTF protein level was reduced significantly (P < 0.001) in the SCs of EAE rats treated with vehicle (812.8 ± 80 pg/mg) compared with healthy controls (1871.5 ± 235.33 pg/mg) and was reversed by treatment with metformin (1028.71 ± 42.99 pg/mg). These data provide evidence that metformin treatment provides myelinogenic milieu for OLs and, thereby, restores CNS integrity in EAE animals.
Figure 2.
Metformin enhances expression of the signatory genes of OL lineage cells and of the neurotrophic factors in the SCs of EAE rats. Induction of EAE in rats and treatment with metformin or vehicle were as detailed in the legend to Figure 1. Composite means ± SEM of three to four samples per group analyzed in triplicate for levels of PDGF-αR (A), Olig2 (B), MBP (C), SOX10 (D), CNTF (E), and PDGF (F) mRNA transcripts in the SCs of EAE rats on day 23 after immunization. CON, control. ∗∗P < 0.01, ∗∗∗P < 0.001.
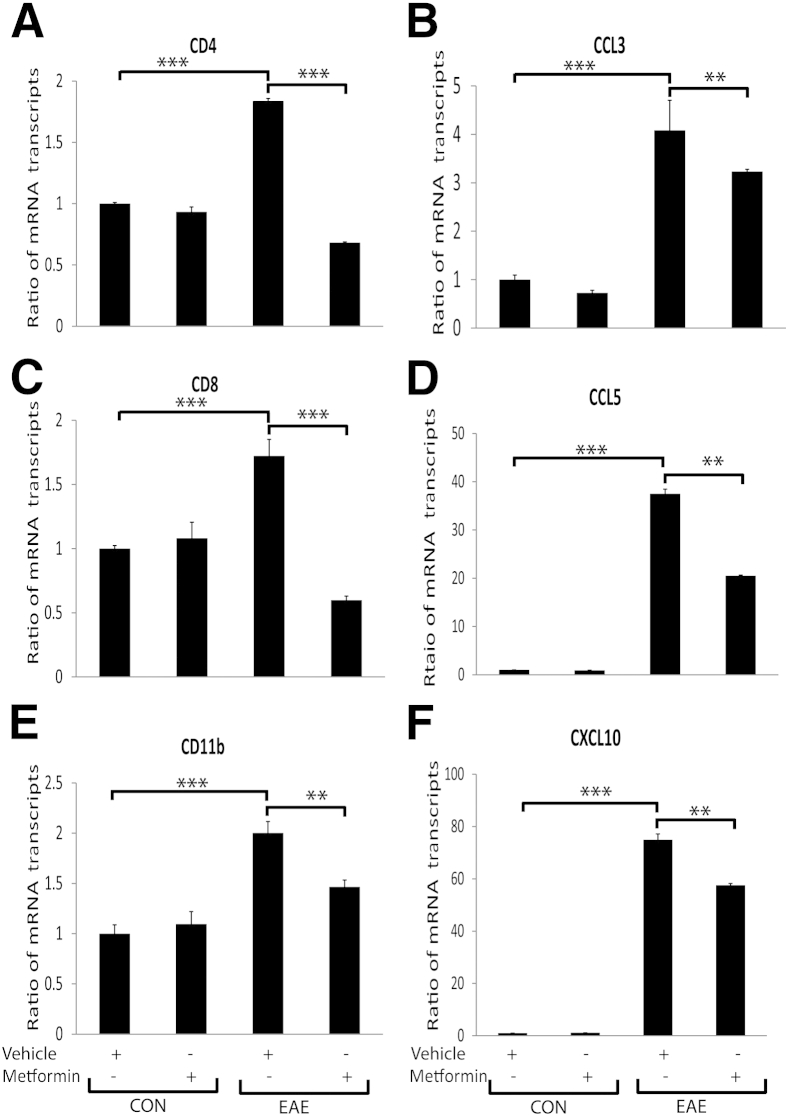
Next, to determine whether the observed protection of CNS integrity and functions in EAE animals is ascribed to the regulation of inflammatory response, we performed gene expression profiling of inflammatory mediators. Levels of the mRNA transcripts of the signatory genes of T cells (CD4 and CD8) and macrophages or microglia (Cd11b) were elevated in the SCs of EAE rats treated with vehicle compared with healthy controls and were attenuated by treatment with metformin (Figure 3, A, C, and E). Comprehensive analysis using gene arrays further demonstrated that the mRNA transcripts for proinflammatory cytokines that participate in the activation, proliferation, and maturation of T cells (CD4+ and CD8+) and B cells and in the activation of dendritic and CNS glial cells accompanied by TNF ligand family cytokines were elevated in the SCs of EAE rats treated with vehicle and were attenuated by treatment with metformin (Table 2). In contrast, levels of the mRNA transcripts for anti-inflammatory cytokines were reduced in the SCs of EAE rats treated with vehicle and were rescued by treatment with metformin (Table 2). Furthermore, levels of the mRNA transcripts for CC and CXC family chemokines, including CX3CL1, were elevated in the SCs of EAE rats treated with vehicle and were attenuated by treatment with metformin (Table 3). These chemokine expression data were validated by measuring the levels of the mRNA transcripts of a few chemokines, ie, CCL3, CCL5, and CXCL10, in the SCs of treated EAE rats using real-time PCR analyses, and that demonstrated the attenuation of their increased expressions in the SCs of EAE rats by treatment with metformin (Figure 3, B, D, and F). Together, these data suggest that metformin attenuates autoimmunity in the CNS compartments of EAE animals.
Figure 3.
Metformin attenuates the expressions of inflammatory mediators in the SCs of EAE rats. Induction of EAE in rats and treatment with metformin or vehicle were as detailed in the legend to Figure 1. Composite means ± SEM of three to four samples per group analyzed in triplicate for levels of CD4 (A), CCL3 (B), CD8 (C), CCL5 (D), CD11b (E), and CXCL10 (F) mRNA transcripts in the SCs of EAE rats on day 23 after immunization. CON, control. ∗∗P < 0.01, ∗∗∗P < 0.001.
Table 2.
Levels of Cytokine mRNA Transcripts (Arbitrary Units) in the SCs of EAE Animals Treated with Vehicle or Metformin (Day 23 after Immunization)
| Gene | EAE + vehicle vs control | EAE + metformin vs control |
|---|---|---|
| Anti-inflammatory cytokines | ||
| IL-1RA | 1.57 ± 0.02 | 2.56 ± 0.3∗ |
| IL-10 | <1.5 | 2.18 ± 0.03∗ |
| Proinflammatory cytokines | ||
| IL-1α | 1.65 ± 0.81 | <1.5 |
| IL-1β | 6.59 ± 1.14 | 4.33 ± 1.01∗∗ |
| IL-7 | <1.5 | −1.51 ± 0.34 |
| IL-15 | <1.5 | −1.65 ± 0.3∗ |
| IL-17F | <1.5 | −1.69 ± 0.1∗ |
| IL-2 | <1.5 | −1.73 ± 0.14∗ |
| IL-16 | <1.5 | −1.82 ± 0.05∗ |
| IL-22 | <1.5 | −1.95 ± 0.01∗ |
| IL-9 | <1.5 | −1.95 ± 0.11∗ |
| IL-21 | <1.5 | −2.04 ± 0.1∗ |
| LIF | <1.5 | −2.04 ± 0.14∗ |
| Dendritic cell–activating cytokines | ||
| IL-12A | <1.5 | −2.08 ± 0.34∗ |
| IL-23A | <1.5 | −2.39 ± 0.04∗∗ |
| IL-12B | <1.5 | −2.57 ± 0.3∗∗ |
| TNF ligand family cytokines | ||
| TNF-α | 3.42 | 1.54 ± 0.14∗ |
| Tnfsf13b | <1.5 | −2.04 ± 0.04∗ |
| Tnfsf10 | <1.5 | −2.18 ± 0.04∗ |
| Tnfsf11 | <1.5 | −2.23 ± 0.11∗∗ |
| Tnfrsf11b | <1.5 | −3.23 ± 0.1∗∗ |
Shown are the composite means ± SEM of four to five samples analyzed in each group.
