Abstract
Aberrant proliferation of mesangial cells (MCs) is a key finding in progressive glomerular disease. TH1177 is a small molecule that has been shown to inhibit low-voltage activated T-type Ca2+ channels (TCCs). The current study investigates the effect of TH1177 on MC proliferation in vitro and in vivo. The effect of Ca2+ channel inhibition on primary rat MC proliferation in vitro was studied using the microculture tetrazolium assay and by measuring bromodeoxyuridine incorporation. In vivo, rats with Thy1 nephritis were treated with TH1177 or vehicle. Glomerular injury and average glomerular cell number were determined in a blinded fashion. Immunostaining for Ki-67 and phosphorylated ERK were also performed. The expression of TCC isoforms in healthy and diseased tissue was investigated using quantitative real-time PCR. TCC blockade caused a significant reduction in rat MC proliferation in vitro, whereas L-type inhibition had no effect. Treatment of Thy1 nephritis with TH1177 significantly reduced glomerular injury (P < 0.005) and caused a 49% reduction in glomerular cell number (P < 0.005) compared to the placebo. TH1177 also reduced Ki-67-positive and pERK-positive cells per glomerulus by 52% (P < 0.01 and P < 0.005, respectively). These results demonstrate that TH1177 inhibits MC proliferation in vitro and in vivo, supporting the hypothesis that TCC inhibition may be a useful strategy for studying and modifying MC proliferative responses to injury.
Mesangial cells (MCs) and their associated matrix are key structural components of the healthy glomerulus. In normal adult kidney, MCs are relatively quiescent, with autoradiographic studies demonstrating a glomerular cell renewal rate of only 1%.1 In response to a variety of injurious stimuli, the phenotype of these cells changes, leading to an increase in proliferative rate and progressive matrix accumulation, which can lead to overt glomerulosclerosis if left unchecked. These features are seen in diseases such as IgA nephropathy and diabetic nephropathy, which are two of the leading causes of end-stage renal disease. Current therapies focus on limiting the initiating insult and on blockade of the renin-angiotensin system.2 Therapies that target the response of MCs to the initial disease trigger may provide additional benefit in slowing the progression of chronic kidney disease.
Numerous different mitogenic signals have been implicated in triggering this switch to an activated MC phenotype, but a consistent early step is a transient rise in intracellular Ca2+ concentration. This has been shown to be dependent on an influx of Ca2+ from the extracellular space, but the molecular identity of the channels involved is unclear.3 Although calcium channel blockers (CCBs) are known to reduce MC proliferation in vitro, this action does not correlate with the blockade of high voltage-activated L-type channels (LCCs) that are the traditional targets for these agents.4 This suggests that CCBs exert their anti-proliferative effect by interacting with a target other than the LCCs. T-type channels (TCCs) are low voltage-activated, transient current Ca2+ channels with some structural homology to LCCs but with different activation thresholds and kinetics. Three isoforms exist that differ in the structure of their pore-forming α1 subunit and are known as α1G, α1H, and α1I (or Cav3.1, Cav3.2, and Cav3.3, respectively).5 Several lines of evidence link these channels with the regulation of cell cycle progression in cancer cells,6 cardiac myocytes,7,8 and vascular smooth muscle cells.9 We have previously reported the presence of a low-voltage activated, transient calcium current, consistent with a T-type current, in isolated human MCs.10 Others have recorded similar currents in rat MCs.11 Human MCs express mRNA for the Cav3.2 isoform alone. Pharmacological inhibition of TCCs (but not LCCs) in vitro is anti-proliferative in these cells, as is the knockdown of the Cav3.2 isoform using siRNA.10
The effect of CCBs on animal models of glomerulosclerosis is controversial, with varying outcomes according to the disease model and the particular agent studied.12–14 One potential explanation is that CCBs differ in their selectivity for TCCs or LCCs. TH1177 is a novel CCB, the design of which incorporates key features of dihydropyridines, benzothiazepines, and phenylalkylamines to enhance its ability to block Ca2+ entry.6 This compound reduces the proliferative rate of prostate cancer cells in vitro, with a potency that correlates with its inhibition of calcium influx. Administration of TH1177 to mice inoculated with human prostate cancer cells led to a significant dose-dependent increase in longevity.
In this study, we examined the effect of pharmacological TCC inhibition on rat MC proliferation in vitro and on Thy1 nephritis in vivo. We also studied the expression of the different TCC isoforms in healthy and diseased rat kidneys. The results demonstrate that both Cav3.1 and Cav3.2 are expressed at a similar level in normal renal cortex and the expression of Cav3.2 mRNA increases with disease severity in Thy1 nephritis. However, Cav3.1 is the main isoform present in rat glomeruli and isolated MC, whereas Cav3.2 predominates in the extraglomerular cortex. TH1177 reduces rat MC proliferation in vitro with an apparent ED50 of 15 μmol/L. Treatment of animals with Thy1 nephritis using TH1177 leads to a reduction in glomerular injury and glomerular cell proliferation, which appears to be mediated via suppression of ERK activation. Therefore, we conclude that TH1177 is a useful tool for reducing MC proliferation in vivo as well as in vitro. Further work delineating the exact role of the individual TCC isoforms and their downstream signaling pathways is of interest.
Materials and Methods
Reagents
All reagents were from Sigma-Aldrich (Gillingham, UK), unless otherwise stated.
In Vitro Experiments
Primary rat MCs purchased from Dominion Pharmakine (Bizkaia, Spain) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) F-12 (Invitrogen, Paisley, UK), supplemented with fetal calf serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin (all from Invitrogen). Cells from passage 5 to 15 were used in experiments.
Calcium Channel Inhibitors
Verapamil and nickel chloride (NiCl2) were made up as 10 mmol/L aqueous solutions and were stored at 4°C. TH1177 was made up as a 10 mmol/L solution in 100% ethanol and was stored at −20°C.
MTS Assay
Cell number was measured using the microculture tetrazolium (MTS) assay (Promega, Southampton, UK). After serum deprivation for 48 hours, cells were seeded into 96-well plates at a density of 5000 cells per well and incubated with 0 to 20 μmol/L TH1177 or 0 to 40 μmol/L verapamil with 20% fetal calf serum. Absorbance at 490 nm was measured at 24, 48, 72, and 96 hours in a microplate reader. Experiments were repeated at least three times.
BrdU Incorporation
After 48 hours of serum deprivation, MC proliferation was stimulated with 20% fetal calf serum, and drugs at a range of concentrations were added for another 48 hours. Bromodeoxyuridine (BrdU) (final concentration, 10 μmol/L) was added for the final 16 hours. Cells were washed with PBS, fixed for 45 minutes (3 volume 50 mmol/L glycine pH 2, 7 volume ethanol), washed, incubated in 4 M hydrochloric acid for 10 minutes, and then blocked in 5% goat serum per 0.05% tween per PBS for 15 minutes before being incubated overnight with monoclonal anti-BrdU antibody (1 in 100) at 4°C. After a further three washes, the cells were incubated with 5 μg/mL Alexa Fluor 555 goat anti-mouse antibody (Invitrogen) at room temperature in the dark for 30 minutes. Nuclei were counterstained with 10 μg/mL Hoechst 33342 for 15 minutes. Cells were visualized with a fluorescence microscope. A total of approximately 200 cells were counted from at least four randomly chosen fields. Experiments were repeated at least three times.