∗P < 0.05, ∗∗P < 0.01 versus EAE + vehicle.
Table 3.
Levels of Chemokine mRNA Transcripts (Arbitrary Units) in the SCs of EAE Animals Treated with Vehicle or Metformin (Day 23 after Immunization)
| Gene | EAE + vehicle vs control | EAE + metformin vs control |
|---|---|---|
| CC family | ||
| CCL1 | <1.5 | −2.08 ± 0.3 |
| CCL2 | 33.98 ± 2.34 | 26.09 ± 1.34∗ |
| CCL5 | 5.6 ± 0.01 | 3.84 ± 0.11∗ |
| CCL7 | 12.18 ± 1.65 | 8.69 ± 0.41∗∗ |
| CCL11 | <1.5 | −2.95 ± 0.04∗ |
| CCL17 | 2.17 ± 0.01 | 1.5 ± 0.1∗ |
| CCL19 | <1.5 | −2.57 ± 0.11∗ |
| CCL20 | 4.31 ± 0.4 | 1.5 ± 0.1∗∗ |
| CCL21 | <1.5 | −1.99 ± 0.1 |
| CCL22 | <1.5 | −1.99 ± 0.1 |
| CCL24 | <1.5 | −1.54 ± 0.0 |
| CXC family | ||
| CXCL1 | 1.69 ± 0.01 | 2.01 ± 0.1∗ |
| CXCL3 | <1.5 | −1.51 ± 0.0 |
| CXCL9 | 13.18 ± 2.01 | 9.89 ± 1.02∗∗ |
| CXCL10 | 5.3 ± 0.21 | 3.87 ± 0.014∗ |
| CXCL11 | 17.39 ± 1.6 | 13.06 ± 1.12∗ |
| CXCL12 | <1.5 | −3.63 ± 0.16∗∗ |
| CXCL13 | 2.5 ± 0.14 | 1.79 ± 0.32∗ |
| CXCL16 | 5.48 ± 0.01 | 3.43 ± 0.2∗ |
| CX3C family | ||
| CX3CL1 | <1.5 | −4.57 ± 1.02∗∗ |
Shown are the composite means ± SEM of four to five samples analyzed in each group.
∗P < 0.05, ∗∗P < 0.01 versus EAE + Vehicle.
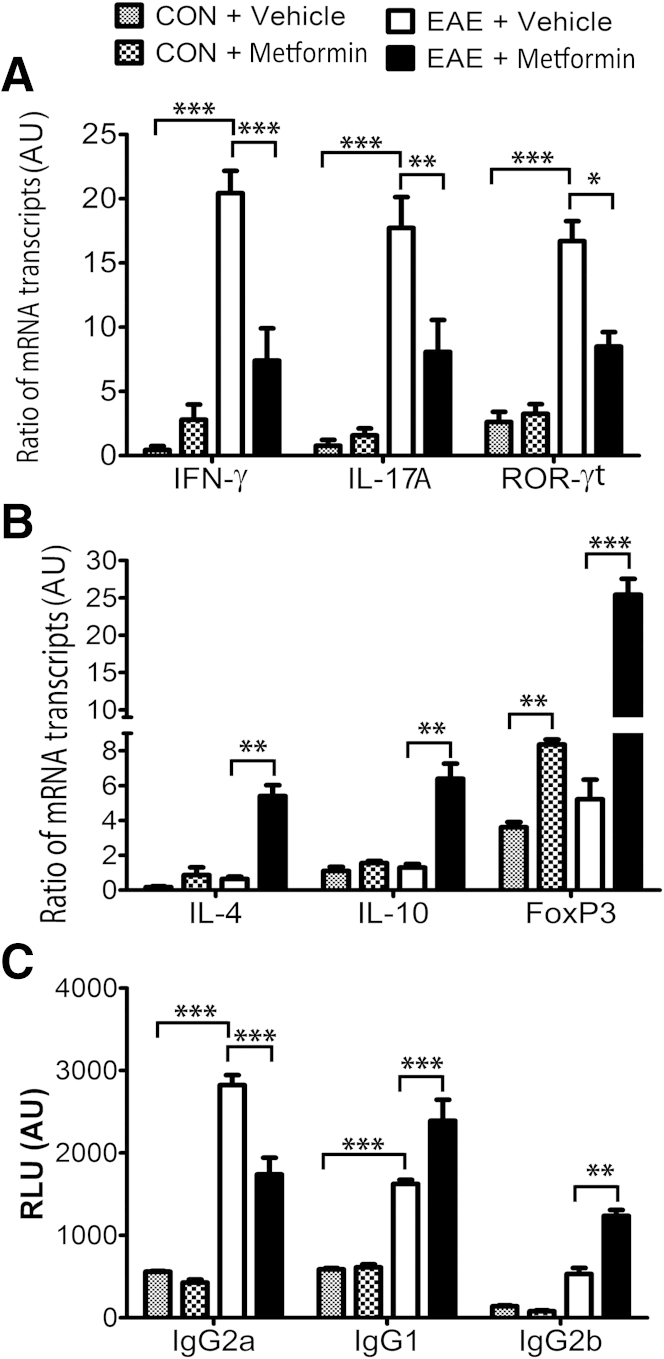
We earlier demonstrated that AICAR and metformin attenuate EAE pathogenesis via attenuation of immune responses and preservation of blood-brain barrier permeability.19–21 Therefore, we next determined to what extent these protective effects of metformin in EAE are, indeed, due to its local effects in the CNS compartment or to effects on the immune response in the peripheral immune system. To address this, spleens of similarly treated EAE rats were analyzed for T-cell autoreactivity. The ratio of the mRNA transcripts of the signatory genes of T helper (Th) 1 (IFN-γ) and Th17 (IL-17A and ROR-γt) cells to CD4 mRNA transcripts were enhanced in the spleens of EAE rats treated with vehicle compared with healthy controls and were attenuated by treatment with metformin (Figure 4A). In contrast, the ratio of the mRNA transcripts of the signatory genes of Th2 (IL-4 and IL-10) and T regulatory (FoxP3) cells to CD4 mRNA transcripts were reduced in the spleens of EAE rats treated with vehicle compared with healthy controls and were reversed by treatment with metformin (Figure 4B). The ratio of the FoxP3 to CD4 mRNA transcripts was also enhanced in the spleens of healthy control rats treated with metformin compared with vehicle (Figure 4B). These data suggest that metformin inhibits Th1 and Th17 cell phenotype responses and enhances the Th2 phenotype response and T regulatory cell generation in the peripheral lymphoid organs of EAE animals.
Figure 4.
Metformin attenuates T-cell autoreactivity in the peripheral compartment of EAE rats. Induction of EAE in rats and treatment with metformin or vehicle were as detailed in the legend to Figure 1. Composite means ± SEM of three to four samples per group analyzed in triplicate for levels of IFN-γ, IL-17A, and ROF-γt mRNA transcripts (A) and of IL-4, IL-10, and FoxP3 mRNA transcripts (B) to CD4 mRNA transcripts ratio in the spleens of EAE rats on day 23 after immunization. Composite means ± SEM of three to four samples per group analyzed in triplicate for levels of anti-MBP Ig, ie, IgG2a, IgG1, and IgG2b (C), in the sera of EAE rats on day 23 after immunization. AU, arbitrary units; CON, control; RLU, relative luminance units. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Because the Th1 responses predominantly elicit IgG2a, whereas Th2 responses produce higher levels of IgG1 in mice,36 we next assessed whether metformin treatment influences the pattern of isotypes of MBP-specific antibodies in EAE rats. The elevated level of Ig isotype, IgG2a detected in the sera of EAE rats treated with vehicle compared with healthy controls was significantly attenuated by treatment with metformin (Figure 4C). Conversely, levels of Ig isotype, IgG1 and IgG2b were significantly increased in the sera of EAE rats treated with metformin compared with vehicle (Figure 4C). These data suggest that metformin treatment biased the anti-MBP Ig response toward IgG1 and IgG2b and against IgG2a, thereby supporting the Th1 to Th2 shift in the blood of EAE animals.
Metformin Protects OLs in Activated Mixed Glial Cultures Via AMPK Activation
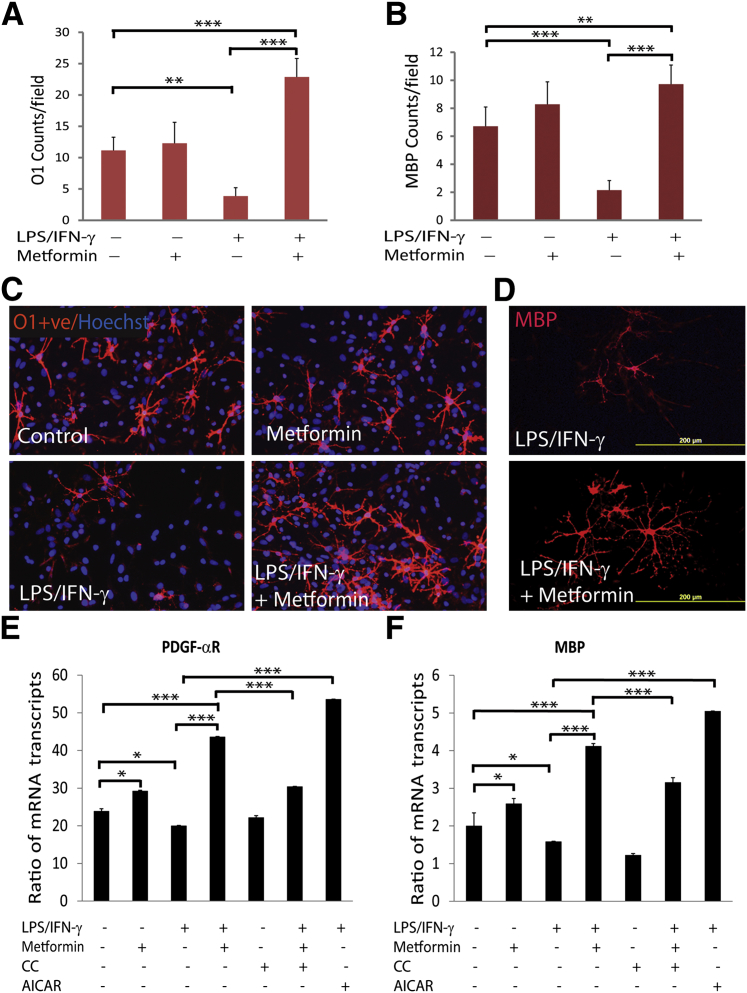
The previously described findings demonstrated that metformin attenuates the inflammatory response in the peripheral (blood and lymphoid organs) and CNS compartments to limit clinical impairments in EAE animals. We earlier reported that AMPK signaling attenuates the expression of inflammatory mediators in astrocytes, microglia, and peritoneal microphages stimulated with LPS in vitro.24 Consistently, AMPK signaling has recently been documented to restrict IFN-γ–induced STAT-1 signaling, thereby inhibiting the activation of astrocytes and microglia.22 To assess the underlying mechanism behind OL protection, and, thus, the restoration of CNS integrity and functions in metformin-treated EAE animals, we treated 20% OPC–enriched mixed glial cultures with metformin before stimulation with LPS and IFN-γ for different durations. As illustrated in Figure 5, A–D, LPS/IFN-γ–stimulated mixed glial cultures had reduced OL lineage cells, ie, O1+ve and MBP+ve OL populations, compared with controls and were rescued by treatment with metformin. These results were consistent with the observed reduction in the levels of mRNA transcripts of the signatory genes of OPCs (PDGF-αR) and OLs (MBP) in mixed glial cultures stimulated with LPS/IFN-γ compared with controls and were rescued by treatment with metformin (Figure 5, E and F). These effects of metformin were reversed by compound C (CC; AMPK inhibitor) and were mimicked by treatment with AICAR (AMPK activator) in similarly activated mixed glial cultures (Figure 5, E and F). Note that the cell death (20% to 25%) observed in mixed glial cultures stimulated with LPS/IFN-γ was also attenuated by treatment with metformin or AICAR (data not shown). Together, these data provide evidence that metformin protects OLs against inflammatory insult in the CNS of EAE animals via AMPK activation.
Figure 5.
Metformin protects OLs in activated mixed glial cultures. Mixed glial cultures (2000/cm2) enriched with OPCs were stimulated with 100 ng/mL of LPS and 100 ng/mL of recombinant IFN-γ protein in the presence or absence of 0.2 mmol/L metformin. Shown are the composite means ± SEM of three to four samples analyzed in triplicate for O1+ve (A) and MBP+ve (B) OL populations in mixed glial cultures treated for 48 and 96 hours, respectively. Representative fields of the slides (n = 6) depict O1+ve (C) and MBP+ve (D) OLs in treated mixed glial cultures for 48 hours and 96 hours, respectively. Slides were counterstained with nuclear Hoechst dye. Shown are the composite means ± SEM of three or four samples analyzed in triplicate for levels of PDGF-αR (E) and MBP (F) mRNA transcripts in treated mixed glial cultures for 96 hours. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
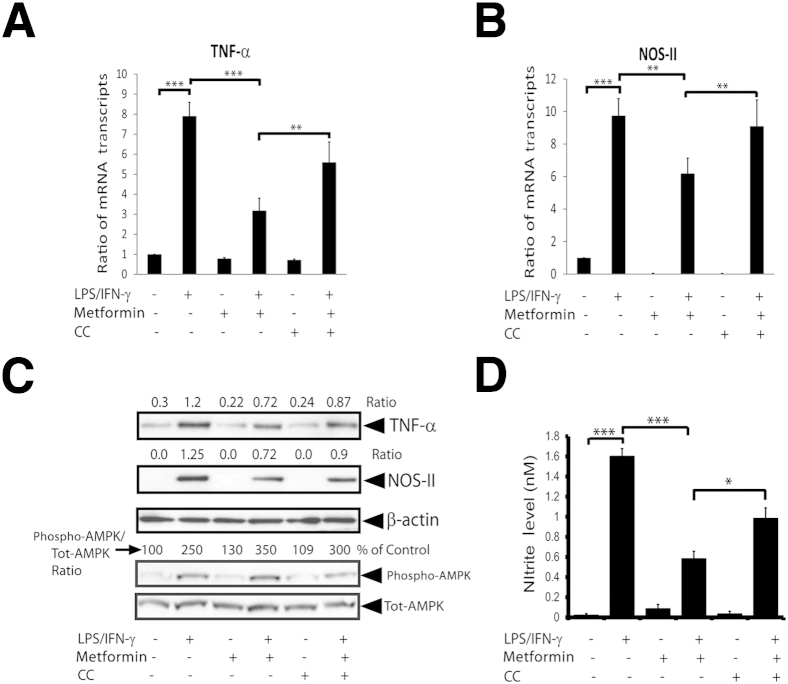
Next, to learn whether OL protection in activated mixed glial cells treated with metformin is ascribed to the attenuation of inflammatory response, we measured the expressions of inflammatory mediators in similarly treated mixed glial cultures. As illustrated in Figure 6,A and B, LPS/IFN-γ–stimulated mixed glial cells had higher levels of the mRNA transcripts of TNF-α and NOS-II compared with controls and were attenuated by treatment with metformin. Consistent with RNA data, TNF-α and NOS-II protein levels were elevated in LPS/IFN-γ–stimulated mixed glial cells compared with controls and were attenuated by treatment with metformin (Figure 6C). Accordingly, the byproduct of NOS-II, NO (measured as nitrite) production, was elevated in the culture supernatants of LPS/IFN-γ–stimulated mixed glial cultures compared with controls and was attenuated by metformin (Figure 6D). Consistent with in vivo data (Figure 1C), AMPK phosphorylation was enhanced in LPS/IFN-γ–stimulated mixed glial cells by treatment with metformin (Figure 6C). This metformin-mediated attenuation of inflammatory response in mixed glial cells stimulated with LPS/IFN-γ was abolished in the presence of CC (Figure 6). These data imply that metformin inhibits the inflammatory response in activated glial cells via AMPK activation.