Apoptosis Assay
Quiescent rat MC were serum-stimulated in the presence or absence of drugs as previously described for 24 hours. Staurosporine (Sigma-Aldrich), at a final concentration of 1 μmol/L, was added to one culture plate and incubated at 37°C for 90 minutes to act as a positive control. Hoechst 33342 was added to the medium of all dishes at a final concentration of 10 μg/mL and incubated for 10 minutes in the dark. Cells were then visualized with a fluorescence microscope, and the proportion of apoptotic cell nuclei was determined in four randomly chosen fields of each dish in a blinded fashion. Each field contained between 100 and 300 nuclei in total. The experiments were repeated four times.
RT-PCR
Total RNA was isolated using the Qiagen RNeasy Mini-Kit (Qiagen Ltd, Crawley, UK) by following the manufacturer’s instructions. For extraction of RNA from tissue, 30 mg of tissue was disrupted and homogenized in lysis buffer/β-mercaptoethanol solution using a Potter’s homogenizer. The resulting lysate was then transferred to a QiaShredder and subsequently treated as previously described. Reverse transcription and DNA amplification steps were performed concurrently in the same tube using the Promega Access RT-PCR system (Promega) by following the manufacturer’s instructions. Primer pairs were designed using the Primer 3 version 0.4.0 web-based primer design program (MIT, Boston, MA), which were as follows: CACNA1G forward 5′-CCTCTTCCGAGTCTCCACTG-3′, reverse 5′-TGGCCTCTTTGTTGCTTTCT-3′; CACNA1H forward 5′-GTATGCGGATCCTGGTCACT-3′, reverse 5′-ACCCTCCTCCGTCTGGTAGT-3′; CACNA1I forward 5′-ACAACCCCTGGATGCTACTG-3′, reverse 5′-GCATAGTAGGGCAGCCTCTG-3′; β-actin forward 5′-CCCACACTGTGCCCATC-3′ reverse 5′-TGATCCACATCTGCTGGAAG-3′. Samples were amplified for 40 cycles and the products were separated on a 1.5% agarose gel containing ethidium bromide and were then imaged under ultraviolet light.
qPCR
Reverse transcription of RNA was performed using the Omniscript reverse transcription kit (Qiagen) by following the manufacturer’s instructions. Quantitative real-time PCR (qPCR) of the resultant cDNA was performed using the TaqMan gene expression assay system (assay numbers: Cacna1g Rn00581051_m1, Cacna1 hours Rn01460351_g1, Cacna1i Rn01648788_m1), and was analyzed using the ABI Prism 7900HT machine (Applied Biosystems, Warrington, UK). Glyceraldehyde-3-phosphate dehydrogenase (assay number Rn99999916_s1) was used as an endogenous control gene after initial studies confirmed that its expression was not influenced by disease induction. Samples were run in triplicate.
Immunocytochemistry
Polyclonal antibodies targeting Cav3.1 and Cav3.2 were kindly donated by Leanne Cribbs (prepared against peptides FVCQGEDTRNITNKSDCAEAS and YYCEGPDTRNISTKAQCRAAH, respectively).9 Primary rat MCs were washed twice with PBS, fixed in 95% ethanol with 5% glacial acetic acid for 5 minutes, and then washed three times in PBS. Cells were permeabilized by incubation with 0.5% Triton X-100 in PBS for 10 minutes. After three more washes in PBS, cells were blocked in 5% goat serum in PBS for 1 hour, and then incubated with primary antibody (diluted 1 in 250 in PBS + 0.1% Triton X-100) or with nonimmune rabbit IgG at 4°C overnight. Cells were again washed three times in PBS and then incubated with a secondary antibody (Alexa Fluor 488 goat anti-rabbit antibody; Invitrogen) at a dilution of 1 in 1000 for 30 minutes. After three more washes, cells were counterstained, with DAPI at a final concentration of 1 μg/mL for 15 minutes, and then visualized under a fluorescent microscope.
Measurement of T-Type Current Inhibition by TH1177
The effects of TH1177 were measured in HEK cells stably expressing Cav3.1 or Cav3.2 channels. T-type Ca2+ currents were measured using whole-cell voltage-clamping with an Axopatch 200A controlled by pClamp software version 6.0 (Molecular Devices, Sunnyvale, CA). Currents were recorded via 1 to 2 MΩ pipettes at room temperature (approximately 22°C) filled with a Cs+-rich pipette solution containing 120 mmol/L CsCl, 5 mmol/L MgCl2, 0.5 mmol/L CaCl2, 5 mmol/L Na2ATP, 0.3 mmol/L Na3GTP, 12 mmol/L EGTA, and 10 mmol/L HEPES, pH 7.2, in combination with a bath solution containing 138 mmol/L NaCl, 10 mmol/L HEPES, 20 mmol/L glucose, and 5 mmol/L CaCl2, pH 7.35. T-type currents were recorded from a holding potential of −90 mV during 200 msec steps to −20 mV.
In Vivo Experiments
Male Wistar rats weighing 200 to 250 g were housed with free access to standard laboratory food and water. All procedures were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act.
Isolation of Glomeruli
Freshly harvested kidneys were used for isolation of glomeruli and subsequent RNA extraction for qPCR. All steps were performed at 4°C and all equipment and solutions were pretreated to minimize RNase activity. Equipment was soaked in 0.1 M NaOH for 60 minutes, then in 1 mmol/L EDTA solution for another 60 minutes before being rinsed in RNase free water. Solutions were made RNase-free by treatment with 0.1% diethylpyrocarbonate overnight. The diethylpyrocarbonate was then degraded by autoclaving.
The kidney capsules were removed and cortices were dissected from each kidney. Cortical sections were then minced with a razor blade and subsequently pressed through a 180 μm pore size sieve. They were then rinsed through successive sieves (106 μm then 63 μm pores) using ice cold PBS. The tissue consisting of glomeruli on the 63 μm mesh was collected and checked for purity using light microscopy. RNA was then extracted from the isolated glomeruli using the Qiagen RNeasy Mini-Kit (Qiagen Ltd) as previously described.
Treatment of Thy1 Nephritis
Thy1 nephritis was induced in 16 male Wistar rats Ox7 antibody (1 mg/kg, i.v.). Eight animals were treated with intraperitoneal injections of TH1177 (dissolved in 100% ethanol then diluted 10 times in PBS), starting on the day of disease induction, at a dose of 20 mg/kg twice daily for the first 3 days and then 20 mg/kg once daily until sacrifice. The other 8 rats were treated with equivalent injections of vehicle (100% ethanol diluted 10 times in PBS). Spontaneously voided urine samples were collected on days 2 and 7 and were analyzed for protein:creatinine ratio. Half of each group was sacrificed on day 7 and the other half on day 10. Organs were harvested for histological analysis and serum samples taken by cardiac puncture to measure serum creatinine.
Assessment of Severity of Glomerular Injury
Sections (4 μm) were cut and stained with H&E. A histopathologist (N.D.) scored 30 glomeruli per section using the following system: 0 = normal appearance; 1 = mesangial expansion and/or hypercellularity; 2 = microaneurysms, necrosis, capsular hemorrhage, matrix or cellular crescents; Total glomerular cell number and cross-sectional area were quantified in 20 glomeruli per animal using NIS-Elements Basic Research Software version 2.3 (Nikon, Kingston-on-Thames, UK). All analyses were performed in a blinded fashion.
Measurement of Serum Creatinine and Urinary Protein:Creatinine Ratio
Blood samples were centrifuged to separate the serum from the red cells. Urine samples were also centrifuged to remove debris. The supernatants were collected and stored at −80°C. All samples were then taken to the Biochemistry Laboratory at King’s College Hospital (London, UK), and they were analyzed using an automated system.
Immunostaining for Ki-67, ED1, and pERK
Sections (4 μm) of formalin-fixed, paraffin-embedded tissue were rehydrated with xylene and ethanol. Slides were heated in citrate buffer pH 6 in a pressure cooker for 3 minutes. Sections were washed in tris-buffered saline with 0.1% tween-20, blocked in 10% goat serum for 30 minutes, and then incubated with primary antibodies at a dilution of 1 in 100 (ED1, AbD Serotec, Kidlington, UK) and pERK (Cell Signaling Technology, Boston, MA) incubated at 4°C overnight. Ki-67 (Leica Microsystems, Milton Keynes, UK) was incubated at room temperature for 1 hour. After this, slides were washed in TBST and then endogenous peroxide activity was blocked with 2% hydrogen peroxide in methanol for 15 minutes. After a further wash, a peroxidase-conjugated goat anti-mouse secondary antibody (Dako REAL EnVision Detection System; Dako, Ely, UK) was applied to the slides for 30 minutes at room temperature. The slides were washed, developed in Dako REAL DAB+ chromogen for 5 minutes, and then washed in water before being counterstained with Haematoxylin and eosin, dehydrated in ethanols and xylene, and mounted. Positively stained cells were counted in 30 glomeruli per section.
BP Recording
Male rats (weight, 200 to 250 g) were anesthetized with 2% isofluorane in 2 litre O2 per minute with analgesia (buprenorphine, 0.1 mg/kg). After routine laparotomy, the catheter of the PhysioTel PA-C40 pressure transmitter (Data Sciences International, St. Paul, MN) was surgically implanted into the descending abdominal aorta and the body of the probe was fixed to the abdominal wall. After 1 week of recovery, rats housed in individual cages were placed above the telemetric receivers with output to a personal computer. Cardiovascular variables: systolic blood pressure (BP), diastolic BP, mean arterial pressure, and heart rate were monitored and recorded by scheduled sampling for 10 seconds every 5 minutes until the end of the protocol. After 7 days of baseline recording, rats were treated with TH1177 as previously described. Control rats received equivalent vehicle injections.
Statistics
Parametric variables were analyzed using a two-tailed Student’s t-test (for comparison of two groups) or one-way analysis of variance (for multiple group comparisons). For nonparametric variables, the significance of the difference between two groups was determined by a two-tailed U-test and multiple groups compared using the Kruskal-Wallis test. Differences were considered significant if P < 0.05. Statistical analyses were performed using Prism software version 4.0 (Graph-Pad Software, San Diego, CA).
Results
Pharmacological Inhibition of T-Type, But Not L-Type Calcium Channels, Reduces Rat MC Proliferation in Vitro
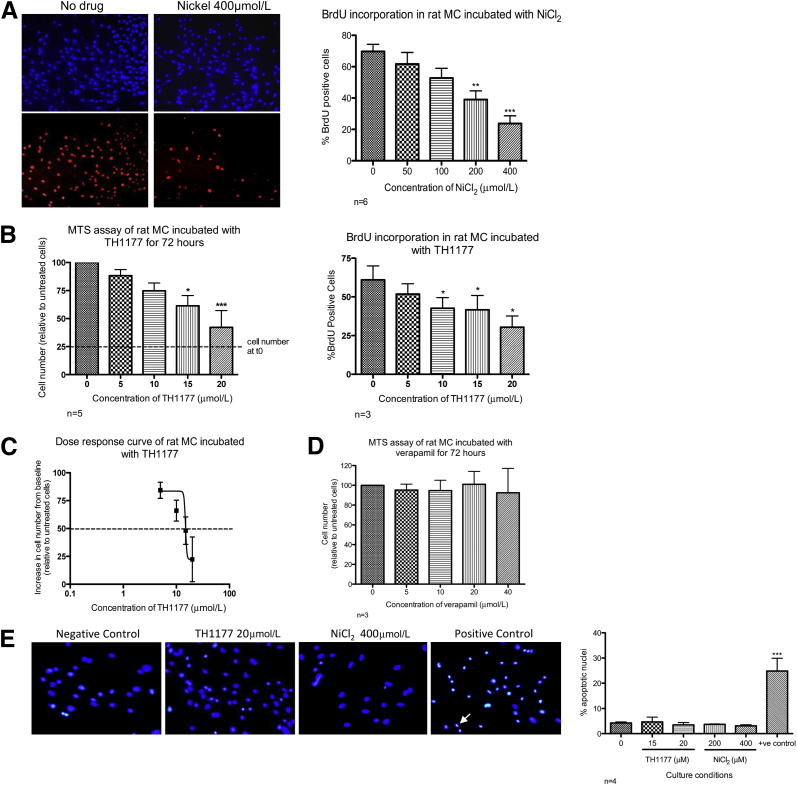
The response of primary rat MCs to co-incubation with nickel ions in vitro is illustrated in Figure 1A. There was a significant dose-dependent reduction in the percentage of cells incorporating BrdU into their DNA (400 μmol/L v control, P < 0.001). Figure 1B illustrates the effect of TH1177 on rat MCs assessed by MTS assay and by BrdU incorporation. In the MTS assay, an increase in cell number from baseline was seen in all conditions, but the rate decreased with increasing TH1177 concentration, leading to a significant dose-dependent reduction in cell number at 72 hours. There was also a dose-dependent reduction in BrdU incorporation, reaching statistical significance at a concentration of 10 μmol/L (P < 0.05). An ED50 of approximately 15 μmol/L can be estimated from these experiments (Figure 1C). This anti-proliferative effect is not seen when rat MCs are treated with verapamil at concentrations that have been shown to completely abolish L-type current (Figure 1D). There was no significant increase in apoptotic nuclei on co-incubation with TH1177 or NiCl2 (up to concentrations of 20 μmol/L and 400 μmol/L), respectively (Figure 1E). No increase in lactate dehydrogenase release was demonstrated when rat MCs were incubated with these concentrations of either drug (data not shown).
Figure 1.
The effect of pharmacological calcium channel blockade on rat MC proliferation and apoptosis in vitro. A: Representative images of BrdU incorporation in primary rat MC co-incubated with 400 μmol/L nickel chloride (NiCl2). Top panel: No drug and 400 μmol/L NiCl2 show all nuclei stained with Hoechst. Bottom panel: No drug and 400 μmol/L NiCl2 show nuclei stained with anti-BrdU antibody. Graphical representation of six independent experiments showed a significant dose-dependent reduction in BrdU incorporation in rat MCs treated with NiCl2. ∗∗P < 0.01, ∗∗∗P < 0.001 versus control cells. B, left panel: A significant reduction in cell number was seen in rat MCs incubated with increasing concentrations of TH1177 for 72 hours (n = 5). ∗P < 0.05, ∗∗∗P < 0.001. Right panel: BrdU incorporation was significantly reduced when these cells were exposed to TH1177 at ≥10 μmol/L (n = 3). ∗P < 0.05 versus vehicle-treated control cells. C: Dose-response curve demonstrated an ED50 of 15 μmol/L for the anti-proliferative action of TH1177 in these conditions. D: Co-incubation of rat MCs with verapamil, an LCCB, had no effect on cell number over 72 hours. E: Representative images of nuclei stained with Hoechst. An example of the apoptotic nucleus is indicated by the arrow in the positive control image. The graph represents four independent experiments demonstrating that nickel (concentration of ≤400 μmol/L) and TH1177 (concentration of ≤20 μmol/L) caused no increase in the proportion of apoptotic nuclei seen compared to vehicle-treated cells.