Figure 6.
Metformin attenuates the inflammatory response in activated glial cells. Mixed glial cultures enriched with OPCs were stimulated with LPS/IFN-γ in the presence or absence of metformin as detailed in the legend to Figure 5. Shown are the composite means ± SEM of three to four samples analyzed in triplicate for levels of TNF-α (A) and NOS-II (B) mRNA transcripts in treated mixed glial cultures for 12 hours. The CC concentration was 5 μmol/L. C: Representative blot depicts the levels of TNF-α, NOS-II, phospho-AMPK, and total AMPK (Tot-AMPK) protein in treated mixed glial cultures for 24 hours. Shown are the composite means ± SEM of three or four samples analyzed in triplicate for nitrite level in the culture supernatants of mixed glial cultures treated for 24 hours (D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
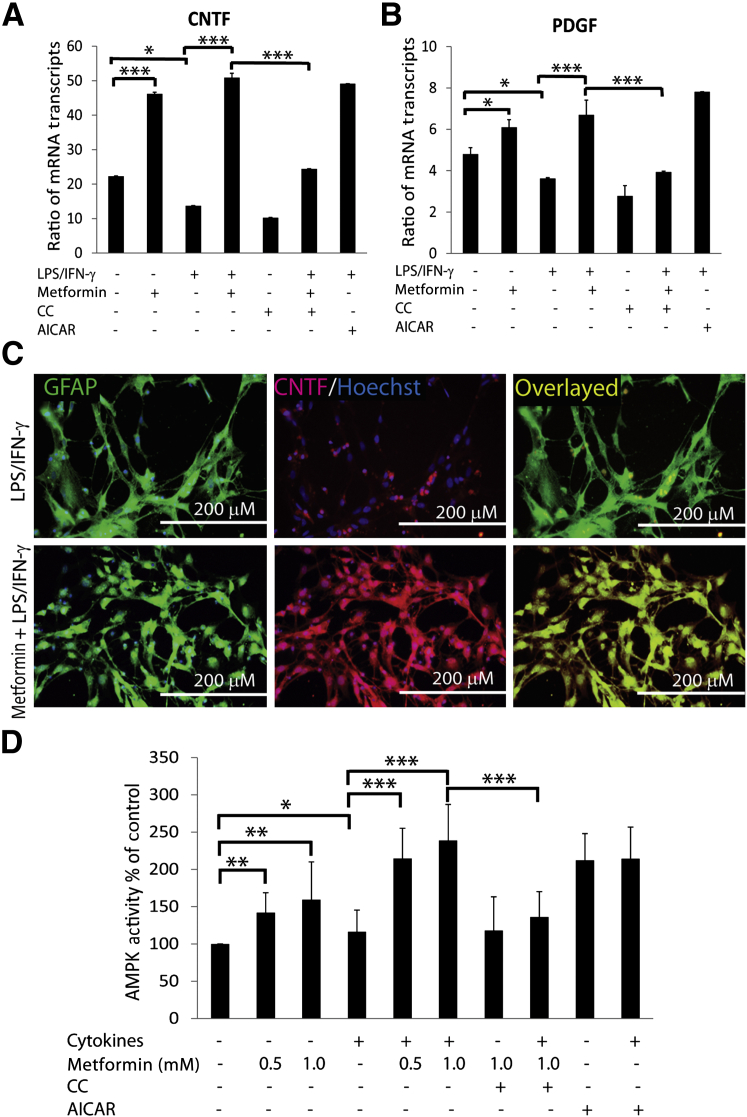
Importantly, neurotrophic factors, ie, PDGF and CNTF, expressed by glial cells are reported to play a significant role in the survival and development of OLs in the brain.37–40 Consistent with in vivo data (Figure 2, E and F), levels of the mRNA transcripts of CNTF and PDGF were reduced in mixed glial cells stimulated with LPS/IFN-γ compared with controls and were abolished by treatment with metformin (Figure 7, A and B). In addition, metformin increased the levels of the mRNA transcripts of CNTF and PDGF in treated control mixed glial cells (Figure 7, A and B). These effects of metformin in activated mixed glial cells were reversed by CC (Figure 7, A and B). However, AICAR mimicked the effect of metformin in activated mixed glial cells (Figure 7, A and B). Consistent with RNA data, mean ± SD CNTF protein levels were reduced significantly (P < 0.01) in the culture supernatants of mixed glial cultures stimulated with LPS/IFN-γ (10.43 ± 1.12) compared with controls (28.33 ± 3.95) and were enhanced by treatment with metformin (143 ± 3.25). These results were consistent with the observed reduced CNTF labeling in GFAP+ve astrocytes in mixed glial cultures stimulated with LPS/IFN-γ compared with controls, which was reversed by metformin treatment (Figure 7C). These data suggest that metformin provides myelinogenic milieu for OLs in activated mixed glial cultures via AMPK activation.
Figure 7.
Metformin provides myelinogenic milieu in activated mixed glial cultures. Mixed glial cultures enriched with OPCs were stimulated with LPS/IFN-γ in the presence or absence of metformin as detailed in the legend to Figure 5. A and B: Composite means ± SEM of three or four samples analyzed in triplicate for levels of CNTF and PDGF mRNA transcripts in treated mixed glial cultures for 12 hours. C: Representative fields of the slides (n = 4) depict the level of CNTF in GFAP+ve astrocytes in treated mixed glial cultures for 72 hours. Slides were counterstained with nuclear Hoechst dye. Overlaying of images depicts the co-localization of CNTF in GFAP+ve astrocytes (right panel). D: Shown are the composite means ± SEM of three or four samples analyzed in triplicate for AMPK activity in OLs (2000/cm2) exposed to cytokines (10 ng/mL of TNF-α plus 20 ng/mL of IL-1β) and treated with metformin or other compounds for 24 hours. The concentrations of CC and AICAR used in the study were 5 μmol/L and 0.5 mmol/L, respectively. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Metformin Protects OLs against Cytokine Toxicity in Vitro and in Vivo
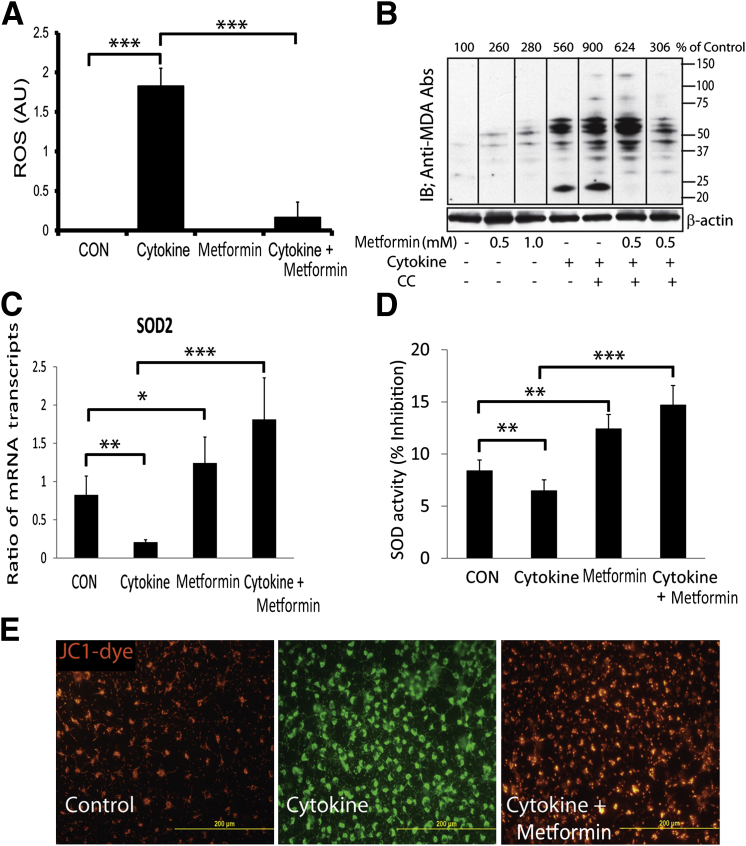
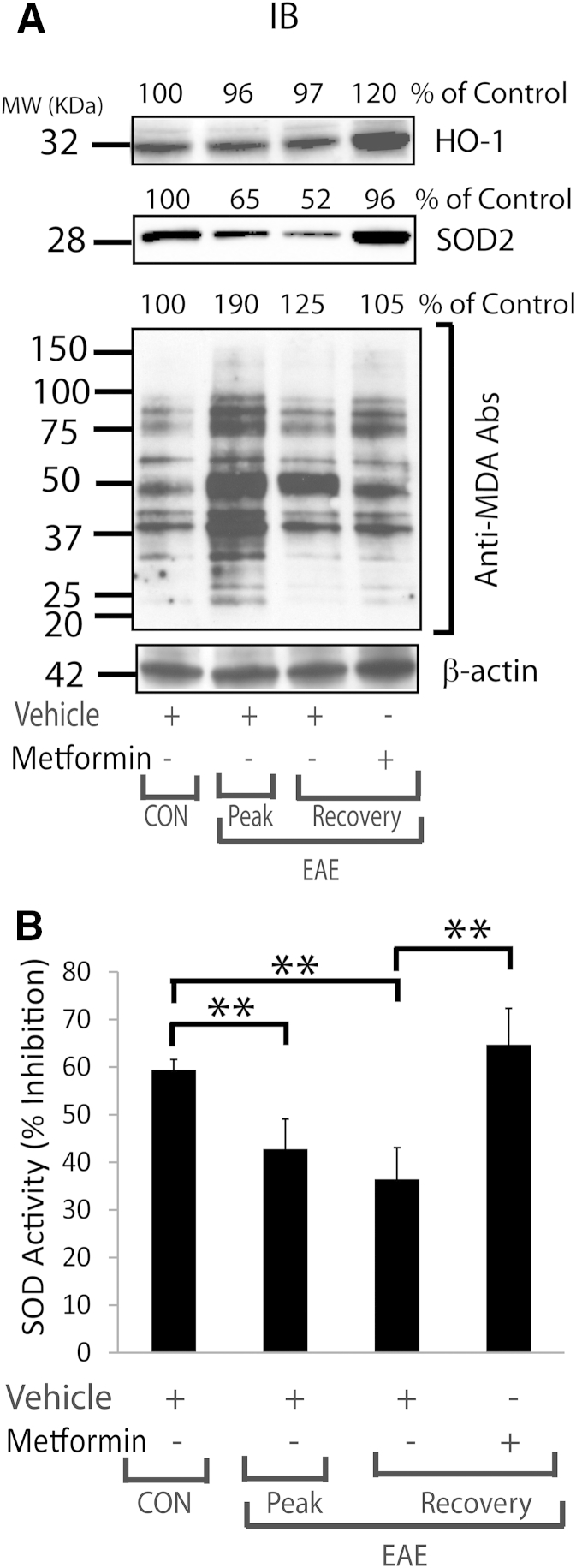