Cav3.1 and Cav3.2 Are Co-Expressed in Rat Renal Cortex, and Cav3.2 mRNA Levels Increase with Increasing Disease Severity in Thy1 Nephritis
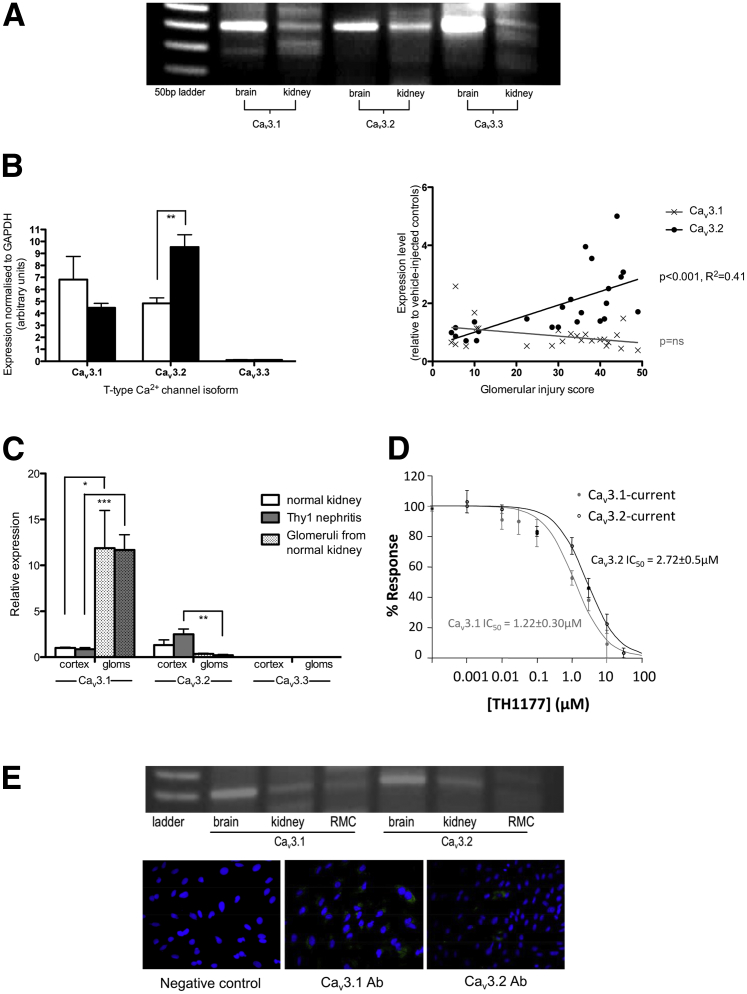
Figure 2A shows a representative gel demonstrating the presence of PCR products for Cav3.1 and Cav3.2 from whole rat kidney cortex mRNA. Some amplification of Cav3.3 mRNA is also detected, but this appears to be at a lower level. These findings were confirmed using qPCR (with different primer sequences) as illustrated in Figure 2B. Cav3.1 and Cav3.2 are detected at similar levels in normal rat kidney, whereas very little Cav3.3 mRNA is present, in keeping with the published literature reporting that it is expressed almost exclusively in neuronal tissue.5 The level of Cav3.2 mRNA increases significantly in kidneys from animals with Thy1 nephritis and this correlates with the severity of renal injury. No significant change is seen in the quantity of mRNA encoding for the other two isoforms in this model. To further investigate the distribution of TCC isoforms in rat kidney, glomeruli were isolated and the RNA extracted from the glomerular fraction was compared to the equivalent whole cortex extract. As illustrated in Figure 2C, Cav3.1 mRNA predominates in glomeruli (12-fold higher than the same isoform in cortical RNA; P < 0.001), whereas Cav3.2 mRNA is primarily found in the nonglomerular cortex (P < 0.01 versus glomerular extract). No significant difference is seen in Cav3.3 mRNA abundance between the compartments, and this isoform remains at significantly lower levels than the other two. The primacy of Cav3.1 in rat glomeruli is consistent with the finding that rat MCs (in contrast to cultured human MCs) express Cav3.1, but not Cav3.2, as demonstrated by PCR and immunocytochemistry (Figure 2E).
Figure 2.
Expression of T-type calcium channel isoforms in the rat kidney. A: Representative gel illustrating the presence of PCR products for T-type calcium channel isoforms in RNA extracted from the rat kidney cortex with rat brain extract as a positive control. Products of the appropriate size were detected for Cav3.1 and Cav3.2, with some amplification of Cav3.3 also present, but apparently at a lower level. B, left panel: Expression of T-type Ca2+ channel isoform mRNA in normal rat kidneys (white bars) and in Thy1 nephritis (black bars). qPCR confirmed the presence of similar amounts of mRNA for Cav3.1 and Cav3.2 in normal rat kidney (n = 6), whereas minimal Cav3.3 expression was detected. ∗∗P < 0.01. Right panel: Expression of T-type calcium channel isoform mRNA in the renal cortex. The expression of Cav3.2 increases significantly in animals with Thy1 nephritis (n = 18), and this increase correlated with disease severity (assessed by glomerular injury score). No association was seen between disease severity and expression of the other two isoforms. C: Expression of T-type channel isoforms in glomeruli. Significantly more Cav3.1 mRNA was found in isolated glomeruli than in the whole cortex, with no difference between normal or diseased glomeruli. Cav3.2 mRNA expression demonstrated the opposite pattern of distribution, with significantly more present in the whole cortex than in the glomerular fraction. The increase in expression of this isoform in disease occurred primarily in the nonglomerular cortex. *P < 0.05, **P < 0.01, and ***P < 0.001. D: Sensitivity of cloned T-type calcium channel isoforms to TH1177. The ability of TH1177 to inhibit T-type current through cloned Cav3.1 and Cav3.2 subunits expressed in HEK cells is illustrated. The IC50s for Cav3.1 and Cav3.2 can be estimated as 1.22 ± 0.3 μmol/L and 2.72 ± 0.5 μmol/L, respectively. E: Primary rat MCs have detectable mRNA for Cav3.1, but not for Cav3.2. Consistently, immunocytochemistry of primary rat MCs demonstrated staining of all cells with anti-Cav3.1 antibody, but minimal staining with anti-Cav3.2. Equivalent concentration of nonimmune rabbit IgG served as a negative control.
Given the differential distribution of the two isoforms in the rat kidney, we felt it was important to clarify the profile of TH1177 inhibitory activity on the individual α1-subunits. This was performed on cloned human subunits expressed in HEK cells. Cav3.1 is the more sensitive of the two isoforms to TH1177 blockade, with an IC50 of 1.22 ± 0.3 μmol/L in this system, whereas Cav3.2 current is inhibited with an IC50 of 2.72 ± 0.5 μmol/L (Figure 2D).
Treatment of Thy1 Nephritis with TH1177 Reduces Glomerular Injury, Glomerular Area, and Glomerular Cell Number
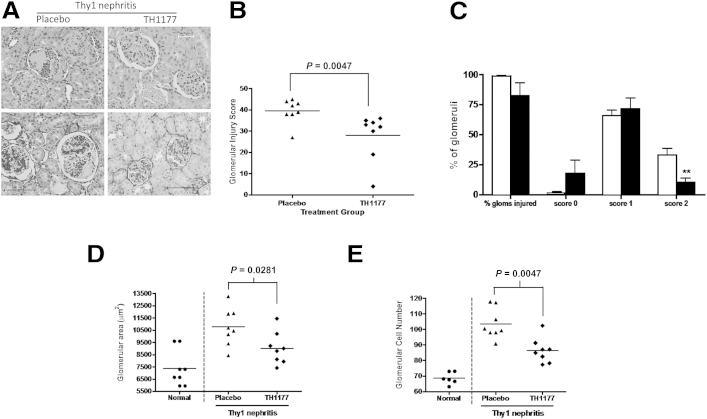
Figure 3A shows representative images of H&E-stained and silver-stained sections of glomeruli from rats with Thy1 nephritis, treated with TH1177 or a vehicle. TH1177 treatment leads to a significant reduction in injury score from an average of 39.5 ± 2.0 in the placebo-treated Thy1 nephritis group to 27.9 ± 3.9 in the TH1177-treated Thy1 nephritis group (P < 0.005) (Figure 3B). Further analysis of the distribution of scores reveals significantly fewer glomeruli with a score of 2 in the TH1177 group, with a corresponding (although not statistically significant) increase in the proportion with a normal appearance (Figure 3C). Average glomerular area is significantly lower in the TH1177-treated group compared to the placebo-treated nephritic controls (P < 0.05) (Figure 3B), as is the total glomerular cell number (P < 0.005) (Figure 3D). The increase in glomerular cellularity seen in placebo-treated Thy1 nephritis is reduced by 49% with TH1177 treatment (normal glomeruli, 68.6 ± 1.57; placebo-treated Thy1 nephritis group, 103.5 ± 3.42; TH1177-treated group, 86.4 ± 2.81) (Figure 3E). Both groups gained weight in an equivalent manner throughout the experiment (data not shown).
Figure 3.
Treatment of Thy1 nephritis with TH1177 reduces glomerular injury. A, Top panels: Representative images of glomeruli from placebo- and TH1177-treated animals with Thy1 nephritis stained with H&E. Bottom panels: Stained with silver stain. B: Glomerular injury score was significantly lower in the TH1177-treated group (average score = 27.9 ± 3.90) than the placebo group (average score = 39.5 ± 2.03). C: The reduction in glomerular injury score in TH1177-treated kidneys (black bars) in comparison to placebo kidneys (white bars) reflects a reduction in the proportion of glomeruli in the most severely damaged group (score 2; evidence of necrosis, microaneurysms, crescent formation, or capsular hemorrhage). There was a corresponding increase in the proportion of glomeruli with a normal appearance (score 0), although this trend did not reach statistical significance. ∗∗P < 0.01. D: Glomerular area increased significantly with Thy1 nephritis compared to normal control kidneys and this was reduced by TH1177 treatment (placebo group, 10765.5 ± 541.54 μm2; TH1177 group, 9020.8 ± 461.77 μm2). E: Glomerular cell number also increased with disease induction, as expected. This increase was reduced significantly in the TH1177 group (normal glomeruli, 68.6 ± 1.57; placebo group, 103.5 ± 3.42; TH1177 group, 86.4 ± 2.81).
Treatment of Thy1 Nephritis with TH1177 Has No Effect On Serum Creatinine or Urinary Protein:Creatinine Ratio
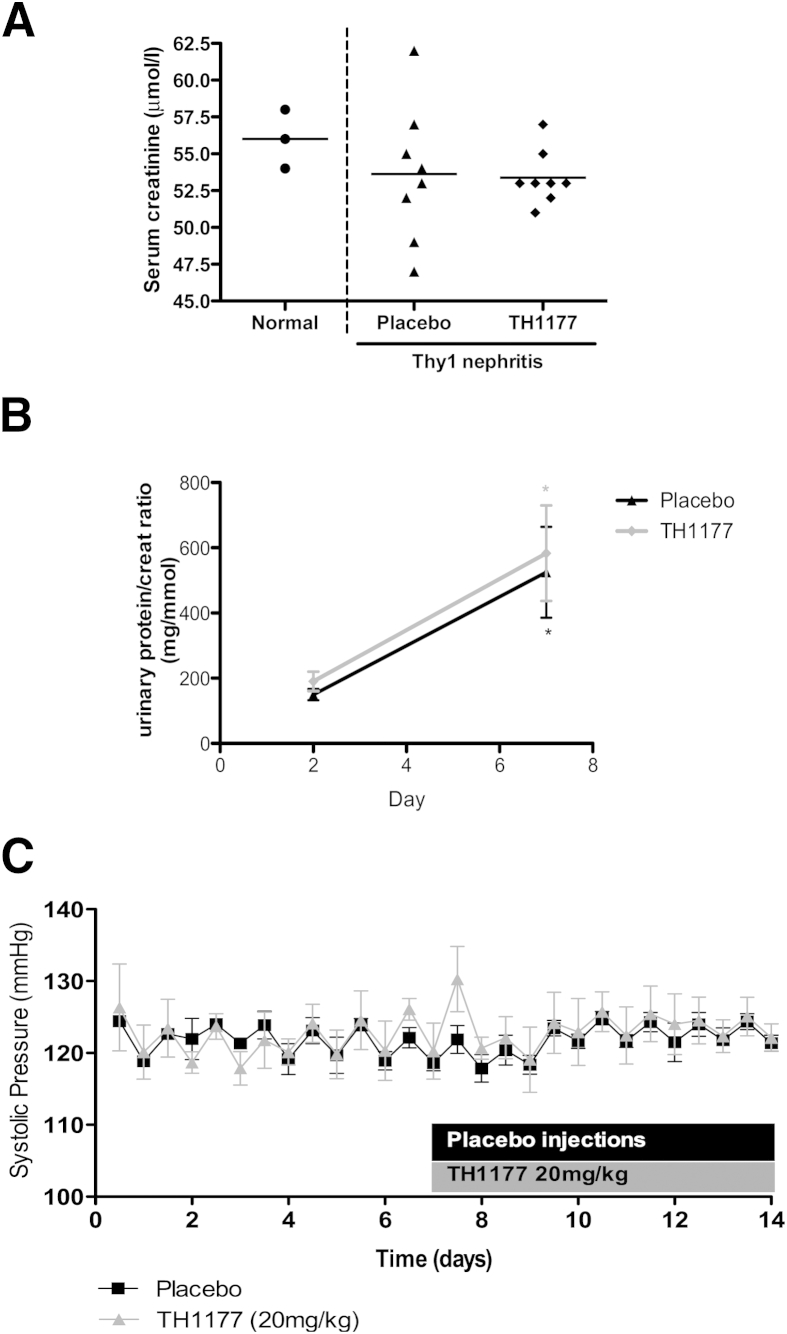
Figure 4A shows the serum creatinine levels from normal Wistar rats, rats with Thy1 nephritis treated with a vehicle, and those treated with TH1177. This acute disease model does not cause an increase in serum creatinine, which is an insensitive measure of renal dysfunction. Therefore, there is no difference between normal animals and those with disease, or between the two treatment groups. Figure 4B illustrates the rise in proteinuria seen in both groups from day 2 to day 7 after disease induction. There is no difference in the level of proteinuria at either time point between the treatment groups.