Th1 cytokines, including TNF-α, are reported to induce oxidative stress in OLs that eventually leads to their apoptotic cell death in in vitro conditions.41,42 We earlier documented that TNF-α reduces cellular glutathione levels and increases the generation of ceramide levels in OLs, which are responsible for their apoptotic cell death.43 In addition, we recently documented that TNF-α– and IL-17–induced signaling mechanisms synergize to exacerbate the apoptotic cell death of OLs.33 The present findings demonstrate increased TNF-α expression in the SCs of EAE animals and in activated mixed glial cells. Therefore, we next assessed whether metformin protects OLs against cytokine toxicity in EAE animals. To answer this question, purified OLs were exposed to TNF-α plus IL-1β in the presence or absence of metformin. The observed increased ROS generation in OLs exposed to cytokines was attenuated by treatment with metformin (Figure 8A). Because inflammatory cytokines are known to deplete intracellular glutathione levels and eventually increase ROS generation and MDA (a byproduct of membrane lipid peroxidation) production in cells,44,45 we next measured the levels of MDA-containing proteins in similarly treated OLs. Levels of MDA-containing proteins were enhanced in OLs exposed to cytokines and were reduced by treatment with metformin (Figure 8B). This effect of metformin in OLs exposed to cytokines was abolished by CC (Figure 8B). Accordingly, the SOD2 mRNA transcript level was reduced in OLs exposed to cytokines compared with controls and was reversed by treatment with metformin (Figure 8C). Consistent with RNA data, SOD enzyme activity was significantly reduced in OLs exposed to cytokines compared with controls and was reversed by treatment with metformin (Figure 8D). In addition, a cytokine-mediated increase in mitochondrial membrane depolarization in OLs exposed to cytokines compared with controls was attenuated by treatment with metformin (Figure 8E). Cytokine-induced AMPK activity in OLs compared with controls was augmented by metformin in a dose-dependent manner and was abolished by CC (Figure 7D). However, AICAR-mediated induction of AMPK activation was not enhanced in OLs exposed to cytokines (Figure 7D). Next, to validate these findings in vivo, we analyzed the levels of MDA-containing proteins in the SCs of EAE rats treated with metformin or vehicle. The observed high levels of MDA-containing proteins in the SCs of EAE rats treated with vehicle on peak clinical day or recovery were attenuated by treatment with metformin (Figure 9A). It was accompanied by reduced levels of heme oxygenase-1 and SOD2 proteins in the SCs of EAE rats treated with vehicle compared with healthy controls on peak clinical day or recovery and was abolished by treatment with metformin (Figure 9A). Likewise, SOD enzyme activity was reduced in the SCs of EAE rats treated with vehicle compared with healthy controls on peak clinical day or recovery and was abolished by treatment with metformin (Figure 9B). Together, these data imply that metformin-induced AMPK signaling attenuates oxidative stress in OLs and enhances ROS detoxifying defenses in the CNS of EAE animals.
Figure 8.
Metformin attenuates oxidative stress and protects mitochondria in OLs against cytokine toxicity. OLs were exposed to cytokines as detailed in the legend to Figure 7D. A: Composite means ± SEM of three to four samples analyzed in triplicate for ROS level in treated OLs for 48 hours using a CM-DCFDA assay kit. B: Representative blot of three samples depicts the level of proteins reacting to anti-MDA antibodies (Abs) in the total lysate of OLs treated for 24 hours. C: Composite means ± SEM of three to four samples analyzed in triplicate for SOD2 mRNA transcript level in treated OLs for 12 hours. D: Composite means ± SEM of three to four samples analyzed in triplicate for SOD activity in treated OLs for 24 hours. E: Representative fields of the slides (n = 4) depict color change in the mitochondrial membrane potential from red to green as depolarization marker in OLs for 24 hours analyzed by JC-1 staining. AU, arbitrary units; CON, control. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Figure 9.
Metformin attenuated oxidative stress with induction of ROS detoxifying defense in the SCs of EAE rats. Induction of EAE in rats and treatment with metformin or vehicle were as detailed in the legend to Figure 1. A: Representative blots of three samples that depict the levels of proteins reacting to anti-HO1, anti-SOD2, and anti-MDA antibodies (Abs) in the SCs of EAE rats. B: Composite means ± SEM of three to four samples analyzed in triplicate for SOD activity in the SCs of EAE rats. CON, control; MW, molecular weight; ∗∗P < 0.01.