Figure 4.
Serum creatinine, urinary protein, and systolic BP are not altered by TH1177 treatment in Thy1 nephritis. A: Serum creatinine was not elevated in animals with acute Thy1 nephritis treated with placebo injections compared to normal controls, demonstrating that this acute model of disease does not induce renal dysfunction, as assessed by the relatively insensitive biomarker. There was also no impact of TH1177 treatment on serum creatinine. B: Urinary protein:creatinine ratio increased significantly from day 2 to day 7 in both treatment groups. The lack of effect of TH1177 on proteinuria in our disease model was surprising, given its impact on histological parameters of disease severity, suggesting that TH1177 was not affecting the damage to the filtration barrier itself, but more specifically was acting on the mesangial cell response to disease. C: Graphical representation of invasive systolic BP measurements of animals treated with TH1177 (20 mg/kg) or equivalent vehicle injections from day 7 to day 14. There was no change in BP from pretreatment levels and no difference was seen between the two groups.
The Dose of TH1177 that Reduces Glomerular Cell Proliferation in Thy1 Nephritis Has No Impact on Systemic BP
Figure 4C shows results of intravascular BP recordings in animals receiving TH1177 or a vehicle. Injections commenced at day 7 and continued until day 14. The initiation of injections had no impact on systolic BP and there is no significant difference between the two groups.
Treatment of Thy1 Nephritis with TH1177 Reduces Glomerular Cell Proliferation and Macrophage Number
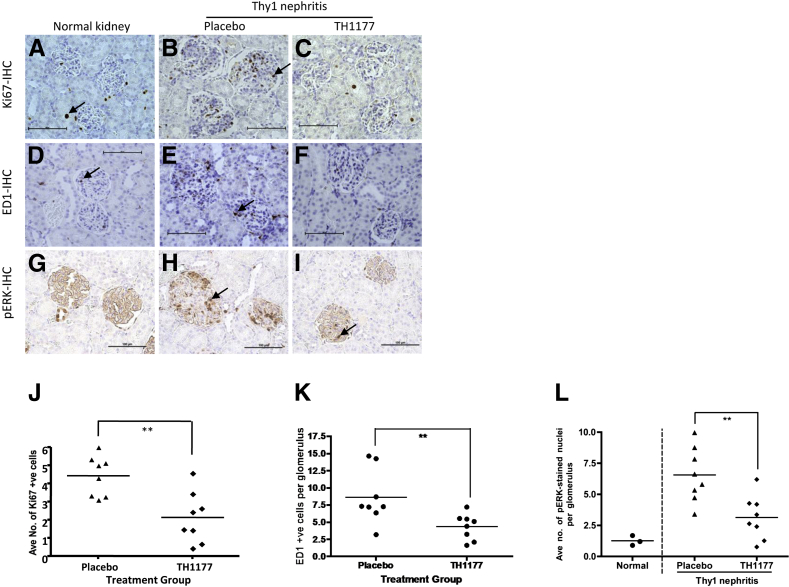
Figure 5 shows representative images of Ki-67 staining in normal kidney, placebo-treated Thy1 nephritis, and Thy1 nephritis treated with TH1177 (Figure 5, A–C, respectively). Very few Ki-67-positive cells are seen in normal glomeruli, in keeping with the quiescent nature of MCs in the healthy kidney. The increase after disease induction is reduced significantly by TH1177 (average number of Ki-67-positive cells per glomerulus in placebo-treated Thy1 nephritis group versus the TH1177-treated group, 4.43 ± 0.39 versus 2.13 ± 0.50, respectively; P < 0.01) (Figure 5J).
Figure 5.
Treatment of Thy1 nephritis with TH1177 reduces glomerular cell proliferation, macrophage infiltration, and ERK activation. Representative images of kidneys show they were immunostained for Ki-67 (A–C), ED1 (D–F), and pERK (G–I). Examples of positively stained cells are marked with arrows. J: TH1177 treatment of Thy1 nephritis caused a significant reduction in Ki-67-positive nuclei per glomerulus. ∗∗P = 0.007 versus placebo. K: There were significantly fewer ED1-positive cells in the TH1177-treated group than the placebo group. ∗∗P = 0.007. L: The reduction in glomerular cell proliferation was paralleled by a 52% reduction in glomerular cells stained positively for phosphorylated ERK. ∗∗P < 0.005 versus placebo.
Results of immunostaining for ED1, a macrophage marker, are also shown in Figure 5, D–F. Again, few macrophages are seen in normal glomeruli, but their numbers increase after induction of nephritis. Treatment with TH1177 reduces the average number of ED1-positive cells per glomerulus from 8.62 ± 1.39 to 4.33 ± 0.67 in the placebo-treated Thy1 nephritis group (P < 0.01) (Figure 5K).
TH1177 Reduces ERK Activation in Thy1 Nephritis
The results of immunostaining for pERK are shown in Figure 5, G–I. The number of cells exhibiting positive staining for pERK increases in the glomeruli of animals with Thy1 nephritis compared to normal kidneys. This is significantly attenuated by TH1177 treatment (average number of pERK-expressing cells per glomerulus in placebo-treated Thy1 nephritis group versus TH1177-treated group, 6.56 ± 0.77 versus 3.13 ± 0.62, respectively; P < 0.005) (Figure 5L).
Discussion
These data demonstrate that TCC inhibition with two independent agents inhibits primary rat MC proliferation in vitro, whereas blockade of LCC has no effect. Furthermore, TH1177 treatment reduces glomerular injury and glomerular cell proliferation in Thy1 nephritis at a dose that does not lower systemic BP. These anti-proliferative actions appear to involve modulation of the ERK signaling pathway. These findings demonstrate that TCC blockade is a useful strategy for studying and modifying MC proliferation in vivo, as well as in vitro.
Twenty years ago, Xu and Best8 first identified an association between T-type current and growth in atrial myocytes. Cells isolated from rats with growth hormone-secreting tumors had increased T-type current density compared to controls. These changes preceded any increase in heart weight or myocyte size, suggesting a causative role. Since these early observational studies, there have been a number of interventional experiments supporting the role of TCC in proliferation. Rodman et al9 demonstrated expression of the Cav3.1 isoform in pulmonary artery myocytes. Knockdown of this isoform using five independent siRNA sequences caused a reduction in proliferation that appeared to correlate with the degree of knockdown achieved. Overexpression of the Cav3.2 isoform in vascular smooth muscle cells markedly increases their proliferative rate.15 T-type currents have also been recorded in human MCs and knockdown of the Cav3.2 isoform in these cells is anti-proliferative. This is associated with an increase in the proportion of cells in G1 and a reduction in S-phase, suggesting a role for TCCs in facilitating progression across the G1/S boundary of the cell cycle.10
The in vitro results presented here support a similar role for TCCs in regulating rat MC proliferation. We cannot exclude the possibility that TH1177 may affect ion channels other than TCCs. However, published data in two different prostate cancer cell lines demonstrate that TH1177 inhibits influx of extracellular calcium, but it has no effect on calcium release from internal stores.6 LCCs are known to be present in MCs and are an important pathway for the influx of extracellular calcium leading to MC contraction. Importantly, the anti-proliferative effect seen with TH1177 and nickel is not found with concentrations of verapamil that are known to completely abolish L-type current.16 Expression of TRP channels in MCs has also been described,17 but a comprehensive study of the expression of the individual TRP channel isoforms and their role in MC proliferation has not been undertaken and is beyond the scope of the current study. These channels have been implicated in proliferation in other cell types,18 and it is possible that these channels also have a role in regulating the proliferative rate of MCs. However, the use of two independent TCC blocking agents that are unlikely to have overlapping off-target profiles, along with the specificity of low concentrations of nickel for TCCs over other ion channels, make TCC inhibition the most likely mechanism of action in this study. Previous work using siRNA to knockdown TCCs with high specificity in human MCs also supports the role of these channels in the regulation of MC proliferation.10
TCC inhibition has been shown to be nephroprotective in a number of different animal models of renal disease. Sugano et al19 studied subtotal nephrectomy in spontaneously hypertensive rats and found that the Cav3.1 isoform was upregulated in glomeruli of diseased animals. Selective blockade of TCCs, using the R(-)-enantiomer of efonidipine, reduced glomerular hypertrophy and interstitial fibrosis in this model. A study of renal injury in deoxycorticosterone acetate-salt hypertensive rats showed significant benefit of mibefradil (a combined T and L-type channel blocker) in comparison with amlodipine on glomerular injury and proteinuria.20 This occurred despite both agents reduction of systemic BP to the same degree. Importantly, in this study both agents also equally reduced glomerular capillary pressure, suggesting that the benefit of TCC blockade cannot be attributed solely to improved intraglomerular hemodynamics. Mibefradil inhibits proliferation of both rat (our unpublished observations) and human MCs in vitro,10 and therefore may be exerting its beneficial effect in part by reduction of aberrant MC proliferation in this model.
The in vivo data detailed here demonstrate that TH1177 reduces glomerular injury and glomerular cell proliferation in acute Thy1 nephritis in rats. This occurs at doses that have no impact on systemic BP, making it unlikely that this dose of TH1177 has any significant LCC blocking action in these animals. These findings support the hypothesis that TCC blockade inhibits MC proliferation in vivo, as well as in vitro. Despite increasing interest in the involvement of TCC in proliferation in a variety of cell types, the intracellular signaling pathways responsible for mediating the effect of this tiny and transient Ca2+ influx are poorly understood. The mitogen-activated protein kinase cascade is recognized as being a central pathway in mesangioproliferative disease and inhibition of this pathway at various levels has been shown to reduce MC proliferation.21,22 Moreover, extracellular calcium influx is capable of stimulating ERK signaling via protein kinase C, Pyk2, or CaMKII, as well as potentially through calmodulin-dependent activation of Ras-GRF. The demonstration that TH1177 reduces the abundance of pERK in the glomeruli of animals with Thy1 nephritis supports the hypothesis that its anti-proliferative actions are mediated via suppression of this signaling pathway. Further work is required to delineate the exact mechanism by which calcium influx through TCCs influences ERK activation in glomeruli.
This work also demonstrates the novel finding of distinct expression patterns of Cav3.1 and Cav3.2 TCC isoforms within the rat kidney, and an isoform-specific response to disease. The increase in expression of the Cav3.2 isoform in Thy1 nephritis directly correlates with glomerular injury, but surprisingly occurs predominantly in the nonglomerular cortex. It is probable, therefore, that the correlation with glomerular injury reflects a correlation of both parameters with disease severity. TCC inhibition has been shown to reduce proliferation in fibroblasts23 and peripheral blood mononuclear cells,24 as well as vascular smooth muscle cells.14 Therefore, the increase in Cav3.2 expression may be due, in part, to an influx of leukocytes into the interstitial compartment, or to activation and proliferation of resident cells such as fibroblasts. It is possible that TH1177 has some impact on these cells in this disease. However, the key pathology in this model is glomerular injury with increased glomerular cellularity, and TH1177 has a clear impact on this process. This is most likely due to a direct effect on glomerular MC proliferation as demonstrated in vitro.
It is important to note that although MC proliferation is a key finding in diseases such as IgA nephropathy and mesangiocapillary glomerulonephritis, there is some controversy as to whether this is a causal process or simply a response to the injury of other glomerular cells. In certain circumstances, the proliferative response of MC to injury may be necessary to allow repopulation and repair of the glomerulus, as is seen in postinfectious glomerulonephritis. Reducing MC proliferation in this setting may exacerbate rather than ameliorate disease. However, a number of studies have demonstrated that stimulating MC proliferation in vivo, by administration of mitogens such as platelet-derived growth factor and basic fibroblast growth factor, leads to glomerular damage and increased matrix deposition. This suggests that simply stimulating aberrant MC activation and proliferation can induce a disease phenotype.25 Modification of the persistent activation of MC in the context of ongoing stimulation (such as that seen in IgA nephropathy) is likely, therefore, to be of benefit, even if this is not the primary pathological entity.26
The finding of predominant Cav3.1 expression in rat glomeruli is in keeping with published data from Sugano et al,19 but these authors did not describe the expression patterns of the other isoforms in this model. This is also consistent with our observation that Cav3.1 is the main isoform identified in primary rat MCs in culture, in contrast to primary human MCs, which express the Cav3.2 isoform only.10 This suggests that there is an interspecies variation in the expression of TCC isoforms, which may become an important consideration as isoform-specific T-type inhibitors are developed and tested with the ultimate aim of clinical application. TH1177 inhibits both of the TCC isoforms found in the rat kidney cortex, but with a higher affinity for Cav3.1 blockade, suggesting that this may be the key target for the amelioration of glomerular injury by TH1177 in this disease model.
We have found that TH1177 reduces glomerular cell proliferation in vivo, as well as in vitro. Further work is needed to clarify the exact roles of the different TCC isoforms and their downstream signaling pathways in renal disease to provide information for the development and study of appropriate selective TCC inhibitors. Studies in chronic disease models in animals, as well as large clinical trials with long-term follow-up, are needed to determine whether TCC inhibitors are superior to LCC blockers in slowing the progression of chronic kidney disease.
Limitations of This Study
Although we have demonstrated distinct expression of Cav3.1 and Cav3.2 in the rat kidney at the RNA level, attempts to delineate their exact localization using immunohistochemistry have been unsuccessful in our hands. The existing antibodies that we have worked with have a degree of cross-reactivity between isoforms and have produced conflicting results when used on tissue sections and in Western blot analysis. Therefore, we cannot confidently say that the expression of T-type channel protein mirrors the changes seen in mRNA.
Footnotes
Supported by funding from the Medical Research Council and King’s College London.
The author N.D. is deceased.
Disclosures: M.J.S. is on the Expert Advisory Board of Tau Therapeutics. TH1177 was a gift from Tau Therapeutics, LLC (Charlottesville, VA).