Discussion
The present study describes that AMPK signaling protects OLs, and, thus, CNS integrity and functions, to limit clinical impairments in the EAE model. The CNS has an inherent capacity to generate myelin-forming OLs after episodes of demyelination under pathologic conditions.5 Persistent CNS demyelination is the characteristic entity for the MS brain and SC trauma.6,7 Consistent with a previous report,19 metformin attenuated clinical impairments in EAE animals, and these impairments were accompanied by the attenuation of increased inflammation and demyelination in the CNS compartment (Figure 1, A, B, and D–F). In addition, expressions of the signatory genes of OL lineage cells and of CNTF and PDGF, including AMPK activity, were enhanced in the SCs of EAE animals treated with metformin (Figures 1B and 2). Importantly, the increased T- and B-cell autoreactivity and the activation of macrophages and resident glial cells in the CNS of EAE animals were attenuated by metformin as revealed by the gene expression profiling of their signatory genes and of cytokines and chemokines (Figure 3 and Tables 2 and 3). Accordingly, the observed increased T-cell autoreactivity in the spleens of EAE animals was attenuated by metformin, as revealed by the reduced expressions of the signatory genes of Th1 and Th17 cells and corresponding enhanced expressions of the signatory genes of Th2 and T regulatory cells (Figure 4, A and B). Likewise, metformin biased the elicited anti-MBP antibodies from IgG2a to IgG1 and IgG2b in the blood of EAE animals, thereby suggesting a Th1 to Th2 shift in the peripheral immune system (Figure 4C). These in vivo findings were supported by the reduction in OL lineage cells, ie, O1+ve and MBP+ve OL populations and expressions of their signatory genes in activated mixed glial cells and were rescued by metformin or AICAR treatment (Figure 5). In addition, expressions of the inflammatory mediators, ie, TNF-α and NOS-II including NO production, were enhanced in mixed glial cultures stimulated with LPS/IFN-γ and were attenuated by metformin via AMPK activation (Figure 6). Consistent with in vivo data, CNTF and PDGF expressions were also reduced in the mixed glial cultures stimulated with LPS/IFN-γ and were abolished by metformin or AICAR treatment (Figure 7, A–C). These effects of metformin in activated mixed glial cells were reversed by CC (Figure 7, A–C). Further studies demonstrated that the increased ROS generation and membrane lipid peroxidation in OLs exposed to cytokines was attenuated by metformin (Figure 8, A and B). It was accompanied by increased SOD2 expression and its enzymatic activity, thereby protecting mitochondrial health, including AMPK activity, in similarly treated OLs (Figures 7D and 8, C–E). These effects of metformin on OLs exposed to cytokines were reversed by CC (Figures 7D and 8, A–C). Consistent with in vitro data, levels of MDA-containing proteins were enhanced in the SCs of EAE animals on peak clinical day or recovery and were reduced by metformin treatment (Figure 9A). It was accompanied by increased levels of ROS detoxifying enzymes, ie, heme oxygenase-1 and SOD2, and of SOD enzyme activity in the SCs of EAE animals treated with metformin compared with vehicle (Figure 9). Altogether, these data provide evidence that AMPK signaling is crucial to protect OLs and, thus, CNS integrity and functions in EAE animals.
AMPK activity is tightly regulated by the cellular AMP/ATP ratio that plays a central role in the regulation of energy homeostasis and metabolic stress.46 Accumulating evidence suggests that AMPK activation attenuates the inflammatory response in different disease conditions and the cytokine-induced inflammatory response in endothelial cells via the inhibition of NF-κB activation.25,47,48 We earlier documented that AMPK signaling attenuates the inflammatory response in EAE mice and rats via T-cell immunomodulation and the protection of blood-brain barrier permeability.19–21 Because metformin is reported to cross the blood-brain barrier,49 we observed that it attenuates the expressions of cytokines that participate in the activation and proliferation of Th1, Th17, and B cells and macrophages, including expressions of their signatory genes in the SCs of EAE animals (Table 2 and Figure 3). In addition, increased expressions of the chemokines that participate in the infiltration of autoreactive T and B cells into the CNS were attenuated by metformin in the SCs of EAE animals (Table 3 and Figure 3). In agreement with a previous report in EAE mice,19 T-cell autoreactivity was modulated in the spleens of EAE rats by metformin that demonstrated Th1 to Th2 biased with corresponding inhibition of Th17 cells and generation of T regulatory cells (Figure 4, A and B). In addition, metformin biased the elicited anti-MBP antibodies from IgG1 and IgG2b against IgG2a, thereby supporting the Th1 to Th2 shift in the blood of EAE rats (Figure 4C). These data suggest that metformin limits the expansion of T- and B-cell autoreactivity in the peripheral and CNS compartments of EAE animals via attenuation of the expressions of cytokines and chemokines, ie, CC family (β-chemokine) and CXC family (α-chemokine), in the CNS compartment. In particular, α- and β-chemokines are reported to be critical for MS/EAE pathogenesis that participates in the attraction of autoreactive T and B cells, neutrophils, and dendritic cells into the CNS.50–52 Consistently, metformin has recently been documented to suppress endotoxin-induced uveitis in Lewis rats via attenuation of inflammatory cytokines and chemokines.53 Importantly, AMPK signaling is reported to inhibit IFN-γ–induced expression of TNF-α and chemokines, ie, CCL2 and CXCL10, in astrocytes and microglia.22 In addition, metformin-mediated induction of AMPK signaling is reported to attenuate the inflammatory response in vascular muscle cells.54 Consistently, we earlier documented that AMPK attenuates LPS-induced inflammation in the glial cells and in the brain via inhibition of CCAAT/enhancer-binding protein transcription activity.24 Consistent with these observations, the observed LPS/IFN-γ–induced TNF-α and NOS-II expressions and NO production in mixed glial cells were attenuated by metformin via AMPK activation (Figure 6). These findings suggest that metformin attenuates autoimmunity in the peripheral (blood and lymphoid organs) and CNS compartments of EAE animals via AMPK activation.
Neurotrophic factors, including CNTF, are known to protect OLs against cytokine toxicity, which is reported to enhance OPC differentiation to promote myelin repair in various CNS demyelinating disorders.37,38 The transplantation of CNTF-expressing OPCs has been reported to promote remyelination and functional recovery in an SC injury model.55 In addition, CNTF is reported to attenuate the inflammatory response in EAE and optic neuritis models.56,57 We found that metformin increases the expressions of CNTF and PDGF in activated mixed glial cultures (Figure 7, A–C) and in the SCs of EAE animals (Figure 2, E and F). Accordingly, OL lineage cell populations were increased in activated mixed glial cultures (Figure 5, E and F) or in the SCs of EAE animals treated with metformin (Figure 2, A–D). These findings provide evidence that the immunomodulatory activities of AMPK signaling are accompanied by the induction of neurotrophic factor expression in CNS cells (astrocytes) that eventually provide myelinogenic milieu for OLs to protect CNS integrity. We recently documented that s-nitrosoglutathione induces CNTF expression in astrocytes via a peroxisome proliferator–activated receptor (PPAR)-γ–dependent mechanism.32 PPAR activity is the important target of AMPK in different cell types.58,59 Based on this information, we infer that AMPK-induced PPAR activities may regulate the expressions of neurotrophic factors in glial cells. However, these conclusions warrant detailed investigation.
Proinflammatory cytokine signaling tends to affect OL survival and can be antagonized by anti-inflammatory cytokine-induced signaling mechanisms.10,12,13 In addition, we earlier demonstrated that cytokine induces the apoptotic cell death of OLs under CNS inflammatory disease conditions.33,43 Metformin protected OLs against cytokine toxicity via the attenuation of ROS generation and mitochondrial membrane depolarization (Figure 8). AMPK signaling is reported to attenuate ROS generation in immune and endothelial cells via the attenuation of NADPH oxidase activity.60,61 In addition, endothelial cells lacking AMPK are reported to exhibit aberrant NADPH oxidase expression and ROS generation.62 AMPK signaling is reported to induce PPAR-γ co-activator-1α trans-activity via its direct phosphorylation, which, in turn, suppresses ROS generation with induction of ROS detoxifying defense in neurons under pathologic conditions.63–65 Consistently, we observed metformin-mediated increased expressions of ROS detoxifying enzymes in OLs exposed to cytokines and in the SCs of EAE animals. These findings provide evidence that AMPK signaling protects OLs against cytokine toxicity via induction of ROS detoxifying defenses possibly via activation of PGC-1α. Studies in this direction are currently under way in our laboratory (Medical University of South Carolina, Charleston, SC).
In conclusion, these findings provide unprecedented evidence that AMPK signaling protects OLs to restore CNS integrity and functions in EAE animals. These effects of AMPK signaling were ascribed to the attenuation of autoimmunity in the peripheral and CNS compartments that eventually attenuates oxidative stress in OLs with increased ROS detoxifying defenses in the CNS of EAE animals. These findings suggest that AMPK activators, including metformin as the first-line choice of drug for type 2 diabetes mellitus, have the potential to limit neurologic deficits in MS and related CNS disorders, including Alzheimer disease, stroke, traumatic brain injury, and peripheral diabetic neuropathy.
Footnotes
Supported by NIH grants VA-1BX001072, VA-BX001999, NS-22576, NS-37766, and C06-RR018823.
A.S.P. and M.K.P. contributed equally to this study.
References
- 1.Groves A.K., Barnett S.C., Franklin R.J., Crang A.J., Mayer M., Blakemore W.F., Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- 2.Oluich L.J., Stratton J.A., Xing Y.L., Ng S.W., Cate H.S., Sah P., Windels F., Kilpatrick T.J., Merson T.D. Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. J Neurosci. 2012;32:8317–8330. doi: 10.1523/JNEUROSCI.1053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulshagen L., Krysko O., Bottelbergs A., Huyghe S., Klein R., Van Veldhoven P.P., De Deyn P.P., D'Hooge R., Hartmann D., Baes M. Absence of functional peroxisomes from mouse CNS causes dysmyelination and axon degeneration. J Neurosci. 2008;28:4015–4027. doi: 10.1523/JNEUROSCI.4968-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassmann C.M., Lappe-Siefke C., Baes M., Brugger B., Mildner A., Werner H.B., Natt O., Michaelis T., Prinz M., Frahm J., Nave K.A. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- 5.Dubois-Dalcq M., Armstrong R. The cellular and molecular events of central nervous system remyelination. Bioessays. 1990;12:569–576. doi: 10.1002/bies.950121203. [DOI] [PubMed] [Google Scholar]
- 6.Blakemore W.F., Smith P.M., Franklin R.J. Remyelinating the demyelinated CNS. Novartis Found Symp. 2000;231:289–298. doi: 10.1002/0470870834.ch17. [DOI] [PubMed] [Google Scholar]
- 7.Murray P.D., McGavern D.B., Sathornsumetee S., Rodriguez M. Spontaneous remyelination following extensive demyelination is associated with improved neurological function in a viral model of multiple sclerosis. Brain. 2001;124:1403–1416. doi: 10.1093/brain/124.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornek B., Storch M.K., Weissert R., Wallstroem E., Stefferl A., Olsson T., Linington C., Schmidbauer M., Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine K.A., Blakemore W.F. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- 10.Lin W., Kemper A., Dupree J.L., Harding H.P., Ron D., Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 11.Balabanov R., Strand K., Kemper A., Lee J.Y., Popko B. Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-gamma. J Neurosci. 2006;26:5143–5152. doi: 10.1523/JNEUROSCI.0737-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J., Zhang Y., Dutta D.J., Argaw A.T., Bonnamain V., Seto J., Braun D.A., Zameer A., Hayot F., Lopez C.B., Raine C.S., John G.R. Proapoptotic and antiapoptotic actions of Stat1 versus Stat3 underlie neuroprotective and immunoregulatory functions of IL-11. J Immunol. 2011;187:1129–1141. doi: 10.4049/jimmunol.1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butzkueven H., Zhang J.G., Soilu-Hanninen M., Hochrein H., Chionh F., Shipham K.A., Emery B., Turnley A.M., Petratos S., Ernst M., Bartlett P.F., Kilpatrick T.J. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- 14.Wolswijk G. Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain J Neurol. 2000;123:105–115. doi: 10.1093/brain/123.1.105. [DOI] [PubMed] [Google Scholar]
- 15.Paintlia A.S., Paintlia M.K., Khan M., Vollmer T., Singh A.K., Singh I. HMG-CoA reductase inhibitor augments survival and differentiation of oligodendrocyte progenitors in animal model of multiple sclerosis. FASEB J. 2005;19:1407–1421. doi: 10.1096/fj.05-3861com. [DOI] [PubMed] [Google Scholar]
- 16.Paintlia A.S., Paintlia M.K., Singh I., Skoff R.B., Singh A.K. Combination therapy of lovastatin and rolipram provides neuroprotection and promotes neurorepair in inflammatory demyelination model of multiple sclerosis. Glia. 2009;57:182–193. doi: 10.1002/glia.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez M., Varela L., Vazquez M.J., Rodriguez-Cuenca S., Gonzalez C.R., Velagapudi V.R., Morgan D.A., Schoenmakers E., Agassandian K., Lage R., Martinez de Morentin P.B., Tovar S., Nogueiras R., Carling D., Lelliott C., Gallego R., Oresic M., Chatterjee K., Saha A.K., Rahmouni K., Dieguez C., Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronnett G.V., Ramamurthy S., Kleman A.M., Landree L.E., Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109(Suppl 1):17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath N., Khan M., Paintlia M.K., Singh I., Hoda M.N., Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009;182:8005–8014. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath N., Giri S., Prasad R., Salem M.L., Singh A.K., Singh I. 5-Aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:566–574. doi: 10.4049/jimmunol.175.1.566. [DOI] [PubMed] [Google Scholar]
- 21.Prasad R., Giri S., Nath N., Singh I., Singh A.K. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates experimental autoimmune encephalomyelitis via modulation of endothelial-monocyte interaction. J Neurosci Res. 2006;84:614–625. doi: 10.1002/jnr.20953. [DOI] [PubMed] [Google Scholar]
- 22.Meares G.P., Qin H., Liu Y., Holdbrooks A.T., Benveniste E.N. AMP-activated protein kinase restricts IFN-gamma signaling. J Immunol. 2013;190:372–380. doi: 10.4049/jimmunol.1202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nath N., Khan M., Rattan R., Mangalam A., Makkar R.S., de Meester C., Bertrand L., Singh I., Chen Y., Viollet B., Giri S. Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem Biophys Res Commun. 2009;386:16–20. doi: 10.1016/j.bbrc.2009.05.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giri S., Nath N., Smith B., Viollet B., Singh A.K., Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myerburg M.M., King J.D., Jr., Oyster N.M., Fitch A.C., Magill A., Baty C.J., Watkins S.C., Kolls J.K., Pilewski J.M., Hallows K.R. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:676–684. doi: 10.1165/rcmb.2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buler M., Aatsinki S.M., Skoumal R., Komka Z., Toth M., Kerkela R., Georgiadi A., Kersten S., Hakkola J. Energy-sensing factors coactivator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha) and AMP-activated protein kinase control expression of inflammatory mediators in liver: induction of interleukin 1 receptor antagonist. J Biol Chem. 2012;287:1847–1860. doi: 10.1074/jbc.M111.302356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P., Xu T.Y., Guan Y.F., Tian W.W., Viollet B., Rui Y.C., Zhai Q.W., Su D.F., Miao C.Y. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- 28.Fu J., Jin J., Cichewicz R.H., Hageman S.A., Ellis T.K., Xiang L., Peng Q., Jiang M., Arbez N., Hotaling K., Ross C.A., Duan W. Trans-(-)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J Biol Chem. 2012;287:24460–24472. doi: 10.1074/jbc.M112.382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giri S., Khan M., Nath N., Singh I., Singh A.K. The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: implication for Krabbe disease. J Neurochem. 2008;105:1820–1833. doi: 10.1111/j.1471-4159.2008.05279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paintlia A.S., Paintlia M.K., Singh A.K., Stanislaus R., Gilg A.G., Barbosa E., Singh I. Regulation of gene expression associated with acute experimental autoimmune encephalomyelitis by Lovastatin. J Neurosci Res. 2004;77:63–81. doi: 10.1002/jnr.20130. [DOI] [PubMed] [Google Scholar]