References
- 1.Pabst R., Sterzel R.B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983;24:626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- 2.Jaber B.L., Madias N.E. Progression of chronic kidney disease: can it be prevented or arrested? Am J Med. 2005;118:1323–1330. doi: 10.1016/j.amjmed.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Saleh H., Schlatter E., Lang D., Pauels H.G., Heidenreich S. Regulation of mesangial cell apoptosis and proliferation by intracellular Ca(2+) signals. Kidney Int. 2000;58:1876–1884. doi: 10.1111/j.1523-1755.2000.00359.x. [DOI] [PubMed] [Google Scholar]
- 4.Orth S.R., Nobiling R., Bonisch S., Ritz E. Inhibitory effect of calcium channel blockers on human mesangial cell growth: evidence for actions independent of L-type Ca2+ channels. Kidney Int. 1996;49:868–879. doi: 10.1038/ki.1996.120. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Reyes E. Three for T: Molecular analysis of the low voltage-activated calcium channel family. Cell Mol Life Sci. 1999;56:660–669. doi: 10.1007/s000180050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haverstick D.M., Heady T.N., Macdonald T.L., Gray L.S. Inhibition of human prostate cancer proliferation in vitro and in a mouse model by a compound synthesized to block Ca2+ entry. Cancer Res. 2000;60:1002–1008. [PubMed] [Google Scholar]
- 7.Guo W., Kamiya K., Kodama I., Toyama J. Cell cycle-related changes in the voltage-gated Ca2+ currents in cultured newborn rat ventricular myocytes. J Mol Cell Cardiol. 1998:1095–1103. doi: 10.1006/jmcc.1998.0675. [DOI] [PubMed] [Google Scholar]
- 8.Xu X.P., Best P.M. Increase in T-type calcium current in atrial myocytes from adult rats with growth hormone-secreting tumors. Proc Natl Acad Sci USA. 1990;87:4655–4659. doi: 10.1073/pnas.87.12.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodman D.M., Reese K., Harral J., Fouty B., Wu S., West J., Hoedt-Miller M., Tada Y., Li K.X., Cool C., Fagan K., Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res. 2005;96:864–872. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- 10.Mulgrew C.J., Cove-Smith A., McLatchie L.M., Brooks G., Shattock M.J., Hendry B.M. Inhibition of human mesangial cell proliferation by targeting T-type calcium channels. Nephron Exp Nephrol. 2009;113:e77–e88. doi: 10.1159/000232590. [DOI] [PubMed] [Google Scholar]
- 11.Linz P. W.S., Amann K., Hilgers K.F., Veelken R. Is there a role for T-type Ca-channels of mesangial cells cultured in high glucose media? (abstract) J Am Soc Nephrol. 2004:727A. [Google Scholar]
- 12.Aguas A.P., Nickerson P.A. Effect of verapamil on blood pressure and lesions in heart and kidney of rats made hypertensive by deoxycorticosterone (DOC) Am J Pathol. 1983;110:48–54. [PMC free article] [PubMed] [Google Scholar]
- 13.Gaber L., Walton C., Brown S., Bakris G. Effects of different antihypertensive treatments on morphologic progression of diabetic nephropathy in uninephrectomized dogs. Kidney Int. 1994;46:161–169. doi: 10.1038/ki.1994.255. [DOI] [PubMed] [Google Scholar]
- 14.Moriyama T., Oka K., Ueda H., Imai E. Nilvadipine attenuates mesangial expansion and glomerular hypertrophy in diabetic db/db mice, a model for type 2 diabetes. Clin Exp Nephrol. 2004;8:230–236. doi: 10.1007/s10157-004-0303-1. [DOI] [PubMed] [Google Scholar]
- 15.Brooks G.H.J., Bates S.E. Over-expression of the voltage-gated T-type calcium channel induces vascular smooth muscle cell proliferation (abstract) Circulation. 1999;100:1–209. [Google Scholar]
- 16.Kuga T., Sadoshima J., Tomoike H., Kanaide H., Akaike N., Nakamura M. Actions of Ca2+ antagonists on two types of Ca2+ channels in rat aorta smooth muscle cells in primary culture. Circ Res. 1990;67:469–480. doi: 10.1161/01.res.67.2.469. [DOI] [PubMed] [Google Scholar]
- 17.Sours S., Du J., Chu S., Ding M., Zhou X.J., Ma R. Expression of canonical transient receptor potential (TRPC) proteins in human glomerular mesangial cells. Am J Physiol Renal Physiol. 2006;290:F1507–F1515. doi: 10.1152/ajprenal.00268.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zeng B., Yuan C., Yang X., Atkin S.L., Xu S.Z. TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Curr Cancer Drug Targets. 2013;13:103–116. [PubMed] [Google Scholar]
- 19.Sugano N., Wakino S., Kanda T., Tatematsu S., Homma K., Yoshioka K., Hasegawa K., Hara Y., Suetsugu Y., Yoshizawa T., Utsunomiya Y., Tokudome G., Hosoya T., Saruta T., Hayashi K. T-type calcium channel blockade as a therapeutic strategy against renal injury in rats with subtotal nephrectomy. Kidney Int. 2008;73:826–834. doi: 10.1038/sj.ki.5002793. [DOI] [PubMed] [Google Scholar]
- 20.Baylis C., Qiu C., Engels K. Comparison of L-type and mixed L- and T-type calcium channel blockers on kidney injury caused by deoxycorticosterone-salt hypertension in rats. Am J Kidney Dis. 2001;38:1292–1297. doi: 10.1053/ajkd.2001.29227. [DOI] [PubMed] [Google Scholar]
- 21.Bokemeyer D., Panek D., Kramer H.J., Lindemann M., Kitahara M., Boor P., Kerjaschki D., Trzaskos J.M., Floege J., Ostendorf T. In vivo identification of the mitogen-activated protein kinase cascade as a central pathogenic pathway in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 2002;13:1473–1480. doi: 10.1097/01.asn.0000017576.50319.ac. [DOI] [PubMed] [Google Scholar]
- 22.Clarke H.C., Kocher H.M., Khwaja A., Kloog Y., Cook H.T., Hendry B.M. Ras antagonist farnesylthiosalicylic acid (FTS) reduces glomerular cellular proliferation and macrophage number in rat thy-1 nephritis. J Am Soc Nephrol. 2003;14:848–854. doi: 10.1097/01.asn.0000057543.55318.8b. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Estacion M., Mordan L.J. Ca2+ influx via T-type channels modulates PDGF-induced replication of mouse fibroblasts. Am J Physiol. 1993;265:C1239–C1246. doi: 10.1152/ajpcell.1993.265.5.C1239. [DOI] [PubMed] [Google Scholar]
- 24.Lijnen P., Fagard R., Petrov V. Mibefradil-induced inhibition of proliferation of human peripheral blood mononuclear cells. J Cardiovasc Pharmacol. 1999;33:595–604. doi: 10.1097/00005344-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Floege J., Eng E., Young B.A., Alpers C.E., Barrett T.B., Bowen-Pope D.F., Johnson R.J. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest. 1993;92:2952–2962. doi: 10.1172/JCI116918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson R.J., Floege J., Yoshimura A., Iida H., Couser W.G., Alpers C.E. The activated mesangial cell: a glomerular “myofibroblast”? J Am Soc Nephrol. 1992;2:S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]