- 31.Paintlia A.S., Paintlia M.K., Singh A.K., Orak J.K., Singh I. Activation of PPAR-gamma and PTEN cascade participates in lovastatin-mediated accelerated differentiation of oligodendrocyte progenitor cells. Glia. 2010;58:1669–1685. doi: 10.1002/glia.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paintlia M.K., Paintlia A.S., Singh A.K., Singh I. S-Nitrosoglutathione induces ciliary neurotrophic factor expression in astrocytes, which has implications to protect the central nervous system under pathological conditions. J Biol Chem. 2013;288:3831–3843. doi: 10.1074/jbc.M112.405654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paintlia M.K., Paintlia A.S., Singh A.K., Singh I. Synergistic activity of interleukin-17 and tumor necrosis factor-alpha enhances oxidative stress-mediated oligodendrocyte apoptosis. J Neurochem. 2011;116:508–521. doi: 10.1111/j.1471-4159.2010.07136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paintlia A.S., Paintlia M.K., Singh I., Singh A.K. Immunomodulatory effect of combination therapy with lovastatin and 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside alleviates neurodegeneration in experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:1012–1025. doi: 10.2353/ajpath.2006.051309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paintlia A.S., Paintlia M.K., Singh I., Singh A.K. IL-4-induced peroxisome proliferator-activated receptor gamma activation inhibits NF-kappaB trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions: implication for CNS-demyelinating diseases. J Immunol. 2006;176:4385–4398. doi: 10.4049/jimmunol.176.7.4385. [DOI] [PubMed] [Google Scholar]
- 36.Hooper D.C., Morimoto K., Bette M., Weihe E., Koprowski H., Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72:3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louis J.C., Magal E., Takayama S., Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- 38.Linker R.A., Maurer M., Gaupp S., Martini R., Holtmann B., Giess R., Rieckmann P., Lassmann H., Toyka K.V., Sendtner M., Gold R. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–624. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- 39.Vana A.C., Flint N.C., Harwood N.E., Le T.Q., Fruttiger M., Armstrong R.C. Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J Neuropathol Exp Neurol. 2007;66:975–988. doi: 10.1097/NEN.0b013e3181587d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paintlia A.S., Paintlia M.K., Singh A.K., Singh I. Inhibition of rho family functions by lovastatin promotes myelin repair in ameliorating experimental autoimmune encephalomyelitis. Mol Pharmacol. 2008;73:1381–1393. doi: 10.1124/mol.107.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews T., Zhang P., Bhat N.R. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Pang Y., Cai Z., Rhodes P.G. Effect of tumor necrosis factor-alpha on developing optic nerve oligodendrocytes in culture. J Neurosci Res. 2005;80:226–234. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- 43.Singh I., Pahan K., Khan M., Singh A.K. Cytokine-mediated induction of ceramide production is redox-sensitive: implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J Biol Chem. 1998;273:20354–20362. doi: 10.1074/jbc.273.32.20354. [DOI] [PubMed] [Google Scholar]
- 44.Millan-Plano S., Garcia J.J., Martinez-Ballarin E., Reiter R.J., Ortega-Gutierrez S., Lazaro R.M., Escanero J.F. Melatonin and pinoline prevent aluminium-induced lipid peroxidation in rat synaptosomes. J Trace Elem Med Biol. 2003;17:39–44. doi: 10.1016/S0946-672X(03)80044-5. [DOI] [PubMed] [Google Scholar]
- 45.Topal T., Oter S., Korkmaz A., Sadir S., Metinyurt G., Korkmazhan E.T., Serdar M.A., Bilgic H., Reiter R.J. Exogenously administered and endogenously produced melatonin reduce hyperbaric oxygen-induced oxidative stress in rat lung. Life Sci. 2004;75:461–467. doi: 10.1016/j.lfs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Wilson W.A., Hawley S.A., Hardie D.G. Glucose repression/derepression in budding yeast: sNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP: ATP ratio. Curr Biol. 1996;6:1426–1434. doi: 10.1016/s0960-9822(96)00747-6. [DOI] [PubMed] [Google Scholar]
- 47.Hattori Y., Suzuki K., Hattori S., Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 48.Nerstedt A., Johansson A., Andersson C.X., Cansby E., Smith U., Mahlapuu M. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3) Diabetologia. 2010;53:2406–2416. doi: 10.1007/s00125-010-1856-z. [DOI] [PubMed] [Google Scholar]
- 49.Correia S., Carvalho C., Santos M.S., Proenca T., Nunes E., Duarte A.I., Monteiro P., Seica R., Oliveira C.R., Moreira P.I. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008;4:358–364. doi: 10.2174/157340608784872299. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G.X., Baker C.M., Kolson D.L., Rostami A.M. Chemokines and chemokine receptors in the pathogenesis of multiple sclerosis. Mult Scler. 2000;6:3–13. doi: 10.1177/135245850000600103. [DOI] [PubMed] [Google Scholar]
- 51.Bagaeva L.V., Rao P., Powers J.M., Segal B.M. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176:7676–7685. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- 52.Izikson L., Klein R.S., Charo I.F., Weiner H.L., Luster A.D. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalariya N.M., Shoeb M., Ansari N.H., Srivastava S.K., Ramana K.V. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2012;53:3431–3440. doi: 10.1167/iovs.12-9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S.A., Choi H.C. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;425:866–872. doi: 10.1016/j.bbrc.2012.07.165. [DOI] [PubMed] [Google Scholar]
- 55.Cao Q., He Q., Wang Y., Cheng X., Howard R.M., Zhang Y., DeVries W.H., Shields C.B., Magnuson D.S., Xu X.M., Kim D.H., Whittemore S.R. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maier K., Rau C.R., Storch M.K., Sattler M.B., Demmer I., Weissert R., Taheri N., Kuhnert A.V., Bahr M., Diem R. Ciliary neurotrophic factor protects retinal ganglion cells from secondary cell death during acute autoimmune optic neuritis in rats. Brain Pathol. 2004;14:378–387. doi: 10.1111/j.1750-3639.2004.tb00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhlmann T., Remington L., Cognet I., Bourbonniere L., Zehntner S., Guilhot F., Herman A., Guay-Giroux A., Antel J.P., Owens T., Gauchat J.F. Continued administration of ciliary neurotrophic factor protects mice from inflammatory pathology in experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:584–598. doi: 10.2353/ajpath.2006.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burns K.A., Vanden Heuvel J.P. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng R., Pei Z., Zhang A., Zhou Y., Cai X., Chen B., Liu G., Mai W., Wei J., Dong Y. AMPK activation enhances PPARalpha activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway. Arch Biochem Biophy. 2011;511:1–7. doi: 10.1016/j.abb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Piwkowska A., Rogacka D., Jankowski M., Dominiczak M.H., Stepinski J.K., Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun. 2010;393:268–273. doi: 10.1016/j.bbrc.2010.01.119. [DOI] [PubMed] [Google Scholar]
- 61.Li X.N., Song J., Zhang L., LeMaire S.A., Hou X., Zhang C., Coselli J.S., Chen L., Wang X.L., Zhang Y., Shen Y.H. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S., Zhang M., Liang B., Xu J., Xie Z., Liu C., Viollet B., Yan D., Zou M.H. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canto C., Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jager S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jager S., Handschin C., Zheng K., Lin J., Yang W., Simon D.K., Bachoo R., Spiegelman B.M. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]